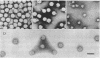
Abstract
Labeling experiments with formyl-[35S]-methionyl sulfone methylphosphate and crosslinking studies with dimethylsuberimidate suggest that the spike glycoproteins of Semliki Forest virus extend through the viral membrane into close contact with the nucleocapsid. Based on this finding, we present a mechanism for the formation of virus-specific patches in the host cell plasma membrane during virus assembly.
Keywords: formyl-[35S]methionyl sulfone methylphosphate, dimethylsuberimidate, peptide mapping, sodium dodecyl sulfate-gel electrophoresis
Full text
PDF
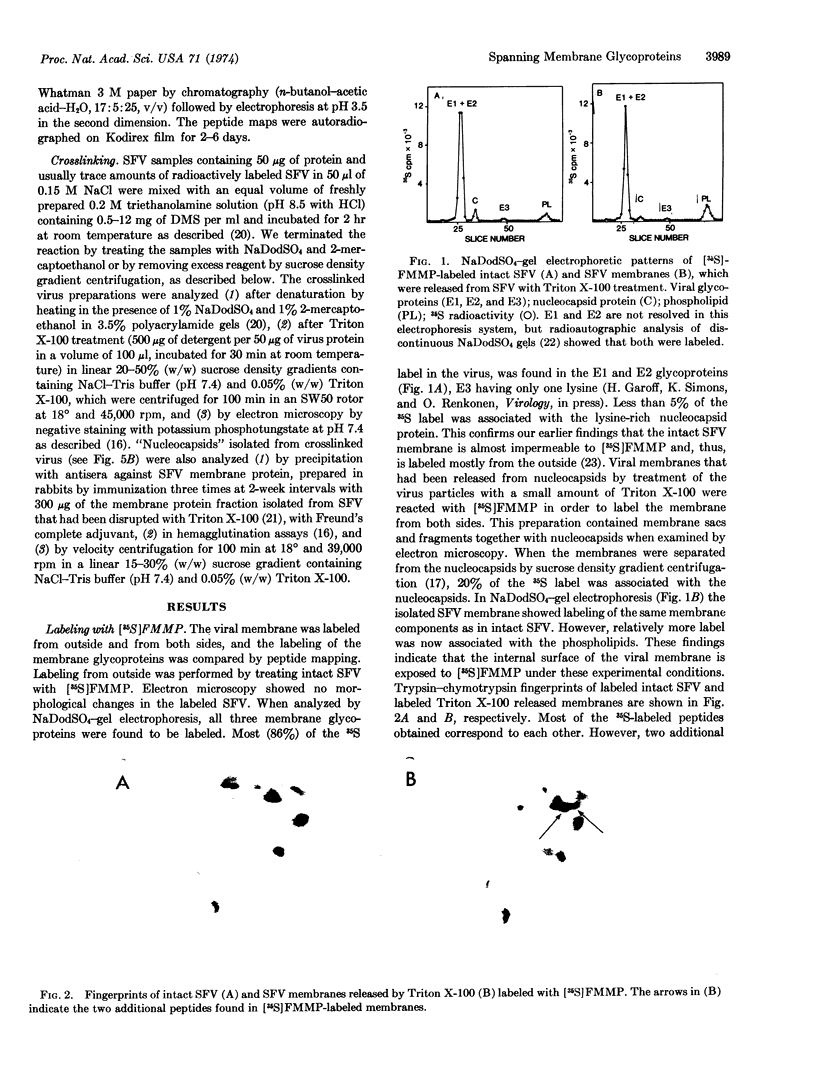
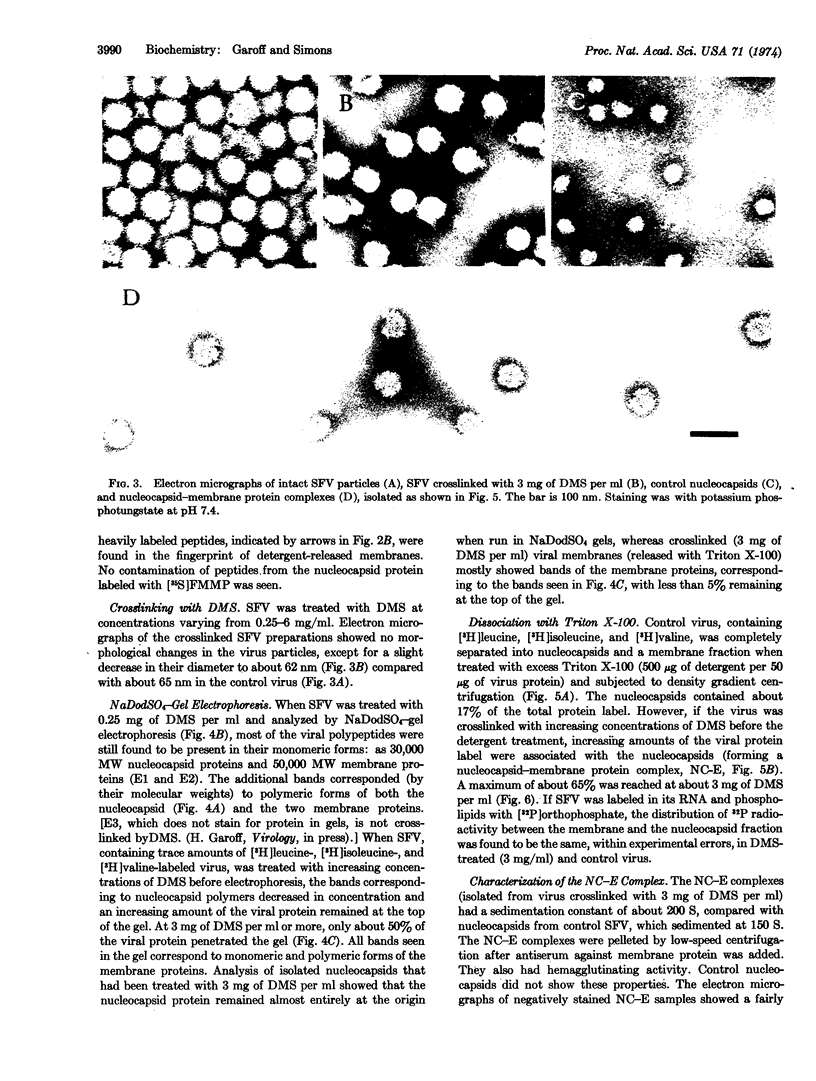

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Hershey J. W., Traut R. R. Spatial arrangement of ribosomal proteins: reaction of the Escherichia coli 30S subunit with bis-imidoesters. Proc Natl Acad Sci U S A. 1972 May;69(5):1327–1331. doi: 10.1073/pnas.69.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Human erythrocyte membranes: specific labelling of surface proteins. J Mol Biol. 1971 Jun 28;58(3):775–781. doi: 10.1016/0022-2836(71)90039-8. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Phosphatidyl-ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane-impermeable reagent. J Mol Biol. 1972 Nov 28;71(3):523–528. doi: 10.1016/s0022-2836(72)80020-2. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Mosser A. G. Proteins associated with the poliovirus RNA replication complex. Virology. 1971 Nov;46(2):375–386. doi: 10.1016/0042-6822(71)90039-0. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Adams M., Singer S. J. Bifunctional imidoesters as cross-linking reagents. Biochem Biophys Res Commun. 1966 Jun 13;23(5):730–739. doi: 10.1016/0006-291x(66)90462-1. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Simons K., Renkonen O., Käriäinen L. Exposure of proteins and lipids in the Semliki forest virus membrane. Virology. 1972 Oct;50(1):259–262. doi: 10.1016/0042-6822(72)90367-4. [DOI] [PubMed] [Google Scholar]
