Significance
There are no effective treatments for dry mouth, a common problem that affects millions of people in the United States. Salivary gland hyposecretion is caused by multiple diseases, including Sjögren's syndrome, as well as by radiation therapy and medications. The resulting dry mouth is often associated with dysphagia, reduced taste sensation, and opportunistic infections. The mammalian fluid secretion model predicts that Ca2+-activated Tmem16A and/or cAMP-dependent Cftr Cl- channels are critical in this process. We demonstrate that activation of the Tmem16A channel is required for muscarinic, Ca2+-dependent salivation, but that β-adrenergic receptor-mediated salivation is independent of Tmem16A, Cftr, and ClC-2 Cl- channels. A better understanding of the β-adrenergic stimulated secretion mechanism may prove important in the development of therapeutic targets for dry mouth.
Keywords: acinar cell, secretion, Cl− channel, Tmem16A/Ano1, Cftr channel
Abstract
Activation of an apical Ca2+-activated Cl− channel (CaCC) triggers the secretion of saliva. It was previously demonstrated that CaCC-mediated Cl− current and Cl− efflux are absent in the acinar cells of systemic Tmem16A (Tmem16A Cl− channel) null mice, but salivation was not assessed in fully developed glands because Tmem16A null mice die within a few days after birth. To test the role of Tmem16A in adult salivary glands, we generated conditional knockout mice lacking Tmem16A in acinar cells (Tmem16A−/−). Ca2+-dependent salivation was abolished in Tmem16A−/− mice, demonstrating that Tmem16A is obligatory for Ca2+-mediated fluid secretion. However, the amount of saliva secreted by Tmem16A−/− mice in response to the β-adrenergic receptor agonist isoproterenol (IPR) was comparable to that seen in controls, indicating that Tmem16A does not significantly contribute to cAMP-induced secretion. Furthermore, IPR-stimulated secretion was unaffected in mice lacking Cftr (Cftr∆F508/∆F508) or ClC-2 (Clcn2−/−) Cl− channels. The time course for activation of IPR-stimulated fluid secretion closely correlated with that of the IPR-induced cell volume increase, suggesting that acinar swelling may activate a volume-sensitive Cl− channel. Indeed, Cl− channel blockers abolished fluid secretion, indicating that Cl− channel activity is critical for IPR-stimulated secretion. These data suggest that β-adrenergic–induced, cAMP-dependent fluid secretion involves a volume-regulated anion channel. In summary, our results using acinar-specific Tmem16A−/− mice identify Tmem16A as the Cl− channel essential for muscarinic, Ca2+-dependent fluid secretion in adult mouse salivary glands.
Mammalian salivary gland fluid secretion is highly regulated by the autonomic nervous system (1). The amount and composition of saliva varies depending on the autonomic division that triggers salivation. Parasympathetic-dependent secretion is associated with large fluid volume and low protein concentration, whereas sympathetic-dependent secretion is characterized by lower fluid volume and higher protein concentration (2).
The major ion-transporting proteins involved in fluid secretion have been identified in exocrine glands (1, 3). It was recently proposed in salivary glands that Tmem16A (4–6) encodes the apical Ca2+-activated Cl− channel (CaCC) efflux pathway required for Ca2+-dependent fluid secretion (6, 7), whereas the molecular identity of the apical Cl− efflux pathway involved in β-adrenergic receptor-activated secretion is unknown.
To evaluate the physiological role of Tmem16A in salivary glands, we generated mice with disrupted Tmem16A expression in secretory acinar cells. Our results demonstrate that muscarinic Ca2+-dependent fluid secretion is abolished in the salivary glands of Tmem16A null (Tmem16A−/−) mice, whereas β-adrenergic receptor-stimulated cAMP-dependent fluid secretion is normal. Given that cAMP-dependent Cftr Cl− channels are gated on β-adrenergic receptor stimulation and are highly expressed in salivary glands (8), we also analyzed fluid secretion in mice carrying the homologous ∆F508 mutation of the human CFTR channel, the mutation most frequently observed in cystic fibrosis patients (9). Saliva secretion induced by β-adrenergic receptor agonist isoproterenol (IPR) was unaffected in mice lacking Cftr and ClC-2 Cl− channels (Cftr∆F508/∆F508 and Clcn2−/− mice, respectively). In contrast, cAMP-dependent fluid secretion was inhibited by 4-[(2-Butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid (DCPIB) and 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), blockers of volume-regulated anion channel (VRAC). These results provide conclusive evidence that Tmem16A plays an essential role in muscarinic, Ca2+-dependent fluid secretion in adult mice. They also demonstrate that β-adrenergic induced secretion does not depend on Tmem16A, Cftr, or ClC-2 Cl− channels, but likely involves the ubiquitously expressed VRAC.
Results
Conditional Knockout of Tmem16A in Mouse Salivary Gland Acinar Cells.
A systemic Tmem16A null mutation has established that this gene encodes for the apical CaCC expressed in acinar cells of the mouse salivary gland (7); however, the role of the Tmem16A Cl− channel in fluid secretion cannot be directly assessed in a mature gland because systemic Tmem16A−/− mice die shortly after birth. To test the functional importance of this channel in adult salivary glands, we generated Tmem16A conditional knockout mice using the Cre/loxP system (Tmem16Aflox/flox; Fig. S1) (10). To selectively disrupt Tmem16A expression in acinar cells, we took advantage of a mouse that expresses Cre-recombinase under control of the Aquaporin 5 (Aqp5) gene promoter (ACID) (11). In salivary glands, the Aqp5 H2O channel is expressed exclusively in secretory acinar cells (12, 13).
To validate Aqp5 promoter-driven expression of Cre-recombinase in acinar cells, we crossed ACID mice with mT/mG reporter mice. mT/mG cells express a membrane-bound version of the red tomato protein (mT) except in cells that express Cre-recombinase, which deletes the mT encoding sequence (flanked by loxP sites) and activates the expression of a membrane-bound version of green fluorescent protein (mG) (14). Fig. 1A shows that all submandibular gland (SMG) cells in the mT/mG reporter mouse express the red tomato protein in the absence of Cre-recombinase. In contrast, mG is expressed in acinar and intercalated cells in the ACID-mT/mG reporter mouse (green cells in Fig. 1B), whereas no green staining is observed in duct cell populations (red cells in Fig. 1B). These results confirm that Cre-recombinase expression in ACID mice coincides with acinar expression of native Aqp5 and Tmem16A in the mouse SMG (6, 7, 15).
