Significance
Extracellular matrix (ECM) stiffness is an influential factor in many biological processes. Temporal changes in ECM stiffness are observed in cancer, cardiovascular disease, and wound healing, and are likely involved in disease progression. However, no cell culture systems exist to appropriately model temporal changes in ECM stiffness to determine the biological relevance and mechanisms involved. Here, we present a 3D hydrogel cell culture system in which the matrix stiffness can be tuned by light. Our approach offers both spatial and temporal control of stiffness, is compatible with cell culture, and can be used transdermally for in vivo applications. This system is amenable to many applications to investigate the influence of matrix stiffness on cell behavior and fate.
Keywords: hydrogel, dynamic microenvironment, cell spreading, transdermal
Abstract
Hydrogels are widely used as in vitro culture models to mimic 3D cellular microenvironments. The stiffness of the extracellular matrix is known to influence cell phenotype, inspiring work toward unraveling the role of stiffness on cell behavior using hydrogels. However, in many biological processes such as embryonic development, wound healing, and tumorigenesis, the microenvironment is highly dynamic, leading to changes in matrix stiffness over a broad range of timescales. To recapitulate dynamic microenvironments, a hydrogel with temporally tunable stiffness is needed. Here, we present a system in which alginate gel stiffness can be temporally modulated by light-triggered release of calcium or a chelator from liposomes. Others have shown softening via photodegradation or stiffening via secondary cross-linking; however, our system is capable of both dynamic stiffening and softening. Dynamic modulation of stiffness can be induced at least 14 d after gelation and can be spatially controlled to produce gradients and patterns. We use this system to investigate the regulation of fibroblast morphology by stiffness in both nondegradable gels and gels with degradable elements. Interestingly, stiffening inhibits fibroblast spreading through either mesenchymal or amoeboid migration modes. We demonstrate this technology can be translated in vivo by using deeply penetrating near-infrared light for transdermal stiffness modulation, enabling external control of gel stiffness. Temporal modulation of hydrogel stiffness is a powerful tool that will enable investigation of the role that dynamic microenvironments play in biological processes both in vitro and in well-controlled in vivo experiments.
The extracellular matrix (ECM) undergoes dynamic remodeling during many developmental and disease processes. Dynamic changes to both the structure and composition of the ECM result in alterations to the biophysical properties of the matrix, which are known to have significant influence on cellular behavior, including migration (1), alignment (2), proliferation (3), morphology (4), and differentiation of progenitors (5, 6). Temporal changes in matrix stiffness occur in tissue development (7), liver fibrosis (8), tumor progression (9, 10), and myocardial infarction (11). As a clinically relevant example, healthy mammary gland tissue has an elastic modulus of 100–200 Pa, whereas mammary tumors are much stiffer, 1–4 kPa (9, 10). As a result of ECM remodeling and increased deposition of matrix proteins such as collagen I, tumor stiffness increases over time and correlates strongly with the severity of disease (9). Recently, it has been demonstrated that stiffness changes can contribute to disease progression and are not merely outcomes of disease (9, 12). For instance, in a rat liver fibrosis model, increases in tissue stiffness were seen several days before any histological evidence of fibrosis was observed (8). However, the mechanisms by which stiffness changes contribute to disease are still largely unknown. Ideally, dynamic processes could be studied in engineered cell culture systems in which stiffness changes could be controlled or induced, yet development and implementation of these systems remains challenging.
Hydrogels are ideal candidates to mimic ECM conditions because they can be fabricated from a variety of synthetic and natural polymers and can span the range of stiffness seen in soft tissue (13). Most hydrogel materials are tunable initially by changing polymer concentration or chain length, but have constant mechanical properties throughout the culture period. Degradation offers a means to soften gels over time, although in a passive and uncontrolled manner. Simple dynamic hydrogels were designed to degrade either hydrolytically (14) or enzymatically (15), and later engineered with degradation sites tailored to specific proteases (16). Recently, cell-mediated degradation was replaced with user-controlled degradation through a photocleavable moiety incorporated within the gel backbone, resulting in temporal softening of the gel (17). To model more physiologically relevant stiffening events, UV light-triggered secondary cross-linking mechanisms (18, 19) or kinetically slow reactions have been implemented (20). However, compared with near-infrared (NIR) light, UV and visible wavelengths suffer from decreased penetration depth through water-based gels and especially through tissue, making translation to in vivo applications difficult (21). Further, the photoinitiators used to produce free radicals for polymerization can be cytotoxic (22). Additionally, covalent cross-links are irreversible by nature, and can abrogate some effects of matrix stiffness, such as directing stem cell differentiation, compared with ionically cross-linked gels (6, 19).
