Significance
Peroxiredoxins (Prxs) are highly abundant proteins, which serve two seemingly mutually exclusive roles as peroxidases and molecular chaperones. Little is known about the precise mechanism of Prxs’ activation as chaperone and the physiological significance of this second function. Here we demonstrate that in Leishmania infantum, reduced Prx provides a crucial, stress-specific chaperone reservoir, which is activated rapidly upon exposure to unfolding stress conditions. Once activated, Prx protects a wide range of different clients against protein unfolding. Clients are bound in the center of the decameric ring, providing experimental evidence for previous claims that Prxs serve as likely ancestors of chaperonins. Interference with client binding impairs Leishmania infectivity, providing compelling evidence for the in vivo importance of Prx’s chaperone function.
Keywords: chaperone, Leishmania, peroxiredoxin
Abstract
Cytosolic eukaryotic 2-Cys-peroxiredoxins have been widely reported to act as dual-function proteins, either detoxifying reactive oxygen species or acting as chaperones to prevent protein aggregation. Several stimuli, including peroxide-mediated sulfinic acid formation at the active site cysteine, have been proposed to trigger the chaperone activity. However, the mechanism underlying this activation and the extent to which the chaperone function is crucial under physiological conditions in vivo remained unknown. Here we demonstrate that in the vector-borne protozoan parasite Leishmania infantum, mitochondrial peroxiredoxin (Prx) exerts intrinsic ATP-independent chaperone activity, protecting a wide variety of different proteins against heat stress-mediated unfolding in vitro and in vivo. Activation of the chaperone function appears to be induced by temperature-mediated restructuring of the reduced decamers, promoting binding of unfolding client proteins in the center of Prx’s ringlike structure. Client proteins are maintained in a folding-competent conformation until restoration of nonstress conditions, upon which they are released and transferred to ATP-dependent chaperones for refolding. Interference with client binding impairs parasite infectivity, providing compelling evidence for the in vivo importance of Prx’s chaperone function. Our results suggest that reduced Prx provides a mitochondrial chaperone reservoir, which allows L. infantum to deal successfully with protein unfolding conditions during the transition from insect to the mammalian hosts and to generate viable parasites capable of perpetuating infection.
Peroxiredoxins (Prxs) are highly conserved and ubiquitous peroxidases that constitute some of the most abundant proteins in the cell (1). Typical 2-Cys-Prxs, the subfamily of Prxs addressed in this study, are obligate homodimers of two inverted subunits that rely on two cysteines for hydroperoxide detoxification: the peroxidatic cysteine (Cp), which interacts directly with peroxides, and the resolving cysteine (Cr), which condenses with the oxidized Cp of the other subunit by forming a disulfide bond. The disulfide bond subsequently is regenerated by a thiol-containing oxidoreductase, such as thioredoxin or another member of the thioredoxin family (2). 2-Cys-Prxs have been studied extensively in regard to their role in the detoxification of reactive oxygen and nitrogen species (3). More recently, however, they also have been implicated in other biological activities, including H2O2 signaling, protein oxidation, and chaperoning functions under stress conditions (4, 5). The chaperone activity of 2-Cys-Prxs was first reported in 2004, when Jang et al. (6) proposed that peroxide-mediated overoxidation of the active cysteine Cp in yeast cytosolic 2-Cys-Prx (Tsa 1) inactivates the peroxidase function and triggers its conversion into high-molecular-weight (HMW) oligomers that prevent in vitro protein aggregation (6). Since this initial discovery, other factors, including phosphorylation or exposure to low pH, have been shown to prompt the functional switch from peroxidases to chaperones in various organisms (7–9), raising the possibility that cysteine overoxidation is only one of several mechanisms that activate the chaperone function of eukaryotic Prx.
Leishmania infantum, an intracellular protozoan parasite with a digenetic life cycle (i.e., it shuttles between two hosts, insects and mammals), encodes several 2-Cys-Prxs (10–12), one of which is the mitochondrial homolog called mitochondrial tryparedoxin-peroxidase mTXNPx (13). We showed that mTXNPx-deficient promastigotes (i.e., the insect form) are significantly more sensitive to a temperature shift from 25 °C to 37 °C than promastigotes harboring functional mTXNPx (13). This shift in temperature is similar to the shift parasites encounter upon transitioning from the insect to the mammalian host. Consistent with this result, we found that mTXNPx-deficient amastigotes (i.e., the mammalian form) are unable to survive within their mammalian host (13). These phenotypes could not be attributed to the peroxidase function of mTXNPx, because an L. infantum line expressing a peroxidase-inactive mutant variant of mTXNPx lacking Cp (mTXNPxC81S) was fully capable of surviving the temperature shift to 37 °C and infecting mice (13). These results suggested that the essential function observed in vivo is not based on mTXNPx’s peroxidase activity but more likely involves a second function of mTXNPx, potentially as molecular chaperone. Moreover, the ability of the mTXNPxC81S variant to rescue the in vivo noninfective phenotype of mTXNPx-depleted parasites excluded the possibility that overoxidation of Cp was responsible for the functional switch and raised the intriguing question as to how the dual functions of mTXNPx might be regulated in Leishmania mitochondria.
Here we demonstrate that reduced, decameric mTXNPx acts as powerful molecular chaperone, specifically protecting proteins against temperature-induced aggregation. Our studies provide compelling experimental evidence that mTXNPx is a fully integrated member of the mitochondrial proteostasis network of parasites and that its role as chaperone is crucial for parasite infectivity.
Results
Reduced mTXNPx Functions as an Efficient Molecular Chaperone in Vitro.