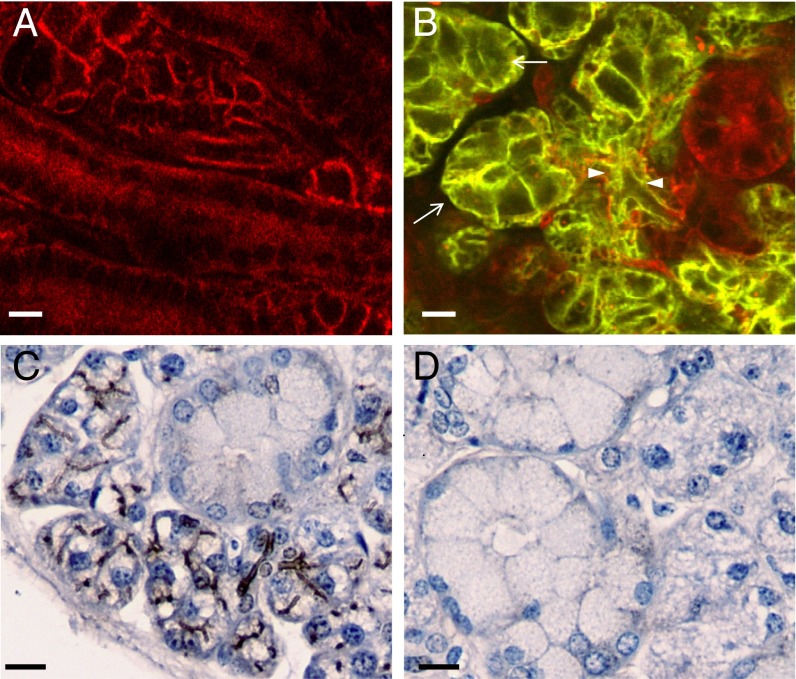
Fig. 1.
Generation of mice lacking Tmem16A Cl− channels in salivary gland acinar cells. (A) SMG sections from mT/mG reporter mice show that mT is expressed in the absence of Cre-recombinase expression. (B) SMG sections from mice obtained by crossing mT/mG reporter mice with ACID mice display mG expression in acinar (white arrows) and intercalated cells (white arrowheads), whereas other duct cells remain red-stained. (C and D) Immunolocalization of Tmem16A on paraffin-embedded SMG sections from littermate controls shows apical brown staining of acinar cells (C), whereas Tmem16A-specific staining is absent in Tmem16A−/− mice (D). (Scale bars: 10 µm.)
We next generated mice lacking Tmem16A in salivary glands (Tmem16A−/−) by crossing ACID and Tmem16Aflox/flox mice. Tmem16Aflox/flox mice were generated by flanking exon 12 of Tmem16A with loxP sites. Exon 12 encodes for a putative transmembrane domain that forms the pore-forming region of the Tmem16A Cl− channel (6), and its systemic excision produced a Tmem16A null mutation (16). The apical Tmem16A-associated immunostaining present in control acinar cells (Fig. 1C) was absent in Tmem16−/− mice (Fig. 1D), verifying that Cre-mediated Tmem16A gene ablation occurred in SMG secretory cells.
Ca2+-Activated Cl− Current Is Abolished in the Acinar Cells of Adult Tmem16A−/− Mice.
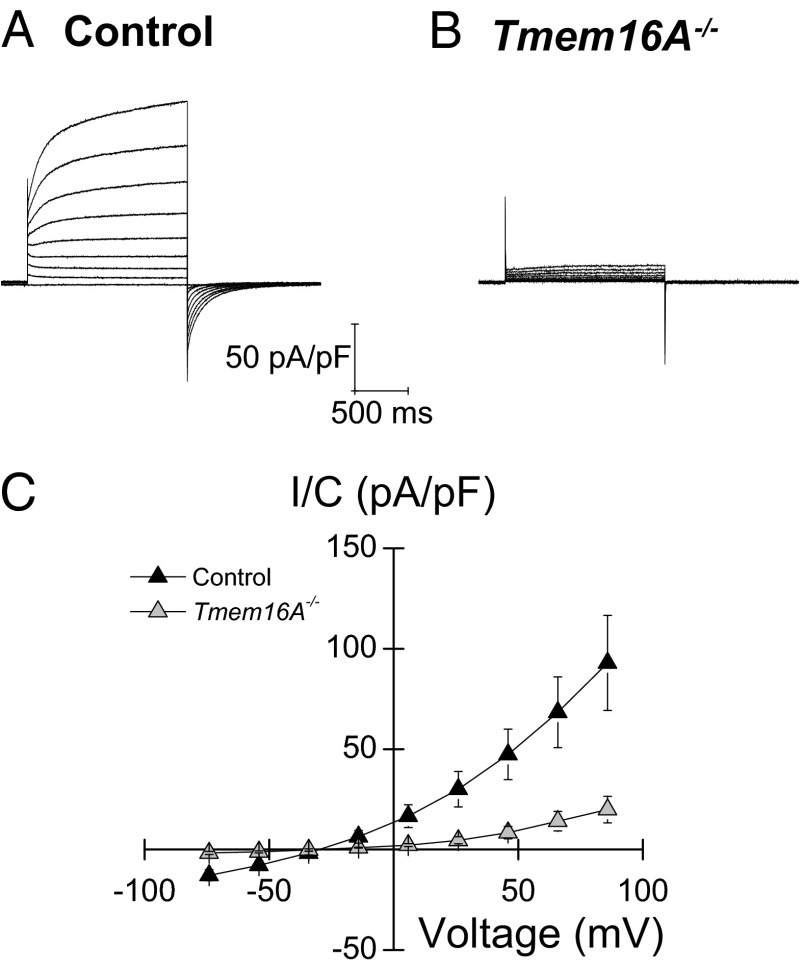
It was previously shown that the outward-rectifying Ca2+-dependent Cl− conductance is absent in acinar cells isolated from 2- to 3-d-old systemic Tmem16A knockout mice (7). In the present study, whole-cell Cl− currents were recorded in mouse SMG acinar cells to determine whether the outward-rectifying Ca2+-dependent Cl− conductance is also lacking in adult conditional Tmem16A knockout mice. Whole-cell currents activated by a pipette solution containing [Ca2+]free = 285 nM exhibited a large time-dependent, outward-rectifying Cl− conductance in acinar cells, with a reversal potential of −28.5 ± 2.9 mV (n = 7 cells), close to the expected equilibrium potential for Cl− (ECl = −23.6 mV) (Fig. 2A). This Cl− conductance was dependent on the intracellular [Ca2+]; that is, outward-rectifying Cl− currents increased markedly when the intracellular Ca2+ concentration was raised by permeabilizing the plasma membrane with the Ca2+ ionophore ionomycin in the presence of 1 mM extracellular Ca2+ (Fig. S2). This outward-rectifying conductance is consistent with the activation of Tmem16A channels (7). In contrast, Ca2+-dependent outward-rectifying Cl− currents were essentially absent in Tmem16A−/− acinar cells (Fig. 2B). Fig. 2C summarizes the current–voltage relationships from littermate control and Tmem16A−/− acinar cells.