- Hartman F. C., Wold F. Cross-linking of bovine pancreatic ribonuclease A with dimethyl adipimidate. Biochemistry. 1967 Aug;6(8):2439–2448. doi: 10.1021/bi00860a021. [DOI] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C., Bode U., Kurland C. G., Stöffler G. Ribosomal protein neighborhoods. 3. Cooperativity of assembly. Mol Gen Genet. 1974 Mar 14;129(2):167–176. doi: 10.1007/BF00268629. [DOI] [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Marinetti G. V., Baumgarten R., Sheeley D., Gordesky S. Cross-linking of phospholipids to proteins in the erythrocyte membrane. Biochem Biophys Res Commun. 1973 Jul 2;53(1):302–308. doi: 10.1016/0006-291x(73)91434-4. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Wold F. Cross-linking of erythrocyte membranes with dimethyl adipimidate. Biochim Biophys Acta. 1970;196(2):170–175. doi: 10.1016/0005-2736(70)90004-0. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Käräinen L., Simons K., Gahmberg C. G. The lipid class composition of Semliki forest virus and plasma membranes of the host cells. Virology. 1971 Nov;46(2):318–326. doi: 10.1016/0042-6822(71)90033-x. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Marchesi V. T., Guyer R. B., Terry W. Red cell membrane glycoprotein: amino acid sequence of an intramembranous region. Biochem Biophys Res Commun. 1972 Nov 15;49(4):964–969. doi: 10.1016/0006-291x(72)90306-3. [DOI] [PubMed] [Google Scholar]
- Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol. 1973 Oct 15;80(1):119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Simons K., Käriäinen L. Characterization of the Semliki Forest virus core and envelope protein. Biochem Biophys Res Commun. 1970 Mar 12;38(5):981–988. doi: 10.1016/0006-291x(70)90818-1. [DOI] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]