We sought to design a dynamically tunable system that could be stiffened or softened temporally in 3D constructs. We chose to use alginate in our system because guluronic regions of the polymer backbone physically cross-link in the presence of divalent cations. Therefore, the gel stiffness depends on the calcium concentration and can be temporally modulated by controlled introduction of additional calcium or calcium chelators. Here, we present a mechanism for NIR light-triggered release of calcium or chelator from temperature-sensitive liposomes using encapsulated gold nanorods to induce local heating upon irradiation. Our design allows for both stiffening and softening over time, either in bulk gels or patterned regions. Cell viability is maintained during stiffness modulation, and we demonstrate changes in cell morphology in response to stiffening, making the platform well suited for 3D cell encapsulation experiments. Finally, the use of NIR light allows for transdermal gelation and stiffness tuning, providing an avenue for in vivo translation of this technology.
Results and Discussion
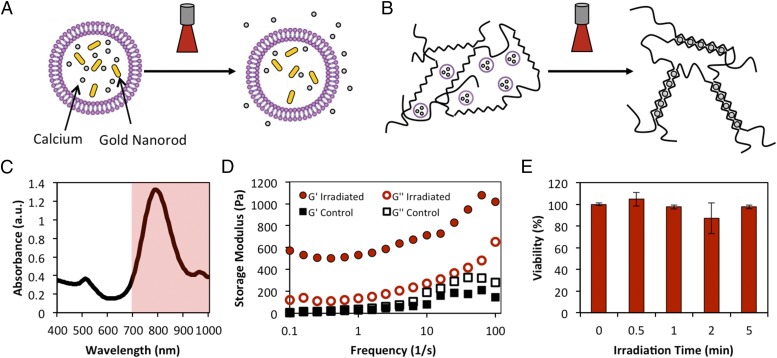
Previously, calcium has been caged and released via irradiation with UV light to gel alginate in microfluidic systems (23), but the bound calcium concentration is low and the costs are prohibitive for macroscale studies. We overcame these limitations by encapsulating high concentrations of CaCl2 (55 mM, Fig. S1) in large, unilamellar liposomes (1–5 µm) produced via the interdigitation fusion method (24). The liposomes were formed from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), which undergoes a gel-to-fluid phase transition at 41 °C. Gold nanorods were also encapsulated in the liposomes as the triggering agent (Fig. 1A and Fig. S2). Upon irradiation with NIR light near the absorption peak (Fig. 1C), the gold nanorods undergo surface plasmon resonance and locally heat their surroundings. Thus, local heating induced by the irradiation of the gold nanorods allows previously entrapped Ca2+ to permeate the vesicle bilayer (25), resulting in gelation and increased cross-linking density in alginate (Fig. 1B and Fig. S3). Calcium and gold nanorod-loaded liposomes were mixed with a solution of alginate and irradiated with a continuous wave 808-nm laser (1.78 W/cm2). The irradiated solution rapidly formed a gel, with a storage modulus much greater than the unirradiated control solution (Fig. 1D). To assess whether heating and calcium release negatively affected viability or proliferation, Live/Dead and MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assays were performed on MCF10A mammary epithelial cells distributed within a 3D alginate gel along with liposomes. The gels were irradiated for various times, and assays were carried out 24 h after irradiation. We observed no decrease in viability or proliferation due to the presence of liposomes or irradiation (Fig. 1E and Figs. S4 and S5).
Fig. 1.
Mechanism for light-triggered release from liposomes. (A) Schematic of temperature-sensitive liposome loaded with gold nanorods and calcium. Upon irradiation, gold nanorods heat the lipid bilayer past its transition temperature, allowing calcium to permeate the lipid bilayer. (B) Irradiation of liposomes mixed with an alginate solution causes release of calcium and an increase in cross-linking density. (C) Absorbance spectrum of gold nanorods, demonstrating the narrow peak in NIR region. Shading denotes the optical window of highest penetration depth through tissue. (D) Rheometry of alginate:liposome solutions after irradiation. Irradiation causes release of calcium and gelation, shown by increased G′ over controls. (E) MTS assay demonstrates no loss of viability after irradiation of cell-seeded gels.