Fig. 2.
Ca2+-activated Cl− currents are abolished in adult Tmem16A−/− acinar cells. (A and B) Whole-cell recordings in isolated acinar cells generated as described in SI Materials and Methods from control (A) and Tmem16A−/− (B) SMGs showing that the Ca2+-activated Cl− conductance is essentially abolished in Tmem16A−/− mice. (C) Current–voltage relations obtained from experiments like those shown in A and B (n = 7 control and n = 4 Tmem16A−/− cells, respectively). Currents are normalized by cell capacitance (pA/pF). Data are presented as mean ± SEM.
Ca2+-Dependent Fluid Secretion Is Abolished in Salivary Glands Lacking Tmem16A Channels.
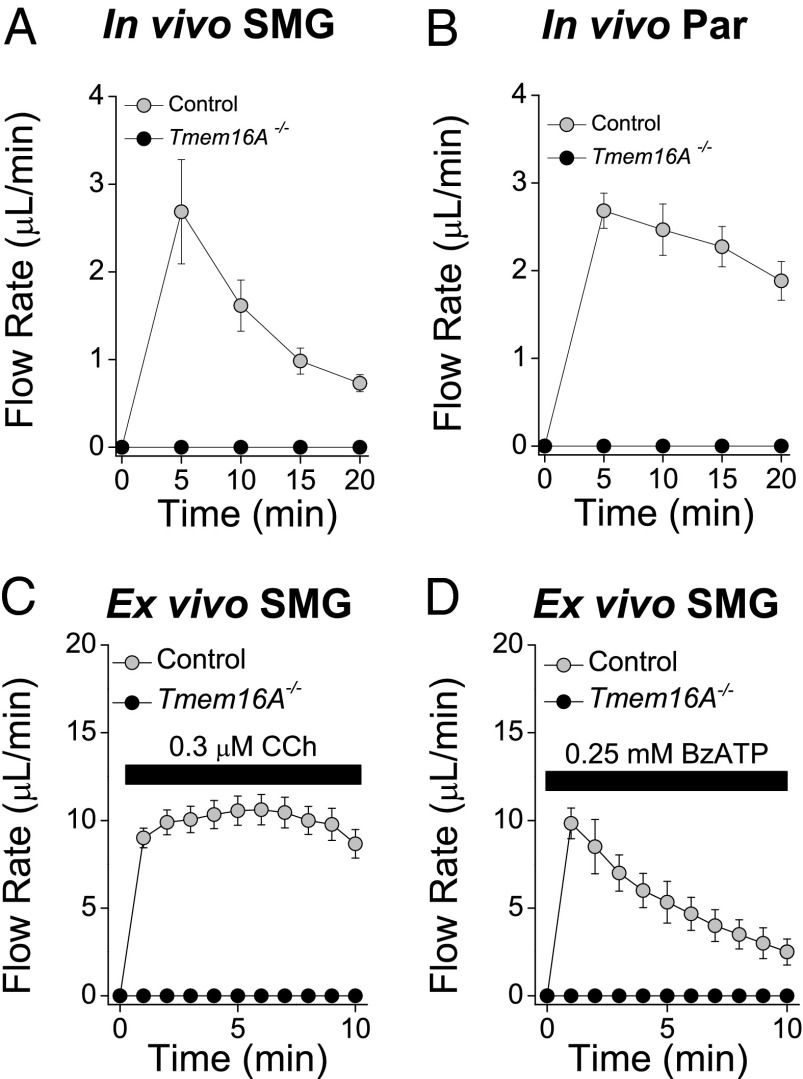
We next evaluated the impact of disrupting Tmem16A expression on adult whole-organ function in conditional Tmem16A null mice. In vivo saliva secretion induced by the muscarinic receptor agonist pilocarpine was totally abolished in both the SMGs and parotid glands of conditional Tmem16A−/− mice (Fig. 3 A and B). To eliminate possible in vivo systemic factors (e.g., bioactive circulatory factors and central neural input that often confound interpretation of secretion results), we assessed organ function using an ex vivo perfused-SMG preparation. Consistent with in vivo results, salivation induced by the muscarinic receptor agonist carbachol (CCh) was abolished in the ex vivo SMGs of Tmem16A−/− mice (Fig. 3C). We also stimulated secretion via the purinergic receptor P2X7 using the selective agonist 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) (0.25 mM), which activates Ca2+-dependent secretion not through a G protein-coupled mechanism like the muscarinic receptor, but by direct activation of the P2X7 receptor channel (17). Salivation in response to BzATP was also abolished in Tmem16A−/− mice (Fig. 3D), suggesting that increasing the intracellular Ca2+ concentration cannot activate fluid secretion in Tmem16A−/− mouse acinar cells.
Fig. 3.