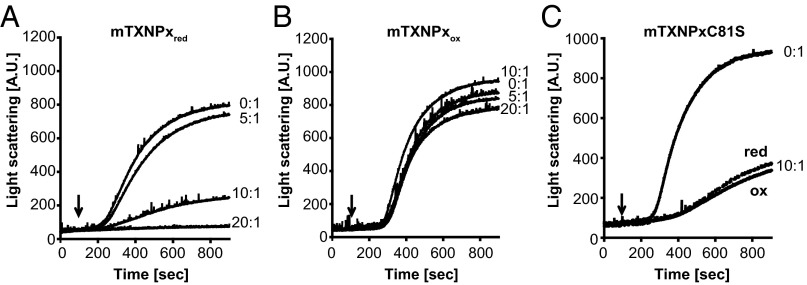
Previous findings indicated that mTXNPx displays ATP-independent chaperone activity in vitro, preventing citrate synthase from nonspecific aggregation at elevated temperatures (13). To characterize this activity further and to gain insights into the mechanism by which this chaperone function is regulated, we purified mature mTXNPx from Escherichia coli and analyzed its ability to protect several in vitro chaperone client proteins from thermal aggregation. Because Leishmania lines expressing the peroxidase-inactive variant mTXNPxC81S retained the capacity to infect mice and withstand heat-shock treatment (13), we reasoned that overoxidation of the active site cysteine is unlikely to be the physiological stimulus triggering mTXNPx’s conversion into a chaperone. Therefore we focused only on the reduced and oxidized subpopulations of wild-type mTXNPx, knowing that substantial amounts of both species are constitutively present in Leishmania promastigotes (13). As observed with other Prxs, purification of mTXNPx in the absence of reducing agents yields >90% disulfide-bonded, dimeric protein (mTXNPxox), indicative of its high propensity to air-oxidize (SI Appendix, Fig. S1A). We found that large quantities of the reducing agent DTT were necessary to generate mTXNPxred (SI Appendix, Fig. S1A) and that the constant presence of moderate amounts of DTT (0.2 mM) was required to prevent spontaneous reoxidation of mTXNPxred during activity assays. To compare the chaperone function of mTXNPxred and mTXNPxox directly, we tested their influence on the aggregation of thermally unfolding luciferase, which aggregates rapidly in the absence of Prx (Fig. 1A, 0:1). In the presence of a 10-fold molar excess of mTXNPxred, the thermal aggregation of luciferase was decreased significantly, and with a 20-fold excess of mTXNPxred aggregation was suppressed completely (Fig. 1A). Similarly, when purified mTXNPx was reduced by its physiological reducing system, it almost completely suppressed the aggregation of luciferase (SI Appendix, Fig. S1B). In contrast, however, when the same amount of mTXNPxox was added to the incubation reaction, no significant effect on luciferase aggregation was detected (Fig. 1B). Similar results were obtained when two other classical chaperone client proteins, malate dehydrogenase (SI Appendix, Fig. S1C) and citrate synthase (SI Appendix, Fig. S1D), were tested as client proteins. In each case, mTXNPxred prevented thermal aggregation of the model proteins, whereas mTXNPxox had only negligible effects. We concluded from these results that reduced mTXNPx works as an effective ATP-independent molecular chaperone and that overoxidation of the active site cysteine is not a crucial factor in the activation process.
Fig. 1.
Reduced mTXNPx prevents thermal aggregation of luciferase. Influence of mTXNPxred (A) or mTXNPxox (B) on the thermal aggregation of luciferase. Native luciferase (0.1 µM) was incubated in the absence (0:1) or presence of different ratios of mTXNPx at 41.5 °C, and light scattering (expressed in arbitrary units, A.U.) was monitored at 360 nm. Upon dilution of mTXNPxred into the assay buffer, the concentration of residual DTT was 0.2 mM. The same amount of DTT was added to the respective controls. (C) Influence of a 10-fold molar excess of reduced or oxidized mTXNPxC81S mutant variant on the in vitro aggregation of thermally unfolding luciferase. The addition of 0.2 mM DTT did not significantly affect luciferase aggregation in the absence of chaperones. The ratio of chaperone to client protein was calculated based on the monomeric form of mTXNPx. Arrows indicate the time point at which luciferase was added to the reactions.
The mTXNPx Chaperone-Active Species Is the Decamer.
Leishmania mTXNPx, like most other typical 2-Cys-Prxs (14), undergoes redox-dependent changes in its quaternary structure, cycling between reduced ∼230-kDa decamers and oxidized 46-kDa disulfide-bonded dimers (SI Appendix, Fig. S2A). We conducted isothermal titration calorimetry of both oxidized and reduced mTXNPx and confirmed that the reduced mTXNPx undergoes the typical, highly cooperative decamerization process also observed for other members of the Prx family. We determined a critical transition concentration (CTC; i.e., the minimal concentration at which mTXNPxred forms decamers) (15, 16) of 0.75 µM (SI Appendix, Fig. S2B), which is well below the intracellular concentration of mTXNPx in promastigotes (2–6 µM) or amastigotes (49–102 µM) (SI Appendix, Fig. S2C). Because the CTC also was found to be below the concentration of mTXNPxred that we typically use for our activity assays, we concluded that mTXNPxred is decameric in our assays. In contrast, we did not observe any decamerization for mTXNPxox even at concentrations as high as 100 µM, indicating that mTXNPxox is dimeric in our assays. This observation, together with the data shown above, implied that the minimal chaperone-active Prx species is likely the reduced decamer. Absence of the active site cysteine, as in the peroxidase-inactive mutant mTXNPxC81S (13), maintains the protein in a mixture of decameric and higher oligomeric conformations under nonreducing conditions and in a purely decameric conformation upon its reduction (SI Appendix, Fig. S2A). Analysis of the in vitro chaperone function of purified mTXNPxC81S revealed that this mutant variant is constitutively chaperone active, exerting a very similar propensity to prevent protein aggregation in both the reduced and oxidized form (Fig. 1C). These results are in excellent agreement with our previous in vivo studies showing that this mutant variant rescues the heat-shock phenotype of mTXNPx-deficient promastigotes and restores their infectivity (13). These findings also suggest that no oligomeric structure higher than the reduced decamer is necessary to confer chaperone activity to Leishmania mTXNPx. Our titration experiments using increasing amounts of wild-type mTXNPxred were consistent with this conclusion and showed that a 10-fold molar excess of mTXNPx monomers is both necessary and sufficient to suppress significantly the aggregation of one molecule of client protein (Fig. 1A). Together, our data demonstrate that the redox status of mTXNPx is crucial for its chaperone function, most likely via its influence on the oligomeric structure of the Prx.
Reduced mTXNPx Maintains Clients in Folding-Competent Conformation.