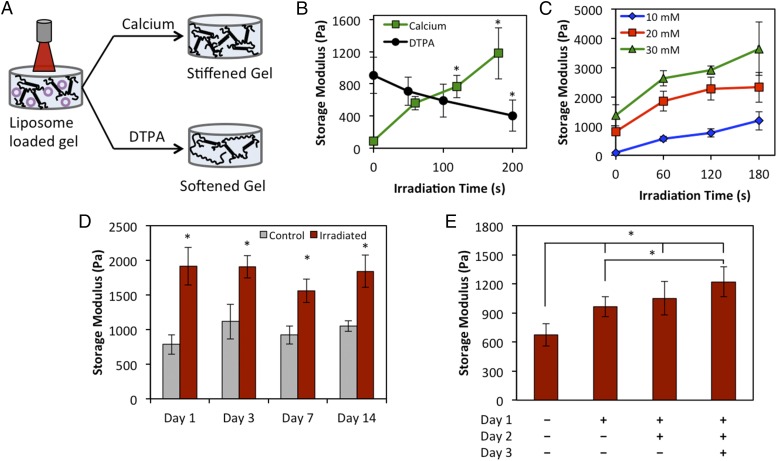
Dynamic tuning of gel stiffness was demonstrated by distributing liposomes within a 3D (8-mm-diameter, 3-mm-thick) alginate gel, irradiating the gel, and performing rheometry after overnight swelling in Dulbecco's phosphate-buffered saline (DPBS) (Fig. 2A). The initial stiffness of the gels can be varied by altering the initial calcium concentration (Fig. S6). Relatively compliant gels (10 mM Ca2+ initially) could be stiffened from 91-Pa storage modulus to 1,179 Pa after 180 s of irradiation (Fig. 2B). The stiffness of the gel increases with the irradiation time, due to the increased release of calcium from the liposomes. Initially stiffer gels consisting of 20 mM and 30 mM Ca2+ stiffened at a faster rate and with a greater overall change in magnitude, generating changes in storage modulus of 1,511 and 2,279 Pa, respectively (Fig. 2C). The stiffening profile and net change in stiffness also increase with increasing concentration of liposomes in the gels, as more calcium is available for release (Fig. S7). Thus, the user has a high degree of tunability for modeling physiological systems by varying initial stiffness, liposomes concentration, and irradiation time. In general, our approach is suitable for modulating hydrogels within the range of stiffness of soft tissues (storage modulus of 10–5,000 Pa), with the understanding that the maximum stiffness obtainable depends on the initial gel stiffness. The range of stiffening for our gels spans that of observed stiffness increases during fibrotic events like tumor progression (9, 10). Additionally, differential stem cell lineage commitment in 3D hydrogels (6) has been reported within the range of our system, where adipogenesis is predominant from 2.5- to 5-kPa elastic modulus and osteogenesis is favored as low as 11 kPa.
Fig. 2.
Modulation of matrix stiffness in 3D gels. (A) Liposomes loaded with gold nanorods and either CaCl2 (stiffening) or DTPA (softening) are distributed throughout a 3D alginate gel and irradiated for various times. (B) Irradiation induces release of liposomal cargo and either stiffening or softening. (C) Stiffening rate and magnitude can be varied by changing the initial stiffness of the gels. All irradiation timepoints are statistically significantly greater than unirradiated controls (P < 0.05) except 10 mM, 60 s. (D) Longitudinal study to demonstrate stiffening can be induced at least 14 d after initial gelation. (E) Multiple irradiations of the same gel on consecutive days cause gradual stiffening. * denotes statistical significance of P < 0.05.
Unlike covalently cross-linked hydrogels, alginate cross-links are reversible and the cross-linking density can be reduced to soften gels. To achieve softening of bulk 3D gels, we loaded diethylenetriaminepentaacetic acid (DTPA), a calcium chelator, into the liposomes instead of CaCl2. Upon irradiation and release, DTPA chelated calcium from the alginate cross-links, resulting in increasingly softer gels with irradiation time (ΔG′ = 502 Pa, Fig. 2B). The smaller overall change in stiffness for DTPA release compared with calcium is likely due to competition between alginate and DTPA for calcium ions. However, this difference is still within the range of stiffness changes measured during tumor progression (9) and will be useful for dynamic modeling. Finally, we verified that release of DTPA does not compromise viability (Figs. S4 and S5).