In vivo and ex vivo salivary gland secretion in response to Ca2+-mobilizing agonists. (A and B) In vivo SMG (A) and parotid gland (B; Par) secretions from control (gray circles; n = 7 glands) and Tmem16A−/− (black circles; n = 6 glands) mice in response to i.p. injection of pilocarpine (10 mg/kg body weight). (C and D) Ex vivo SMG secretions collected as described in SI Materials and Methods from control (gray circles) and Tmem16A−/− (black circles) mice in response to 0.3 μM CCh (C; n = 12 control glands and n = 6 Tmem16A−/− glands) and 0.25 mM BzATP (D; n = 4 glands from control and Tmem16A−/− mice). Data are presented as mean ± SEM.
The loss of agonist-induced saliva secretion of Tmem16A−/− mice is consistent with the Tmem16A apical Cl− channel acting as the rate-limiting step in Ca2+-dependent salivation. These results do not address the (albeit unlikely) possibility that Tmem16A disruption in acinar cells may negatively affect fluid secretion by altering Ca2+ signaling or other critical transport pathways. Consequently, we measured the muscarinic-stimulated [Ca2+] changes in Tmem16A−/− mouse acinar cells loaded with the ratiometric Ca2+ indicator Fura-2. Fig. S3 shows that there was no difference in the CCh-stimulated Ca2+ response in the acinar cells of littermate control and Tmem16A−/− mice. In addition, we examined the expression patterns of Aqp5 water channels and Nkcc1 Na+/K+/2Cl− cotransporters, two key players in the saliva secretion process (12, 13, 18). Immunolocalization of Aqp5 and Nkcc1 suggested that there may be lower levels of Aqp5 and Nkcc1 proteins in Tmem16A−/− compared with control glands (Fig. S4 A and B). Indeed, Western blot analysis of plasma membrane-enriched protein fractions confirmed that Aqp5 and Nkcc1 protein levels (Fig. S4 C and D) were reduced by 29% and 51%, respectively, in Tmem16A−/− glands, as summarized in Fig. S4E.
A β-Adrenergic Receptor-Induced Fluid Secretion Pathway.
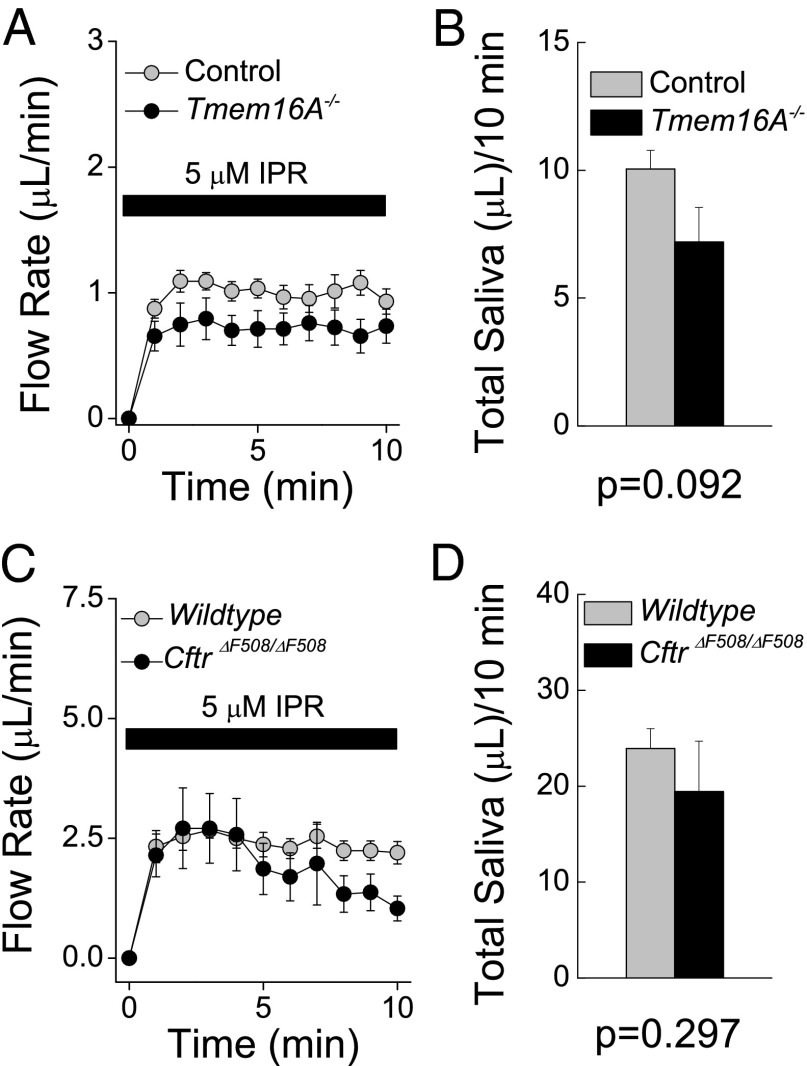
The mechanism underlying β-adrenergic–induced fluid secretion, including whether it is acinar and/or ductal in origin (19), is not well understood. One possibility is that β-adrenergic stimulation activates ion-transporting proteins shared with the muscarinic-dependent pathway, including acinar Tmem16A channels. To test this hypothesis, we measured fluid secretion in the ex vivo SMGs of Tmem16A−/− mice in response to IPR as well as to forskolin, which bypasses receptors to directly activate adenylate cyclase and increase intracellular cAMP levels. IPR stimulated ex vivo secretion of saliva with comparable kinetics (Fig. 4A) and total fluid volumes (Fig. 4B) in littermate control and Tmem16A−/− glands. Furthermore, no differences in saliva secretion were observed when SMGs were treated with forskolin (Fig. S5).
Fig. 4.
Ex vivo SMG secretions in response to β-adrenergic receptor stimulation. (A) Ex vivo SMG secretions collected as described in SI Materials and Methods from control (gray circles; n = 6 glands) and Tmem16A−/− (black circles; n = 6 glands) mice in response to 5 μM IPR. (B) Total saliva secreted in 10 min from the data shown in A. (C) Ex vivo SMG secretions from WT (gray circles; n = 8 glands) and Cftr∆F508/∆F508 (black circles; n = 10 glands) mice in response to 5 μM IPR. (D) Total saliva secreted in 10 min from the data shown in C. Data are presented as mean ± SEM. The P value was obtained by the Student's t test.