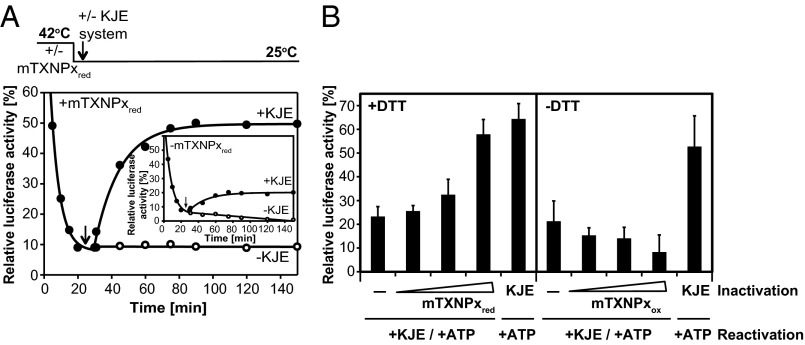
Several studies have shown that Prxs can suppress the aggregation of different client proteins in vitro. However, to our knowledge, few mechanistic studies have addressed the fate of client proteins once bound to 2-Cys-Prxs. To investigate whether mTXNPxred has the ability to delay the unfolding process and/or refold its unfolded client proteins, we examined the influence of mTXNPxred on the inactivation and reactivation of thermally denatured luciferase. We incubated luciferase in the absence or presence of chaperone-active mTXNPxred at 42 °C and monitored luciferase activity over time. Within 20 min of incubation, luciferase activity was below 10%, independent of the absence (Fig. 2A, Inset) or presence (Fig. 2A) of mTXNPxred. This result is reminiscent of other chaperones, such as heat-shock protein 22 (Hsp33), which prevent protein aggregation without slowing protein unfolding. We then shifted the temperature to 25 °C and further monitored luciferase activity. However, we did not observe any significant luciferase reactivation (Fig. 2A, open circles), indicating that mTXNPxred either is unable to release its client proteins or releases them in a refolding-incompetent form. Because many ATP-independent chaperones such as Prx are unable to refold their client proteins and instead transfer them to ATP-dependent foldases, such as the heat-shock protein 77 (Hsp70) system (17), we tested whether mTXNPxred also could cooperate with other chaperone systems in the folding of its client proteins. We supplemented the incubation reaction after its shift to 25 °C with the bacterial DnaK/DnaJ/GrpE (KJE) system, which is highly homologous to the mitochondrial Hsp70 system (18, 19). Addition of the KJE system to preformed mTXNPxred:luciferase complexes resulted in a substantial increase in luciferase reactivation (Fig. 2A, closed circles), which was dependent on the amount of mTXNPxred present during thermal inactivation (Fig. 2B). In the presence of a fourfold molar excess of mTXNPxred decamers to luciferase, nearly 40% of luciferase molecules reactivated upon the shift to non–heat-shock temperatures and the addition of the KJE system (Fig. 2). In contrast, when the thermal inactivation of luciferase was conducted in the absence of mTXNPxred (Fig. 2A, Inset) or in the presence of mTXNPxox (Fig. 2B), KJE-mediated reactivation of luciferase was less than 10%. We concluded from these results that chaperone-active mTXNPxred maintains its client proteins in a folding-competent conformation.
Fig. 2.
mTXNPxred maintains luciferase in a refolding competent state. (A) Luciferase (0.1 µM) was incubated in the absence (Inset) or presence of a 40:1 molar ratio of mTXNPxred for 20 min at 42 °C. Samples were cooled to 25 °C. After 10 min (indicated by the arrow), the samples were supplemented with 2 mM MgATP, 0.1 mg/mL BSA, and, where indicated, with a 20:4:20:1 ratio of DnaK:DnaJ:GrpE (KJE) system to luciferase. At the indicated time points, aliquots were removed, and luciferase activity was determined. (B) Luciferase (0.1 µM) was inactivated in the absence of any chaperone system (−), in the presence of a 10:1, 20:1, or 40:1 molar ratio of mTXNPxred/mTXNPxox to luciferase, or in the presence of a 20:4:20:1 ratio of DnaK:DnaJ:GrpE (KJE) to luciferase at 42 °C. All samples shown in the left panel contained 0.2 mM DTT, whereas samples shown in the right panel did not contain any reducing agents. After 20 min, the temperature was shifted to 25 °C for 10 min, and 2 mM MgATP and 0.1 mg/mL BSA were added to all samples. In addition, all samples except those that already contained the KJE system were supplemented with a 20:4:20:1 ratio of DnaK:DnaJ: GrpE:luciferase. After 120 min of incubation at 25 °C, luciferase activity was measured. Luciferase activity before incubation at 42 °C was set to 100%.
mTXNPx Functions as a General Chaperone both in Vitro and in Vivo.
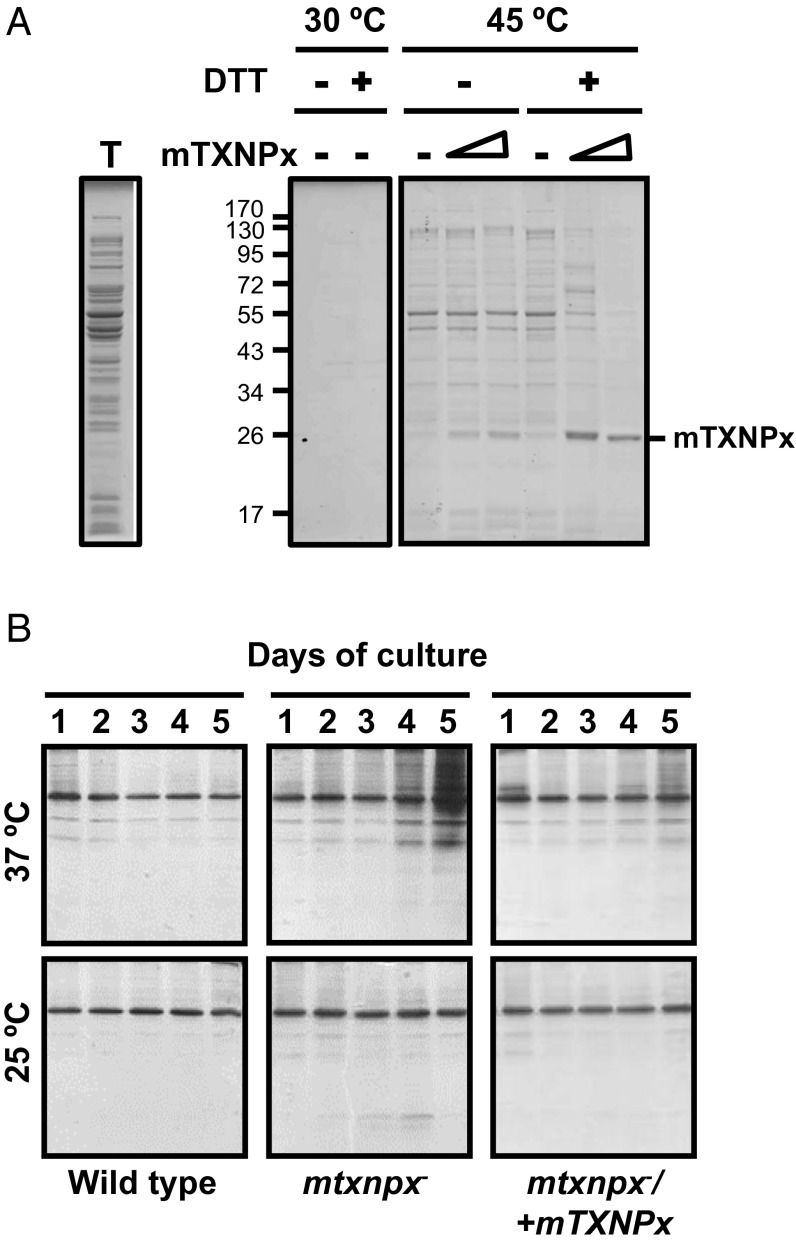
Little is known about the client specificity of Prxs or the extent to which they affect protein aggregation in vivo. To address the potential client specificity of mTXNPx, we first investigated the influence of purified mTXNPx on the heat-induced aggregation of proteins within a soluble bacterial extract under both reducing and nonreducing conditions. We used extracts of a bacterial strain that lacks the heat shock sigma factor σ32 (gene name, rpoH) and therefore contains reduced levels of most E. coli chaperones. This approach allowed us to minimize the influence of other chaperones in the assay and focus primarily on the chaperone function of mTXNPx. We prepared cell lysates of the rpoH-deletion strain (Fig. 3A, lane T) and supplemented these with different amounts of oxidized or reduced mTXNPx. We then incubated the lysates at either 30 °C or 45 °C for 60 min, separated the aggregated from the soluble proteins by centrifugation, and analyzed the precipitated proteins on a reducing SDS/PAGE. As shown in Fig. 3A, mTXNPxred exerts a highly promiscuous protein-protective effect, protecting the great majority of thermolabile E. coli proteins against temperature-induced protein aggregation. In contrast, and in agreement with our data shown above, mTXNPxox was unable to affect the aggregation behavior of thermolabile E. coli proteins. These studies demonstrate that reduced mTXNPx functions as a highly effective general chaperone.