Experiments that would use a dynamic system such as the one presented here would require cell culture within the gels for a period of days to weeks before irradiation to modulate the stiffness. We mimicked this scenario by forming gels with CaCl2-loaded liposomes distributed throughout and incubating them for up to 2 wk before irradiation. Upon irradiation on day 1, 3, 7, or 14, the gels stiffened significantly compared with controls that were maintained for the same duration but not irradiated (Fig. 2D). Thus, longitudinal studies with dynamic stiffening events could be carried out using this platform. Additionally, we were able to gradually stiffen gels over the course of 3 d based on the number of irradiation doses to which the gels were exposed (Fig. 2E).
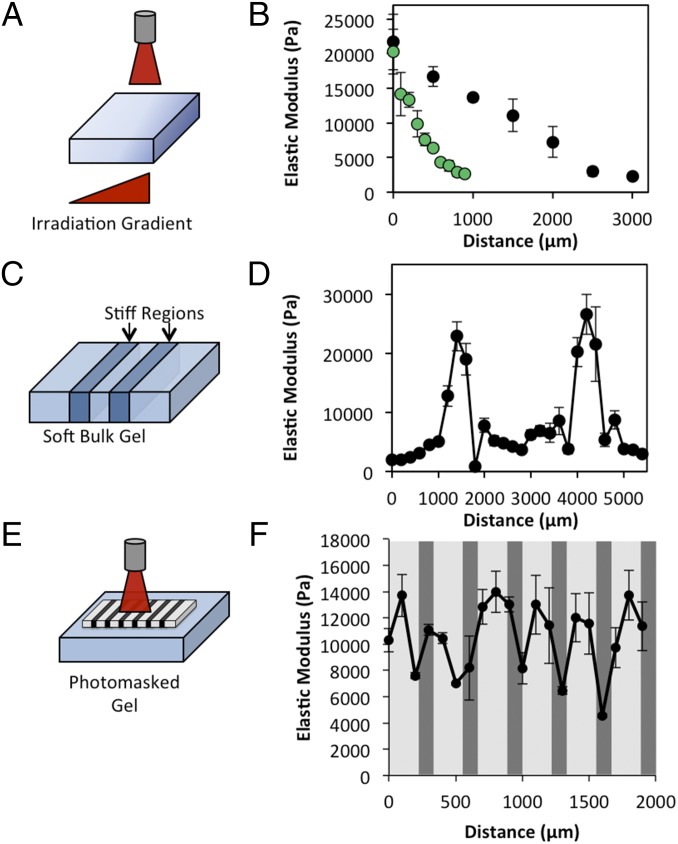
The light-based triggering modality allows direct control of not only when the gels are stiffened but also where stiffening occurs, by spatially controlling the laser profile. A stiffness gradient was formed in a gel slab by varying the irradiation intensity along a gradient (Fig. 3A). We were able to generate gradual or steep gradients of elastic modulus from ∼2 kPa to over 20 kPa, as measured by atomic force microscopy (Fig. 3B). In addition to forming stiffness gradients, our technique is well suited for generating locally stiffened regions in an initially uniform, soft gel by focusing the laser. Stiff regions were patterned in compliant bulk gels by focusing the laser beam to a small spot and translating the gel with a motorized stage (Fig. 3 C and D). Additionally, other common photolithographic techniques such as photomasking can be used to produce patterns as well. Using a metal-on-glass photomask, we were able to generate stiff patterns in an initially uniform, compliant gel (Fig. 3 E and F). The resolution we were able to achieve with these systems was between 200 and 400 μm, similar to other dynamic hydrogel systems (26). Spatial modulation of gel stiffness will allow for unique experimental designs in which the effects of matrix stiffness can be assessed on cells in close proximity and otherwise identical culture conditions.
Fig. 3.
Spatial tuning of gel stiffness. (A and B) Laser intensity gradients can be used to generate gradients in gel stiffness. Gels are stiffened in gradients of varying slopes. (C and D) Stiffness patterns can be made by focusing light on particular gel regions. (E and F) Metal-on-glass photomasks can be used to create defined stiffness patterns.