The foregoing results demonstrate that acinar Tmem16A plays little, if any, role in the β-adrenergic cAMP-activated fluid secretion process. However, mouse SMG duct cells express cAMP-dependent Cftr Cl− channels that are regulated by β-adrenergic receptor activation (8). Consequently, we asked whether IPR-induced fluid secretion might occur in the duct epithelium through a Cftr channel-dependent mechanism. To address this question, we examined IPR-stimulated fluid secretion in Cftr∆F508/∆F508 mice, which carry a ∆F508 functional null mutation of the Cftr channel. Comparable kinetics (Fig. 4C) and total volumes (Fig. 4D) of ex vivo saliva were secreted in response to IPR in Cftr∆F508/∆F508 mice and littermate controls, indicating that ductal Cftr channels are not involved in this process.
VRAC and β-Adrenergic Receptor-Dependent Cell Swelling and Saliva Secretion.
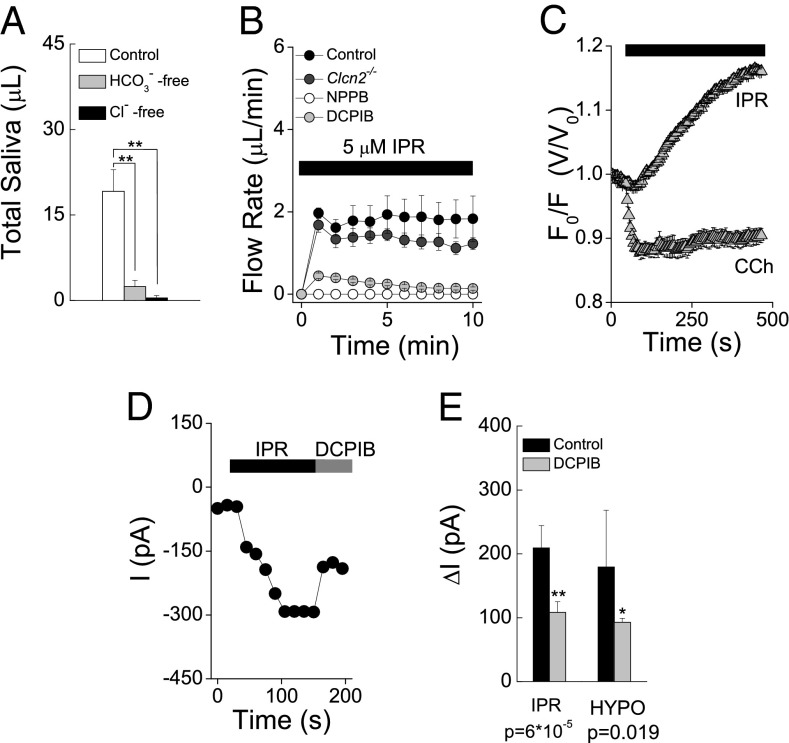
The foregoing results demonstrate that the fluid secretion pathway activated during β-adrenergic receptor stimulation does not involve Tmem16A or Cftr channels (Fig. 4 A–D and Fig. S5). These results raise a fundamental question: Like Ca2+-dependent fluid secretion, is β-adrenergic–stimulated secretion driven by transepithelial Cl− movement? If so, then secretion will be dependent on extracellular Cl− and involve a Cl− channel in the apical membrane. Similar to the Cl− dependence observed in muscarinic receptor-dependent saliva secretion (20), IPR-induced fluid secretion was nearly abolished (98% reduction) when Cl− was replaced by isotonic gluconate in the perfusate (Fig. 5A). A similar pattern was obtained when HCO3− was replaced by gluconate in the perfusate.
Fig. 5.
Involvement of VRAC in β-adrenergic receptor-dependent saliva secretion. (A) Chloride and bicarbonate dependence of β-adrenergic–induced saliva secretion. Shown is the total ex vivo saliva secreted by SMGs in response to 5-μM IPR stimulation for 10 min using control, HCO3−-free, and low-Cl− perfusates (n = 8 glands per condition). Data are presented as mean ± SEM. **P < 0.0003, one-way ANOVA followed by Bonferroni’s post hoc test. (B) Ex vivo SMG secretions from control (black circles; n = 8 glands), Clcn2−/− (dark-gray circles; n = 8 glands), and control glands treated with 50 µM DCPIB (gray circles; n = 8 glands) or 100 µM NPPB (white circles; n = 6 glands). (C) Acinar cell volume changes in response to 0.3 µM CCh or 5 µM IPR. (D) Nystatin-perforated whole-cell recording showing the increase in macroscopic currents at −60 mV in response to 5 µM IPR and blockade by 50 µM DCPIB. (E) Summary of current magnitude and blockade by DCPIB of the Cl− conductance activated by IPR (n = 4) and hypotonic shock (n = 3) at −60 mV. The increase in current magnitude by IPR and hypotonic shock and the decrease in current magnitude by DCPIB-mediated blockade are shown as absolute values. Values are given as ± SEM. P values were obtained using the Student's t test.
Three Cl− channels have been described in rodent salivary gland acinar cells (21): a CaCC encoded by Tmem16A (6, 7), a hyperpolarization-gated Cl− channel encoded by Clcn2 (22), and a volume-regulated anion channel (VRAC). To gain additional insight into the anion channel involved in IPR-induced salivation, we evaluated the β-adrenergic secretory response in SMGs lacking voltage-gated ClC-2 channels (Clcn2−/−) as well as WT glands treated with DCPIB and NPPB, which efficiently block VRAC. Fig. 5B shows that the fluid secretion induced by IPR was unaffected in ex vivo glands lacking ClC-2 channels, whereas in contrast, DCPIB and NPPB severely decreased IPR-evoked secretion.