Fig. 3.
mTXNPx displays general chaperone activity. (A) Aliquots of a soluble extract (T) from the chaperone-deficient E. coli rpoH mutant strain were prepared, supplemented with increasing amounts of mTXNPxred (in the presence of 1.5 mM DTT) or mTXNPxox (in the absence of DTT), and heat treated for 60 min at 45 °C. Samples without mTXNPx served as controls. The insoluble proteins then were separated from the soluble supernatant by centrifugation and were analyzed on reducing SDS/PAGE. The order of the lanes in the gel was rearranged electronically to facilitate data interpretation. (B) Wild-type, mtxnpx−, or mtxnpx−/+mTXNPx L. infantum promastigotes were incubated at 25 °C or 37 °C. Parasites were collected at the indicated time points, and their aggregated proteins were isolated and analyzed by SDS/PAGE. The major protein band that is present in all lanes is likely GP63, a GPI-anchored membrane protein and one of the most abundant proteins in Leishmania.
To determine whether mTXNPx exerts a similarly broad protein-protective effect in Leishmania, and, if so, under which physiological conditions, we analyzed protein aggregation in wild-type and mtxnpx− promastigotes at 37 °C, a temperature at which promastigotes lacking mTXNPx show a thermosensitive phenotype (13). Because temperature-sensitive phenotypes are common in cells lacking chaperones and usually are associated with protein aggregation, we reasoned that we should observe significantly increased protein aggregation in promastigotes of mTXNPx-deletion lines compared with wild-type promastigotes or parasites cultured at 25 °C. We therefore grew wild-type and mtxnpx− promastigotes at both 25 °C and 37 °C for up to 5 d, took aliquots at defined time points, and separated soluble from insoluble proteins. We then analyzed the aggregated proteins on SDS/PAGE (Fig. 3B). Although we did not observe any significant protein aggregation in either strain background at 25 °C (Fig. 3B, Lower), we detected substantially higher levels of protein aggregation in mtxnpx− parasites than in wild-type parasites cultivated at 37 °C. Moreover, the proteins that were protected in wild-type cells and aggregated in heat-treated mtxnpx− promastigotes showed a wide variation in size, in full agreement with our bacterial lysates studies. Introduction of an ectopic copy of mTXNPx into the mtxnpx− deletion strain (mtxnpx−/+mTXNPx) largely diminished protein aggregation at 37 °C (Fig. 3B), consistent with our previous findings that an ectopic copy of mTXNPx completely rescues the temperature-sensitive phenotype (13). These results corroborate the general chaperone function of mTXNPx and demonstrate that mTXNPx plays an important role in preventing temperature-induced protein aggregation in parasites.
mTXNPxred Undergoes Structural Rearrangements at Heat-Shock Temperatures.
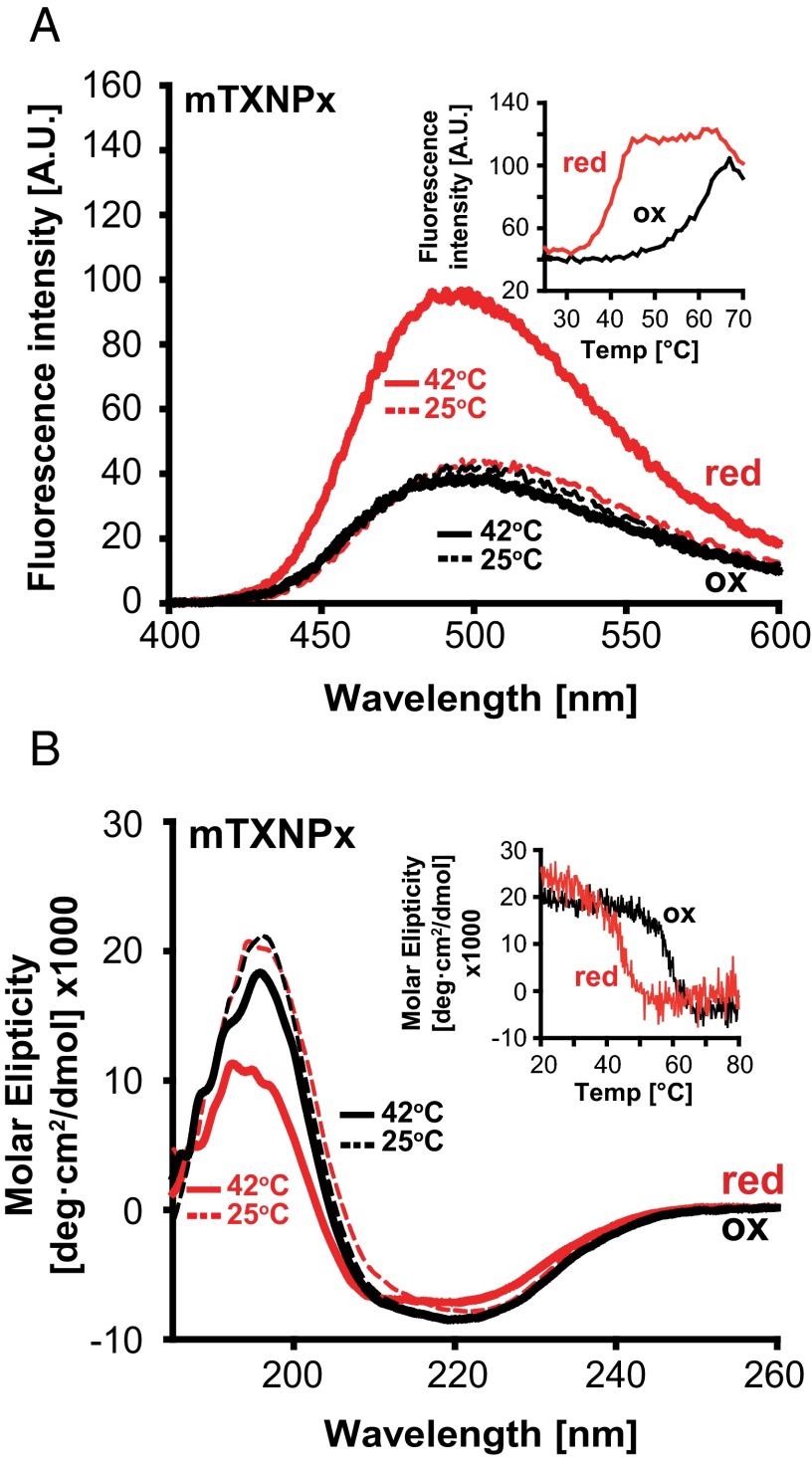
Our data showed that decameric mTXNPxred works as an efficient molecular chaperone under heat-shock conditions both in vivo and in vitro but is unable to suppress aggregation of chemically denatured client proteins at 30 °C, even when present at very high molar excess (SI Appendix, Fig. S3). These results made us wonder whether temperature-induced structural changes might contribute to the chaperone activity of reduced mTXNPx. To address the effect of temperature on mTXNPx structure and function, we incubated dimeric mTXNPxox and decameric mTXNPxred at either 25 °C or 42 °C (i.e., the temperature at which we observed chaperone activity in vitro) and compared surface hydrophobicity and secondary structure. We decided to focus on these two parameters because most molecular chaperones rely on the exposure of hydrophobic patches to bind unfolding clients (20, 21), and many ATP-independent chaperones, including Hsp33, sHsps, and HdeA, have been shown to undergo significant unfolding as part of their stress-specific activation (22). Analysis of the surface hydrophobicity of mTXNPxred using 4,4′-bis-anilino-1,1′-binaphthyl-5,5′-disulfonic acid (bis-ANS) binding revealed a significant increase in the fluorescence signal and a 30- to 40-nm blue shift in the emission maximum at 42 °C compared with 25 °C (Fig. 4A, red traces), fully consistent with the temperature-induced exposure of hydrophobic surfaces (23). In contrast, no significant increase in bis-ANS binding was detected for chaperone-inactive dimeric mTXNPxox irrespective of the temperature (Fig. 4A, black traces). Subsequent analysis of bis-ANS binding as function of the temperature confirmed these observations and revealed