The influence of matrix stiffness on cell morphology in 3D hydrogels is not well understood. Unlike 2D substrates where cell spreading generally increases with stiffness (27, 28), cells seeded within 3D arginylglycylaspartic acid (RGD)-alginate hydrogels showed little variation in morphology across a wide range of stiffness (6). Later, cell-mediated degradation was demonstrated to be necessary for cell elongation within 3D covalently cross-linked gels, independent of matrix stiffness (19). However, recently matrix stiffness was shown to dictate cell morphology in 3D alginate–collagen I interpenetrating networks (29). Thus, the extent to which matrix stiffness or degradability regulates cell morphology in 3D environments is unclear. To address some outstanding questions from these previous studies, we investigated the morphological and phenotypic response of 3T3 fibroblasts to dynamic stiffening in both gels possessing degradable components and nondegradable gels. Alginate does not possess cell adhesive moieties, therefore we introduced cell adhesion sites through two approaches. In one set of gels, Matrigel was mixed with the alginate solution at 25% vol/vol. RGD-conjugated alginate was used in a second set of experiments to reduce the complexity of the system, namely the ability for cells to degrade the matrix and the uncertain nature of mechanotransduction in an alginate–Matrigel composite network. NIH 3T3 fibroblasts were cultured for 24 h in compliant gels and then irradiated for 30, 60, or 120 s or left unirradiated as controls. As demonstrated above, the stiffened gels had a modulus proportional to the irradiation time (Fig. 4A). After an additional 48 h, the cells were stained with calcein acetoxymethyl (AM) ester to ensure viability was maintained and to provide fluorescent images of cell morphology.
Fig. 4.
Stiffening inhibits elongated morphologies in fibroblasts in both degradable and nondegradable gels. (A) NIH 3T3 fibroblasts were encapsulated in alginate gels containing Matrigel as a cell adhesive ligand and CaCl2-loaded liposomes. After 24 h, gels were increasingly irradiated to induce stiffening. After a total of 72 h, the cells were imaged using phase contrast and two-photon microscopy (calcein-AM stained). Z projections of calcein-AM stained cells demonstrate more rounded cells in gels that were stiffened by irradiation, whereas mesenchymal morphologies were observed in soft control gels. Scale bar, 100 µm. (B) Gels made with RGD-conjugated alginate were used in place of the Matrigel–alginate composites from A. The cells transitioned from an elongated, amoeboid morphology to a rounded shape as the gels were stiffened. (C–F) Metrics based on traces of at least 25 cells from each group of A. Cells became more rounded as irradiation time (stiffness) increased. Circularity was calculated as P2/4πA and equals 1 for a perfect circle. * indicates statistical significance of P < 0.05.
In the alginate–Matrigel composites, we observed elongated cells in the unirradiated group (statically soft) that became more rounded with increasing irradiation time and thus increased stiffening (Fig. 4A and Fig. S8). Cell perimeter, aspect ratio, and circularity all decreased significantly after 120 s of irradiation compared with unirradiated controls (Fig. 4 C–F). However, cell area was not affected by irradiation. In compliant gels, the fibroblasts were able to enzymatically degrade or mechanically deform the matrix and form extensions into the gels. As the gels were stiffened, the cells’ ability to remodel the matrix was impaired and the cells were predominately rounded, either because the matrix was too stiff to deform or because degradation sites were blocked by the introduction of additional cross-links (19). Using alginate–collagen I interpenetrating networks, a similar inverse relationship between cell elongation and matrix stiffness has been observed with both static and dynamically cross-linked systems (29, 30). However, it is still unclear if the inhibition of cell elongation is due to matrix stiffness per se, or because the high cross-link density blocks degradation sites.
To address this question, we used nondegradable RGD-conjugated alginate gels. If degradation were necessary for cell elongation in 3D, then the cells would remain rounded independent of matrix stiffening. Interestingly, we again observed increased elongation in soft gels and more rounded morphologies in dynamically stiffened gels (Fig. 4B and Fig. S8). Notably, in RGD-conjugated alginate, the fibroblasts adopted an amoeboid morphology where elongated cells formed blebs extending into the soft gels, morphologically distinct from the mesenchymal migration observed in alginate–Matrigel composites (31). After stiffening the gels, the amoeboid cells were no longer able to deform the polymer network enough to elongate. Thus, we demonstrate that 3D matrix stiffness dictates cell morphology in both degradable and nondegradable gels, whether cells have adopted mesenchymal or amoeboid migration modes.