Our findings show that Tmem16A (Fig. 4 A and B), Cftr (Fig. 4 C and D), and ClC-2 (Fig. 5B) channels are not significantly involved in β-adrenergic–induced fluid secretion. Given that a Cl− channel is required, this leaves open the possibility that VRAC is responsible for β-adrenergic–stimulated saliva secretion. Unlike the rapid CCh-induced acinar cell shrinkage (10 ± 1% decrease), IPR treatment generated a slowly developing increase in acinar cell volume by 12.5 ± 0.2% (Fig. 5C). To test whether IPR activates a Cl− conductance with similar properties to that activated by hypotonic shock-induced cell swelling, we collected nystatin-perforated whole-cell recordings from isolated acinar cells using quasi-physiological external solutions containing HCO3− (SI Materials and Methods). Macroscopic currents in response to voltage increases from −60 mV to 100 mV (in 20-mV steps) were higher after IPR treatment (Fig. S6B) compared with the currents under control conditions (Fig. S6A). The difference in macroscopic currents before and after IPR treatment (Fig. S6C) clearly shows an outward-rectifying conductance with a reversal potential close to ECl (Fig. S6D).
We then tested the sensitivity of this IPR-induced Cl− conductance to the VRAC-selective blocker DCPIB (Tocris Bioscience). As shown in Fig. 5D, DCPIB partially blocked the IPR-induced Cl− current. Significantly, the magnitude and blockade fraction by DCPIB of the current induced by hypotonic shock were similar to the current observed under β-adrenergic receptor stimulation by IPR (Fig. 5E). The blocked fractions of IPR- and hypotonic shock-induced macroscopic currents by DCPIB differ from those of VRAC-mediated currents reported in other cell types, which are almost fully blocked by DCPIB (23, 24). The contribution of other ion channels that are insensitive to DCPIB cannot be ruled out; indeed, this possibility is supported by the finding that DCPIB dramatically inhibited IPR-induced ex vivo saliva secretion.
Discussion
The pioneering work of Lundberg in 1957 (20) described a Cl−-dependent saliva secretion process in which transcellular Cl− transport is coupled to fluid secretion (25). This pump-leak fluid secretion model predicts that Cl− influx occurs through a basolateral “pump” and Cl− ions are extruded via an apical “leak” (26). Numerous studies have confirmed that a mechanism similar to this model accounts for fluid secretion by various epithelia, including salivary glands (1, 27). The apical Cl− efflux pathway involved in saliva secretion remains unidentified, but there is growing evidence that Tmem16A mediates Cl− efflux in salivary gland secretory cells (6, 7). Yang et al. (6) assessed in vivo pilocarpine-induced saliva secretion rates in mice injected with siRNAs and found a ∼35% reduction in saliva secretion rate in mice treated with Tmem16A siRNA. A subsequent study found that muscarinic receptor-evoked Cl− efflux and calcium-activated Cl− current were absent in acinar cells from 2- to 3-d-old Tmem16A systemic knockout mice (7).
Tmem16A and Aqp5 water channels are coexpressed in salivary gland acinar cells (6). To evaluate the functional role of Tmem16A in the acinar cells of the adult salivary gland, we crossed Tmem16Aflox/flox mice with knock-in mice expressing Cre-recombinase under control of the Aqp5 gene promoter (11). The resulting acinar cell-specific Tmem16A−/− mice lacked Tmem16A-associated immunoreactivity. Moreover, Ca2+-activated Cl− current was eliminated in salivary gland acinar cells from Tmem16A−/− adult mice, confirming that functional CaCCs are generated by the Tmem16A gene.
We then used these mice to directly test the hypothesis that Tmem16A channels play a key role in saliva secretion. Muscarinic receptor-mediated saliva secretion was completely abolished in the SMGs and parotid glands of Tmem16A null mice, both in vivo and ex vivo. These results strongly suggest that Tmem16A is critical in the fluid secretion process. However, could Tmem16A disruption alter the expression of other essential ion transport or cellular signal pathways? Given the importance of Ca2+ for activation of the fluid secretion process, we verified that Ca2+ signaling was intact. Ex vivo saliva secretion was lacking in Tmem16A−/− glands stimulated with BzATP, a P2X7 receptor agonist that also induces Ca2+-dependent saliva secretion (17). Unlike G protein-coupled muscarinic receptors, activation of a P2X receptor channel directly raises the intracellular Ca2+ concentration by Ca2+ permeation via its pore. These results indicate that increasing the intracellular Ca2+ concentration through either a G protein-coupled or receptor channel mechanism does not activate fluid secretion in Tmem16A−/− mice. Consistent with these findings, we observed no differences in CCh-induced Ca2+ response in the SMGs of Tmem16A−/− mice. Moreover, the expression and targeting of Aqp5 and Nkcc1, major contributors to the fluid secretion process, were decreased only negligibly in Tmem16A−/− mice. Taken together, our findings demonstrate that Tmem16A channels act as the apical Cl− efflux pathway required for Ca2+-dependent fluid secretion, the major pathway involved in saliva secretion (1).
Salivary glands also secrete saliva in response to a β-adrenergic receptor-activated increase in intracellular cAMP. Unlike Ca2+-dependent salivation, little is known about the mechanism underlying the cAMP-dependent secretory process (19). It is unclear whether an increase in intracellular cAMP is sufficient to produce saliva, or whether cAMP exerts its secretory effect in a Ca2+-dependent manner. For example, α-adrenergic and muscarinic dependent-Ca2+ secretion is potentiated by β-adrenergic receptor activation in rat parotid cells (28). Moreover, it has been proposed that β-adrenergic receptor-induced secretion in rat SMGs depends on intracellular Ca2+, not cAMP (29). We hypothesized that if β-adrenergic, cAMP-induced saliva secretion is Ca2+-dependent, then the fluid secretion mechanism would rely on the calcium-activated Cl− channel Tmem16A. However, saliva secretion was unaffected in Tmem16A−/− mice stimulated with IPR or forskolin, which has been widely used to directly increase intracellular cAMP levels. Thus, our results clearly demonstrate that β-adrenergic, cAMP-dependent saliva secretion does not require Tmem16A channels in mouse SMGs, in contrast to the obligatory role of these channels in muscarinic, Ca2+-dependent salivation.