that mTXNPxred begins to expose hydrophobic surfaces at ∼35 °C with an apparent midpoint of transition at ∼40 °C. In contrast, incubation temperatures above 50 °C were required to detect any increase in bis-ANS binding to mTXNPxox, likely coinciding with mTXNPx unfolding (Fig. 4A, Inset). Consistent with these results, circular dichroism analysis revealed a substantial decrease in the α-helical content of mTXNPxred but not in mTXNPxox upon incubation at physiological heat-shock temperatures (Fig. 4B, Inset). These results strongly suggest that reduced decameric but not oxidized dimeric mTXNPx undergoes major conformational changes in a physiologically relevant temperature range leading to increased surface hydrophobicity and coinciding with the activation of its chaperone function.
Fig. 4.
mTXNPx undergoes temperature-mediated structural changes. (A) Bis-ANS binding to mTXNPxred (red lines) or mTXNPxox (black lines) at either 25 °C (broken lines) or 42 °C (solid lines). (Inset) Bis-ANS binding of mTXNPxred or mTXNPxox as a function of temperature monitored at 500 nm. (B) Far-UV circular dichroism spectra of mTXNPxred (red lines) or mTXNPxox (black lines) at either 25 °C (dashed lines) or 42 °C (solid lines). (Inset) Temperature-dependent changes in the secondary structure of mTXNPxred or mTXNPxox recorded at 197 nm. Temperature was increased at a rate of 1 °C/min. All spectra were buffer corrected.
Reduced mTXNPx Decamer Binds Unfolded Luciferase in the Center of Its Ringlike Structure.
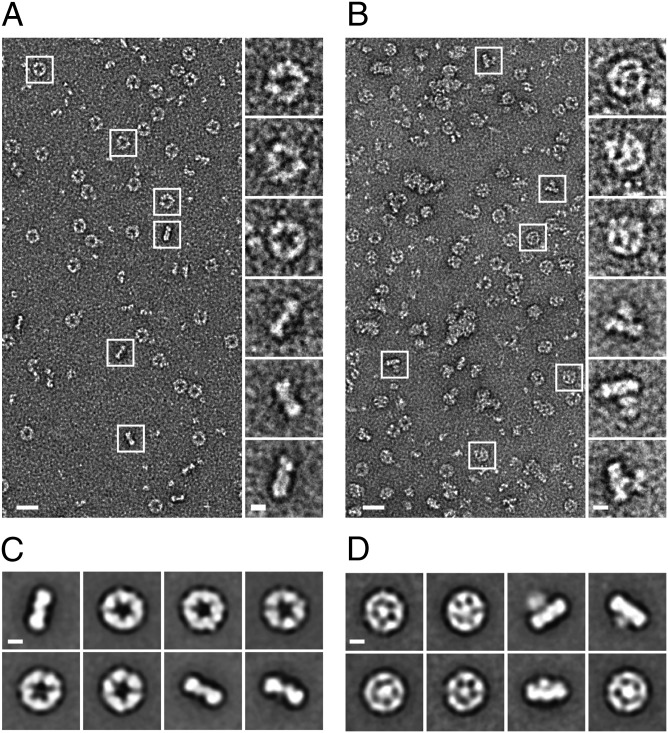
The ringlike structure of chaperone-active Prx decamer, which has been studied extensively by EM and has been solved by X-ray crystallography (24–26), prompted our subsequent studies, which were aimed at directly visualizing client protein binding. For these experiments, we incubated mTXNPxred either alone or in the presence of luciferase at 30 °C or 42 °C, using a 1:1 ratio of mTXNPxred decamer to luciferase. We then analyzed its structure by EM. We found that mTXNPxred preferentially forms typical ringlike structures featuring a large central cavity at both 30 °C and 42 °C in the absence of any client proteins and at 30 °C when the client protein is native and presumably correctly folded (Fig. 5A and SI Appendix, Fig. S4). Top-down orientations are preferred; however many side-views are visible that identify a single-ring arrangement. Stacked ring complexes, which have been characterized previously (27), are not observed under these conditions. Strikingly, upon incubation of mTXNPxred and luciferase at 42 °C many of the mTXNPxred particles contained additional electron density in the center, suggesting that thermally unfolded luciferase is bound within the ringlike structure (Fig. 5B and SI Appendix, Fig. S4). The side-views also showed additional density protruding from one side of the ring. We collected single-particle datasets for mTXNPxred alone (9,963 particles) and with luciferase (10,222 particles) and generated reference-free 2D projection averages. The 2D averages of mTXNPxred alone showed five well-defined lobes of density in the top-down views that are likely dimers arranged as the fivefold symmetric ring (Fig. 5C). The diameter of the rings was measured to be ∼130 Å with a 50-Å internal cavity and an overall width of ∼40 Å, agreeing with previous crystal structures (28). In the 2D averages of mTXNPxred with luciferase, the ring complex appears to contact directly a central, globular luciferase structure that is positioned in its center (Fig. 5D). Although the density for luciferase is structurally variable, we observed as many as five contact points in the complex, strongly suggesting that the interaction is based on mTXNPxred dimers. Notably, in the side-view projection averages, we observed that luciferase protrudes from one side of the decamer ring, indicating that a preferential orientation for client binding likely exists. This result also shows that only portions of the client proteins are bound to mTXNPxred, a finding that agrees well with our earlier results demonstrating that mTXNPx functions as a general chaperone that does not seem to impose any size limitation on its client proteins (see Fig. 3). Overall, these data demonstrate that mTXNPxred forms single decameric rings that use direct contacts to position the luciferase client in the center of its ring. Based on the small interaction region, we concluded that only a defined set of residues that face the center of the mTXNPx ring is likely to be involved in recognizing and binding unfolding client proteins.