We sought to further confirm the morphological changes were a direct result of stiffening by seeding fibroblasts on top of 2D RGD-alginate gels. On stiff 2D substrates, cells tend to spread, whereas they remain rounded on soft substrates (27, 28). Indeed, with our system, the cells remained rounded on the 2D control gels, yet spread after the gels were stiffened by irradiation (Fig. S9). This demonstrates, in the most conventional method, that cell morphology is dictated by dynamic stiffening in our system and validates that the previous results seen in 3D gels are a result of increased stiffness and not other factors, like heating or calcium release. It is interesting that the relationship between gel stiffness and cell spreading is seemingly contradictory between 2D and 3D gels. This highlights the need for more advanced 3D hydrogel models, like the one presented here, to more accurately recapitulate in vivo biology.
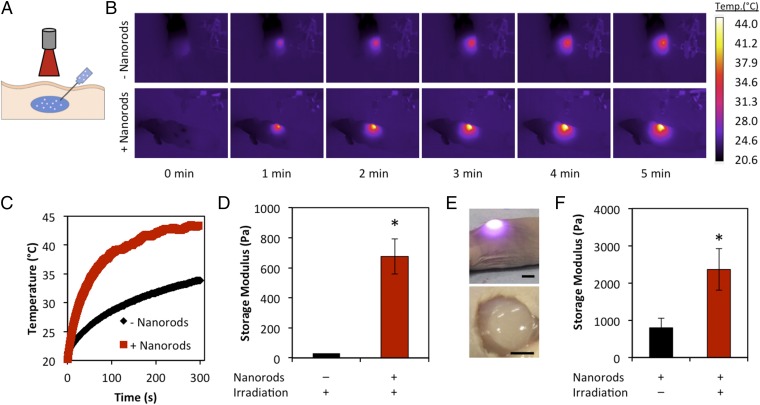
Hydrogels are used in vivo for delivery of therapeutics or cells, as tissue engineering constructs, and in studies interrogating the influence of matrices on disease (32, 33). Transdermal gelation provides a minimally invasive mode of hydrogel delivery (34). Furthermore, external modulation of stiffness could be used to tune drug release rates, alter construct degradation or remodeling, and modulate the biophysical properties of the injected matrix. NIR light falls within the “optical window,” a small range of wavelengths for which light penetration through tissue is maximized (21). We sought to use this feature of our design for transdermal gelation and stiffness modulation. An aqueous solution of alginate was mixed with 20% vol/vol calcium and gold nanorod-loaded liposomes and injected s.c. into the dorsal region of a nude mouse. As a control, liposomes without gold nanorods were used. The laser was positioned 20 mm away from the injection site and the solution was irradiated for 5 min (Fig. 5A). The temperature was monitored during the experiment with an IR camera. The presence of the gold nanorods resulted in a much faster heating rate (Fig. 5 B and C). Heating did occur to a lesser extent in the control group, which can be attributed to absorption of light by the skin. After irradiation, the gels were excised from the animals and rheometry was performed. The solutions with gold nanorod-loaded liposomes formed gels with storage moduli of 675 Pa, whereas the solution without gold nanorods remained un–cross-linked (Fig. 5 D and E). The un–cross-linked solutions could not be recovered for rheometry, so a fresh solution of alginate and liposomes was tested as a substitute.
Fig. 5.
Transdermal light-triggered gelation and stiffening. (A) An alginate solution containing CaCl2-loaded liposomes either with or without gold nanorods was injected s.c. into the dorsal region of a nude mouse. The region was irradiated for 5 min and gels were harvested for mechanical testing. (B) IR images of the mouse indicate significantly faster heating in gold nanorod-loaded liposomes. (C) Temperature profile over the course of irradiation. (D) Gold nanorod-loaded liposomes yielded gels that had significantly higher storage moduli than the ungelled alginate solution. (E) Photograph of mouse during irradiation (Top) and resulting gel after irradiation (Bottom). Scale bar, 5 mm. (F) Gels irradiated transdermally stiffened significantly compared with unirradiated controls.
After demonstrating the feasibility of transdermal gelation, we sought to translate our stiffening strategy to a transdermal model. Alginate was mixed with calcium and gold nanorod-loaded liposomes, 20 mM CaCO3, and 40 mM glucono-δ-lactone in a syringe. The solution was injected into the dorsal region of a nude mouse and allowed to gel. Half of the gels were then irradiated for 5 min to induce stiffening, whereas the other gels were not irradiated as controls. The gels were harvested and the storage modulus was measured via rheometry. We found the irradiated samples to be significantly stiffer than the control gels (2,363 Pa vs. 797 Pa, Fig. 5F) and statistically similar to 20 mM CaCO3 gels from the previous in vitro work (Fig. 2C). Thus, our system for stiffness modulation is translatable to in vivo models and could be broadly used to externally tailor factors that depend on gel stiffness.