Because Tmem16A does not play a major part in β-adrenergic, cAMP-dependent salivation, we reasoned that fluid secretion might require activation of Cftr, a cAMP-dependent Cl− channel involved in fluid secretion in other epithelial tissues (9, 30). Cftr is highly expressed in rodent duct cells (31, 32), and accordingly, if the relevant fluid secretion machinery is present in these cells, then Cftr channel activation should lead to fluid secretion. To test this hypothesis, we used a mouse that expresses the homologous ∆F508 mutation of the human CFTR Cl− channel, the mutation most frequently associated with cystic fibrosis (9). IPR-induced fluid secretion was unaffected in the SMGs of Cftr∆F508/∆F508 mice, in agreement with an earlier study demonstrating that the Cftr Cl− channel is critical for NaCl reabsorption rather than saliva secretion (8); however, this finding contrasts with other reports showing severely impaired in vivo saliva secretion in response to β-adrenergic receptor agonists in a systemic Cftr knockout mouse model as well as the Cftr∆F508/∆F508 mouse model (33, 34). We do not know the basis for this difference, but it may be related to the different experimental approaches used in these studies (in vivo vs. ex vivo saliva secretions).
Our findings reveal that β-adrenergic, cAMP-mediated fluid secretion does not depend on either Tmem16A or Cftr Cl− channels, unmasking a previously unidentified fluid secretion pathway in salivary glands. To gain more insight into this pathway, we evaluated the dependence of β-adrenergic, cAMP-activated fluid secretion on extracellular anions—that is, whether β-adrenergic–stimulated secretion is driven by transepithelial Cl− movement. Similar to muscarinic, Ca2+-dependent secretion, IPR-induced fluid secretion was dramatically reduced by replacing Cl−with the poorly permeant anion gluconate in the perfusate. Similar results were obtained when SMGs were stimulated under HCO3−-free conditions. The Cl− dependency of IPR-induced fluid secretion is in agreement with the current model for saliva secretion; that is, the secretory machinery is composed of basolateral Cl− uptake and apical Cl− efflux pathways. In contrast, the HCO3− dependency suggests either that the basolateral Cl− uptake pathway depends markedly on HCO3− ions or that HCO3− secretion may be linked to fluid secretion. Future experiments are needed to acquire more insight into the mechanism by which HCO3− modulates IPR-induced salivary gland fluid secretion.
Our results clearly demonstrate that neither the Tmem16A channel nor the Cftr Cl− channel is the apical Cl− efflux pathway activated during β-adrenergic–stimulated fluid secretion. Thus, we asked which channel might promote Cl− extrusion across the apical membrane. Voltage-gated ClC-2 channels and VRAC are also expressed in salivary gland acinar cells (21). Ex vivo saliva secretion rates were measured in response to IPR in SMGs lacking ClC-2 channel (Clcn2−/−) and in WT glands treated with DCPIB and NPPB, two blockers of VRAC. IPR-induced saliva secretion was not affected in Clcn2−/− glands, supporting previous reports that ClC-2 channels do not play a major role in saliva secretion (22, 35). In contrast, IPR-induced saliva secretion was severely decreased by both DCPIB and NPPB. The DCPIB and NPPB sensitivity of the IPR-induced secretion and the acinar cell swelling observed on IPR treatment suggest that VRAC might be involved in this secretory process. Although the importance of these latter observations will require further investigation, it can be speculated that IPR activates basolateral Na+/K+/2Cl− cotransporters (36, 37), causing solute accumulation and cell swelling. Such swelling would be expected to activate the cell volume-sensitive Cl− channels present in acinar cells (21) and to provide an apical Cl− efflux pathway important for β-adrenergic, cAMP-stimulated fluid secretion. Indeed, we found that the DCPIB-sensitive Cl− conductance activated by IPR has similar properties as the currents activated by hypotonic shock. Moreover, the relatively slow IPR-induced increase in acinar cell volume contrasted markedly with the rapid cell shrinkage observed on muscarinic stimulation. The IPR-induced rate of cell swelling (t1/2 >1 min) was consistent with the time delay observed before secretion is initiated in the ex vivo gland (t1/2 >1 min), further supporting the involvement of VRAC in IPR-induced saliva secretion.
In summary, we generated a conditional Tmem16A knockout mouse that lacks expression of CaCCs in salivary gland secretory cells. Our conditional Tmem16A−/− mouse confirmed that Tmem16A Cl− channels are absolutely required for muscarinic, Ca2+-dependent saliva secretion in adult mice. On the other hand, stimulation of β-adrenergic receptors activated a saliva secretion pathway that does not depend on Tmem16A, Cftr, or ClC-2 Cl− channels. We speculate that the Cl− efflux required to support cAMP-dependent secretion occurs via VRAC that might correspond to a heteromultimer encoded by the recently discovered Lrrc8 gene family (38, 39).
Materials and Methods
General Materials and Methods.
Tmem16A conditional knockout mice (Tmem16Aflox/flox) were generated by flanking exon 12 of the Tmem16A gene with loxP sites (Fig. S1). Gene targeting protocols for ACID, mT/mG (Jackson Laboratory), Clcn2−/−, and Cftr∆F508/∆F508 mice were as described previously (11, 14, 22, 40). Mice were housed in microisolator cages with ad libitum access to laboratory chow and water during 12-h light/dark cycles. Water used for CftrF508/∆F508 mice and their littermates was supplemented with GoLYTELY (Braintree Laboratories), an oral osmotic laxative used to increase survival of the mutant mice (41). Equal numbers of sex- and age-matched (6–24 wk) animals were used in all experiments except the ex vivo experiments shown in Fig. 3 C and D, Fig. 4 A and B, and Fig. S5, in which only adult males were used. All of the gene-targeted mice had a C57BL/6J background except for the CftrF508/∆F508 and Clcn2−/− mice, which were on a Black Swiss/129 SvJ hybrid background. All animal procedures were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research, National Institutes of Health (ASP 13–686). Reagents were obtained from Sigma-Aldrich unless specified otherwise. Procedures for salivary gland cell isolation, immunohistochemistry, electrophysiology, and imaging are described in detail in SI Materials and Methods.
Statistical Analysis.
Results are presented as mean ± SEM. Statistical significance was determined using the Student's t test. Multiple-sample comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test. A P value < 0.05 was considered statistically significant. Origin 7.0 (OriginLab) was used for statistical calculations. All experiments were performed using preparations from three or more separate mice for each condition.