Fig. 5.
The mTXNPxred decamer binds thermally unfolded luciferase in the center of its ring. (A and B) Representative EM micrographs and selected single-particle images of negatively stained mTXNPxred decamers alone (A) or after incubation with thermally unfolded luciferase (B). (C and D) Reference-free 2D projection averages showing top-down and side views of the mTXNPxred decamer alone or with luciferase. The percentage of mTXNPxred decamers in complex with luciferase varied between experiments. (Scale bars: 200 Å for the micrograph images and 50 Å for the boxed particles and averages.)
mTXNPx Chaperone Function Is Critical for Parasite Infectivity.
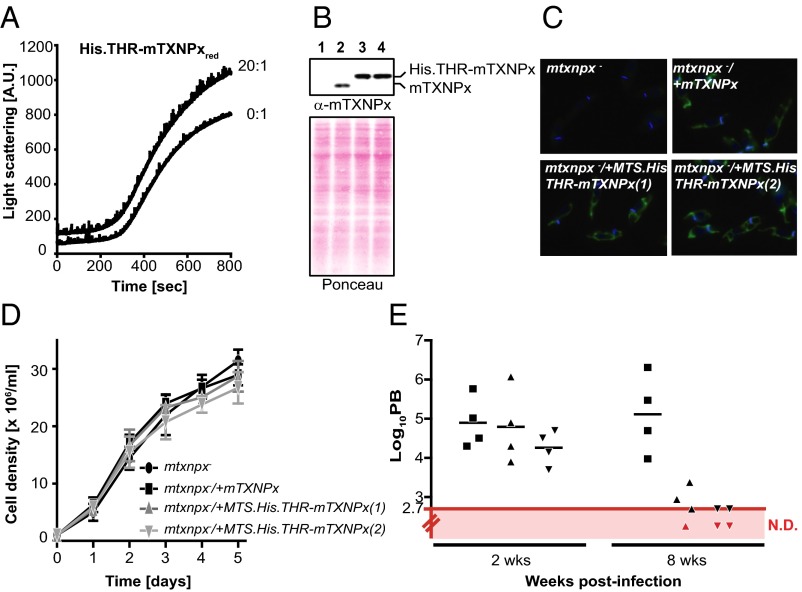
Analysis of the 2D class average particles of mTXNPx in complex with luciferase suggested that the N-terminal extensions of Prx, which face the interior of the decamer (28), might serve a critical role for client binding and therefore for the chaperone function of mTXNPx. This idea also was fully consistent with an earlier observation in our laboratory that an N-terminal His-thrombin tag variant of mTXPNx (His.THR.mTXNPx) failed to protect luciferase against heat-induced aggregation in vitro (Fig. 6A). These results confirmed the involvement of the N terminus of mTXNPx in client interaction and suggested that addition of extra residues abolishes client binding. Fortuitously, His-thrombin tag variants containing the additional mitochondrial targeting sequence (MTS) were found previously to be decameric and to exert full peroxidase activity (29); we now were able to determine directly the specific role of mTXNPx’s chaperone function in Leishmania infectivity and persistence in mammals. We transfected the previously established mtxnpx-knockout cell lines (13) with pSSU-PHLEO-MTS.His.THR-mTXNPx, a plasmid containing the coding sequence for a His-thrombin tag between the predicted mTXNPx MTS and the mature protein (SI Appendix, Fig. S5). Western blot analysis confirmed the presence of a slower-migrating mTXNPx species in the resulting transformants, indicative of the presence of a His-thrombin tag (Fig. 6B). We ascertained the correct targeting of His.THR-mTXNPx into mitochondria by immunofluorescence (Fig. 6C). To test whether the chaperone function of mTXNPx is indeed essential for Leishmania virulence, we then inoculated BALB/c mice with mtxnpx−/+mTXNPx or two different lines of mtxnpx−/+MTS.His.THR-mTXNPx transfectants and evaluated the parasite burden in the spleens at 2 and 8 wk postinfection. Despite permitting wild-typelike growth of promastigotes at 25 °C (Fig. 6D), the chaperone-inactive His.THR-mTXNPx variant was unable to restore the virulence of the mtxnpx− parasites (Fig. 6E). Based on these results, we conclude that the molecular chaperone activity is crucial for Leishmania survival within the mammalian host.
Fig. 6.