We have developed an NIR light-triggered mechanism to increase or decrease the stiffness of 3D hydrogels with both spatial and temporal control. The system is cytocompatible and amenable to modeling dynamic phenomena, such as tumor progression or tissue fibrosis, to isolate the effects of matrix stiffening. We used this system to demonstrate the regulation of fibroblast morphology by matrix stiffness in both degradable and nondegradable gels. Furthermore, we have demonstrated the ability to control the release of cargo from liposomes via transdermal irradiation and remotely modulate the gel stiffness. Thus, it is possible to translate in vitro findings to more relevant in vivo models while maintaining the same hydrogel system.
Materials and Methods
Alginate was purchased from FMC Biopolymer (Pronova UP MVG) and dissolved to 4% wt/vol in DPBS. This solution was diluted to produce a final alginate concentration of 2% wt/vol for all gels. Liposomes were produced using the interdigitation fusion method (24). The samples were irradiated with an 808 nm continuous wave laser (Laser Lab Components, Inc.) at a fluence rate of 1.78 W/cm2. Rheometry was performed with an Anton Paar MCR101 rheometer with an 8-mm parallel plate geometry. Storage moduli are reported at 1.81 Hz throughout the article. Indentation tests were performed with an Asylum Research MFP-3D atomic force microscope using a cantilever (0.08 N/m spring constant) with an attached 10-μm borosilicate bead. The elastic modulus was determined using the Hertz model for a sphere, assuming the Poisson's ratio for the hydrogels was 0.5. For transdermal studies, the solutions and gels were injected s.c. in the dorsal region of nude mice. The injection site was irradiated for 5 min and monitored with an infrared camera (FLIR A325sc). The pixel intensity was converted to temperature after calibration with a blackbody source. For detailed methods, please refer to SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank S. Emelianov for access to the laser, J. Tunnell for use of the motorized stages, G. Luke, S. Lim, V. Pattani, and D. Hernandez for helpful technical discussion and assistance, and K. Eckes and J. Stachowiak for reviewing the manuscript. This work was supported by the Cancer Prevention and Research Institute of Texas (RP130372).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421897112/-/DCSupplemental.
References
- 1.Lo C-M, Wang H-B, Dembo M, Wang Y-L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi YS, et al. The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices. Biomaterials. 2012;33(29):6943–6951. doi: 10.1016/j.biomaterials.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3(2):77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- 4.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121(10):3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 8.Georges PC, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 9.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Berry MF, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290(6):H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6(1):290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 15.West JL, Hubbell J. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32(1):241–244. [Google Scholar]
- 16.Lutolf MP, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 19.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32(4):1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(4):316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 22.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26(11):1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Chueh BH, et al. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed Microdevices. 2010;12(1):145–151. doi: 10.1007/s10544-009-9369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahl PL, et al. Interdigitation-fusion: A new method for producing lipid vesicles of high internal volume. Biochim Biophys Acta. 1994;1195(2):237–244. doi: 10.1016/0005-2736(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 25.Andersen T, Kyrsting A, Bendix PM. Local and transient permeation events are associated with local melting of giant liposomes. Soft Matter. 2014;10(24):4268–4274. doi: 10.1039/c4sm00410h. [DOI] [PubMed] [Google Scholar]
- 26.Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31(32):8228–8234. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Pelham RJ, Jr, Wang Yl. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engler A, et al. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1 Pt 1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Branco da Cunha C, et al. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials. 2014;35(32):8927–8936. doi: 10.1016/j.biomaterials.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 30.Gillette BM, Jensen JA, Wang M, Tchao J, Sia SK. Dynamic hydrogels: switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv Mater. 2010;22(6):686–691. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 31.Friedl P, Wolf K. Plasticity of cell migration: A multiscale tuning model. J Cell Biol. 2010;188(1):11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32-33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater. 2012;11(8):734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gramlich WM, Holloway JL, Rai R, Burdick JA. Transdermal gelation of methacrylated macromers with near-infrared light and gold nanorods. Nanotechnology. 2014;25(1):014004. doi: 10.1088/0957-4484/25/1/014004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.