Supplementary Material
Acknowledgments
We thank the University of Cincinnati Animal Core Facility for the design and generation of the exon 12-floxed Tmem16A mouse. The Secretory Mechanisms and Dysfunction Section is supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH). Z.B., E.C., and P.F. were supported by the Hastings and Whittier Foundations and the NIH. G.E.S. was supported by NIH Grant DK050594.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415739112/-/DCSupplemental.
References
- 1.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 2.Schneyer LH, Young JA, Schneyer CA. Salivary secretion of electrolytes. Physiol Rev. 1972;52(3):720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- 3.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012;92(1):39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 7.Romanenko VG, et al. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285(17):12990–13001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalán MA, et al. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588(Pt 4):713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinton PM. Physiological basis of cystic fibrosis: A historical perspective. Physiol Rev. 1999;79(1) Suppl:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 10.Nagy A. Cre recombinase: The universal reagent for genome tailoring. Genesis. 2000;26(2):99–109. [PubMed] [Google Scholar]
- 11.Flodby P, et al. Directed expression of Cre in alveolar epithelial type 1 cells. Am J Respir Cell Mol Biol. 2010;43(2):173–178. doi: 10.1165/rcmb.2009-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krane CM, et al. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276(26):23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 13.Ma T, et al. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274(29):20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 14.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 15.Larsen HS, et al. Localization of AQP5 during development of the mouse submandibular salivary gland. J Mol Histol. 2011;42(1):71–81. doi: 10.1007/s10735-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321(1):141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Nakamoto T, et al. Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem. 2009;284(8):4815–4822. doi: 10.1074/jbc.M808597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans RL, et al. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem. 2000;275(35):26720–26726. doi: 10.1074/jbc.M003753200. [DOI] [PubMed] [Google Scholar]
- 19.Young JA, Martin CJ. The effect of a sympatho- and a parasympathomimetic drug on the electrolyte concentrations of primary and final saliva of the rat submaxillary gland. Pflugers Arch. 1971;327(4):285–302. doi: 10.1007/BF00588449. [DOI] [PubMed] [Google Scholar]
- 20.Lundberg A. Anionic dependence of secretion and secretory potentials in the perfused sublingual gland. Acta Physiol Scand. 1957;40(2-3):101–112. doi: 10.1111/j.1748-1716.1957.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 21.Arreola J, Park K, Melvin JE, Begenisich T. Three distinct chloride channels control anion movements in rat parotid acinar cells. J Physiol. 1996;490(Pt 2):351–362. doi: 10.1113/jphysiol.1996.sp021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nehrke K, et al. Loss of hyperpolarization-activated Cl(−) current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem. 2002;277(26):23604–23611. doi: 10.1074/jbc.M202900200. [DOI] [PubMed] [Google Scholar]
- 23.Best L, Yates AP, Decher N, Steinmeyer K, Nilius B. Inhibition of glucose-induced electrical activity in rat pancreatic beta-cells by DCPIB, a selective inhibitor of volume-sensitive anion currents. Eur J Pharmacol. 2004;489(1-2):13–19. doi: 10.1016/j.ejphar.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Decher N, et al. DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134(7):1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva P, et al. Mechanism of active chloride secretion by shark rectal gland: Role of Na-K-ATPase in chloride transport. Am J Physiol. 1977;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- 26.Reuss L. Ussing’s two-membrane hypothesis: The model and half a century of progress. J Membr Biol. 2001;184(3):211–217. doi: 10.1007/s00232-001-0096-z. [DOI] [PubMed] [Google Scholar]
- 27.Martinez JR. Cellular mechanisms underlying the production of primary secretory fluid in salivary glands. Crit Rev Oral Biol Med. 1990;1(1):67–78. doi: 10.1177/10454411900010010601. [DOI] [PubMed] [Google Scholar]
- 28.Tanimura A, Nezu A, Tojyo Y, Matsumoto Y. Isoproterenol potentiates alpha-adrenergic and muscarinic receptor-mediated Ca2+ response in rat parotid cells. Am J Physiol. 1999;276(6 Pt 1):C1282–C1287. doi: 10.1152/ajpcell.1999.276.6.C1282. [DOI] [PubMed] [Google Scholar]
- 29.Cook DI, Day ML, Champion MP, Young JA. Ca2+ not cyclic AMP mediates the fluid secretory response to isoproterenol in the rat mandibular salivary gland: whole-cell patch-clamp studies. Pflugers Arch. 1988;413(1):67–76. doi: 10.1007/BF00581230. [DOI] [PubMed] [Google Scholar]
- 30.Greger R. Role of CFTR in the colon. Annu Rev Physiol. 2000;62:467–491. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- 31.Dinudom A, Komwatana P, Young JA, Cook DI. A forskolin-activated Cl− current in mouse mandibular duct cells. Am J Physiol. 1995;268(5 Pt 1):G806–G812. doi: 10.1152/ajpgi.1995.268.5.G806. [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(−) reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1729–R1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]
- 33.Best JA, Quinton PM. Salivary secretion assay for drug efficacy for cystic fibrosis in mice. Exp Physiol. 2005;90(2):189–193. doi: 10.1113/expphysiol.2004.028720. [DOI] [PubMed] [Google Scholar]
- 34.Droebner K, Sandner P. Modification of the salivary secretion assay in F508del mice—the murine equivalent of the human sweat test. J Cyst Fibros. 2013;12(6):630–637. doi: 10.1016/j.jcf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Romanenko VG, et al. Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1058–G1067. doi: 10.1152/ajpgi.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulais M, Turner RJ. Beta-adrenergic upregulation of the Na(+)-K(+)-2Cl- cotransporter in rat parotid acinar cells. J Clin Invest. 1992;89(4):1142–1147. doi: 10.1172/JCI115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanimura A, Kurihara K, Reshkin SJ, Turner RJ. Involvement of direct phosphorylation in the regulation of the rat parotid Na+-K+-2Cl− cotransporter. J Biol Chem. 1995;270(42):25252–25258. doi: 10.1074/jbc.270.42.25252. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Z, et al. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157(2):447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voss FK, et al. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344(6184):634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 40.Zeiher BG, et al. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96(4):2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46(6):612–618. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.