Chaperone activity of mTXNPxs is critical for Leishmania virulence. (A) Influence of a 20-fold molar excess of His.THR-mTXNPxred on the in vitro thermal aggregation of luciferase. See the legend of Fig. 1 for details. (B) Analysis of mTXNPx expression in mtxnpx− (lane 1), mtxnpx−/+mTXNPx (lane 2), and two mtxnpx−/+MTS.His.THR-mTXNPx (lanes 3 and 4, respectively) promastigote clones using a polyclonal antibody raised against the recombinant His.THR-MTS.mTXNPx. Purified mTXNPx and His.THR-mTXNPx were used as controls for the expected size of the proteins, and their position on the blot is indicated. Ponceau S staining of the blot is shown as loading control. (C) Localization of the His.THR-mTXNPx chimera in mtxnpx− promastigotes using indirect immunofluorescence with the anti-mTXNPx antibody (green) merged with DAPI (blue). Controls as described above are included. (D) Growth rate of mtxnpx− (black circles), mtxnpx−/+mTXNPx (black squares), and two mtxnpx−/+MTS.His.THR-mTXNPx clones (dark gray triangles). (E) Parasite burden of BALB/c mice after 2 and 8 wk of infection. BALB/c mice were inoculated i.p. with stationary-phase promastigotes of the mtxnpx−/+mTXNPx clone (squares) or two mtxnpx−/+MTS.His.THR-mTXNPx clones (triangles). After 2 and 8 wk of infection, the number of parasites per gram of spleen (parasite burden, PB) was determined. Each point represents one animal. The red line indicates the detection limit of the technique (log10 = 2.7). Animals with parasite burdens below this limit are “not detected” (ND) and are represented by red symbols.
Discussion
The Reduced Decamer Serves as the Basic Chaperone-Activatable Prx Species.
Following the pioneering work of Jang et al. (6), several studies independently validated the conclusion that cytosolic 2-Cys Prxs serve a dual purpose: as peroxidases that detoxify hydroperoxide and as chaperones that protect proteins against irreversible aggregation. The latter function was associated with the formation of HMW complexes of Prx. The switch between these two apparently mutually exclusive functions of Prx was attributed largely to cysteine-overoxidation (6). However, phosphorylation (7), exposure to low pH (8, 9), and other stimuli (30, 31) also were found to prompt chaperone activity. These results raised the obvious question as to whether all of these mechanisms work in an independent way to generate chaperone-active Prx species or whether they share a single, unifying activation mechanism. Our study now proposes that the reduced Prx decamer serves as the basic, chaperone-activatable scaffold, which is sensitive to structural rearrangements that likely are required for the chaperone to interact with unfolded clients. Our conclusion that mTXNPx does not have to be posttranslationally modified or form HMW complexes to exert chaperone function explains earlier in vivo findings in yeast, which showed that both wild-type and the peroxidase-inactive Tsa1 variant lacking the active site cysteine protect yeast cells against stress conditions at which no significant ROS production (and hence no overoxidation or HMW complex formation) was detected (6). These results also help explain how organisms such as Leishmania, which experience little Prx cysteine overoxidation (13) and lack the sulfinic acid reductase sulfiredoxin, are able to exploit their Prxs as an additional reservoir of chaperone activity under protein-unfolding stress conditions.
Our data clearly demonstrate that reduced mTXNPx decamers serve as potent molecular chaperones under heat-shock conditions in vitro, disagreeing with earlier studies that reported no detectable chaperone activity in reduced Prx (8, 32). Our studies now show that to exert full chaperone activity, Prx needs to be (i) maintained in a reduced, decameric form, (ii) devoid of N-terminal tags, and (iii) used at concentrations that promote decamerization. Therefore, the simple absence of reducing agents in the assay buffer, the presence of N-terminal tags, or even the use of insufficient Prx concentrations (below the kd of Prx decamerization) in the activity assays will abrogate the chaperone function of reduced Prx. In contrast, posttranslational modifications or active site mutations that have been reported to induce chaperone activity are known to stabilize Prx’s decameric structure (7, 30) and likely work simply by maintaining the chaperone activity of reduced Prx under the assay conditions used. Because intracellular conditions are highly reducing and Prx concentrations typically are much higher than the kd of decamerization, much of the cellular Prx will be in its chaperone-activatable form, making posttranslational modifications likely unnecessary to achieve chaperone function in vivo. What appears to be necessary for chaperone activation of Prx, however, is a structural remodeling of the decameric structure, which can be induced by elevated temperatures (an inherent part of most in vitro chaperone assays) and possibly by low pH or other protein-unfolding stress conditions, such as oxidative stress (33). In conclusion, our data suggest that Prxs are capable of integrating different unfolding stimuli and translating them into structural rearrangements that trigger chaperone activation. This ability makes Prx a member of a growing class of stress-specific chaperones whose activation demands local unfolding events (20–22, 34). What remains to be investigated is the extent to which these local unfolding events influence the peroxidase activity of reduced Prx and whether this mode of regulation not only applies to mitochondrial Prx but also extends to the cytosolic members of the 2-Cys Prx family.
Prxs Work as Ringlike Chaperones.
Based on our biochemical and in vivo studies, we now provide the long-sought explanation for the functional relevance of Prx’s oligomeric structures. We found that unfolding luciferase binds to the center of Prx’s ringlike structure and that, by using N-terminal residues that face the interior of the decamer, Prx binds and stabilizes client proteins. These results are in contrast to previous conclusions based on the analysis of the pH-activated Schistosoma mansoni Prx (SmPrxI) structure, which suggested that client proteins might bind to a hydrophobic surface that is exposed upon unfolding of the C terminus (8, 9). Although we cannot exclude the possibility that this region also is involved in client interaction, substantial rearrangements would be required to achieve these additional interactions. In either case, the fact that Prx binds clients in the center of its ringlike structure places Prx in the same category as other ringlike chaperonins, such as GroEL, which equally accommodate their client proteins in a central cavity (35). These results support the previous notion that Prxs are the most likely origin of chaperonins (36).
Physiological Implications of mTXNPx as a Temperature-Sensitive Chaperone.
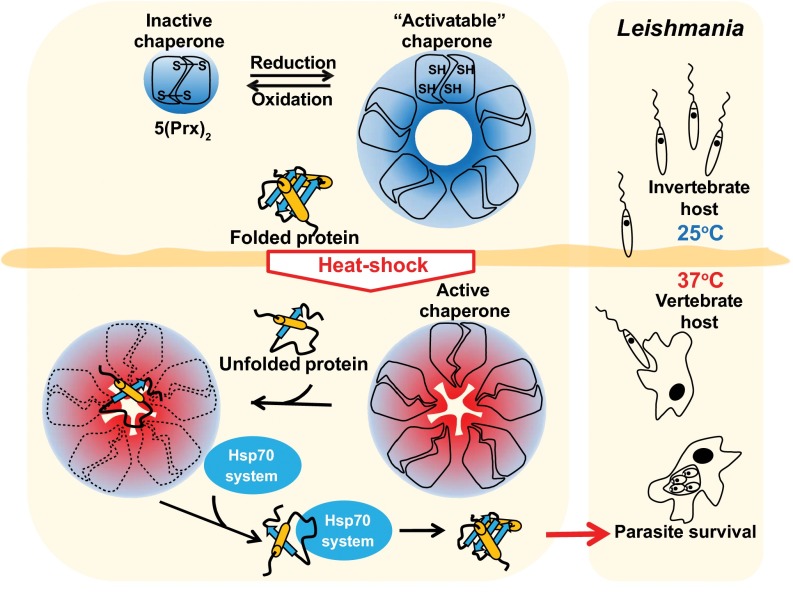
In this study, we demonstrated that mTXNPx prevents protein aggregation in Leishmania promastigotes under temperature conditions that resemble those found at the initiation of an infection within a mammalian host. This result confirms our previous findings that mTXNPx confers thermotolerance to parasites and suggests that the crucial in vivo function of this protein is that of a chaperone. Temperature shifts are part of Leishmania biology, and parasites evolved different mechanisms to cope with this stressful situation. According to our model (Fig. 7), reduced mTXNPx decamers sense the environment changes that occur as promastigotes shuttle from 25 °C to 37 °C and use structural rearrangements to protect a wide range of thermolabile proteins against aggregation. Importantly, mTXNPx is a highly abundant protein in Leishmania, and at least 50% of the mTXNPx found in promastigotes exposed to 37 °C is in its reduced and hence chaperone-activatable decameric form (13). This observation suggests that there is a substantial pool of mitochondrial chaperone activity that can be called upon instantaneously when needed while avoiding time-consuming protein-translation and mitochondrial-trafficking processes. Although the chaperone function of mTXNPx is likely to be important for differentiation and, consequently, for the formation of viable amastigotes, it is worth noting that mTXNPx is expressed throughout the infective process. Hence it is likely that this function provides additional protection to the parasite in other circumstances, such as during fever episodes. In conclusion, this study sheds light on previously undefined aspects of the chaperone function of Prxs and provides unambiguous evidence for the importance of mTXNPx’s chaperone function on Leishmania biology and infectivity.
Fig. 7.
Physiological role of mTXPNx’s chaperone function in Leishmania. Exposure of Leishmania promastigotes (the insect stage) to the mammalian body temperature of 37 °C leads to the protein unfolding. This change in temperature is sensed by reduced mTXNPx decamers and translated into structural rearrangements, which likely contribute to the activation of its chaperone function. Once they are chaperone active, mTXNPx decamers bind the client proteins in the center of their ringlike structure, preventing the client proteins from forming cytotoxic aggregates. Client proteins are maintained in a refolding-competent state and can be reactivated by members of the Hsp70 system in the presence of ATP. The working cycle of mTXNPx chaperone appears to be crucial for Leishmania to adapt to and survive the temperature encountered in the mammalian host and hence to generate viable amastigotes.
Materials and Methods
Protein Purification and Preparation of Oxidized and Reduced mTXNPx.
Mature mTXNPx (i.e., lacking the first 26 amino acids that compose the mitochondrial-targeting sequence MTS) was purified from E. coli under nonreducing conditions. It was subjected to thrombin treatment to remove the N-terminal His tag, leaving behind a four-amino acid scar (GlySerHisMet) at the N terminus (13). The protein was >90% oxidized, dimeric, and fully peroxidase-active (i.e., mTXNPxox). To prepare mTXNPxred, mTXNPxox was incubated in the presence of 5 mM DTT or its physiological reducing system consisting of 200 µM NADPH, 0.5 U/mL L. infantum trypanothione reductase (TR), 50 µM trypanothione disulfide (TS2; Bachem), and 2.5 µM L. infantum tryparedoxin 2 (TXN2) for 30 min at 30 °C. TR and TXN2 were purified as previously described (37). Upon reduction, mTXNPx was diluted into the different assay buffers maintaining a DTT concentration between 0.2 and 1.5 mM in the final activity assay. Equivalent concentrations of DTT or components of the reducing enzymatic cascade were added to control samples lacking mTXNPx. DnaK, DnaJ, and GrpE were purified as previously described (38).
Chaperone Activity Assays.
To investigate the chaperone activity of reduced and oxidized mTXNPx, 100 nM luciferase (Promega) was incubated in 40 mM Hepes (pH 7.5) at 41.5 °C in the absence or presence of different molar ratios of mTXNPx. Light scattering was measured using a fluorescence spectrophotometer (Hitachi F4500) equipped with a temperature-controlled cuvette holder and stirrer (λex/em, 360 nm; slit widths, 2.5 nm). To determine the effect of mTXNPxred on the reactivation of luciferase, 100 nM of luciferase was incubated either alone or in the presence of different ratios of mTXNPxred in 40 mM Hepes (pH 7.5), 50 mM KCl for 20 min at 42 °C. The reaction then was cooled to 25 °C. After 10 min, the samples were supplemented with 2 mM MgATP and 0.1 mg/mL BSA and, where indicated, with 2 µM DnaK, 0.4 µM DnaJ, and 2 µM GrpE. At defined time points, luciferase activity was measured by luminescence using a FLUOstar Omega microplate reader (BMG Labteck) (39). To determine the influence of mTXNPx on ex vivo protein aggregation, 200 µg of a soluble cell lysate from the E. coli ∆rpoH strain was prepared as described (40), supplemented with either 25 or 50 µg of mTXNPx, and incubated at 45 °C for 60 min under constant shaking at 500 rpm. Samples containing mTXNPxred were supplemented with 1.5 mM DTT to prevent the reoxidation of mTXNPx. The insoluble protein fraction was separated by centrifugation at 16,100 × g for 30 min and analyzed by reducing SDS/PAGE.
Preparation of mTXNPxred Client Complexes and EM.
Chaperone-active mTXNPxred (10 µM) alone or in the presence of luciferase (1 µM) was incubated for 1 min on ice and 2 min at room temperature. Samples then were transferred to an incubator and were heated slowly from 30 °C to 42 °C for 10 min to allow complex formation. Samples were centrifuged at 16,100 × g for 30 min at 4 °C to remove large aggregates. Proteins in the soluble supernatant were negatively stained with 0.75% (wt/vol) uranyl formate (pH 5.5–6.0) on 400-mesh carbon-coated copper grids (Pelco) as previously described (41). Samples were imaged under low-dose conditions using a G2 Spirit transmission electron microscope (FEI) operated at 120 kiloelectron volts. Micrographs were taken at 52,000× magnification with 2.16 Å per pixel using a 4 × 4 k CCD camera (Gatan). Single particles of mTXNPxred and mTXNPxred:luciferase complexes were selected manually using Boxer in the EMAN software package (42) and totaled 9,963 and 10,222, respectively. Reference-free 2D classification and projection averages were determined using SPIDER (43).
Spectroscopic Properties of Reduced and Oxidized mTXNPx.
To determine temperature-dependent changes in the surface hydrophobicity of mTXNPx, 3 μM of mTXNPxred or mTXNPxox were incubated with 25 µM bis-ANS in 40 mM Hepes (pH 7.5), and fluorescence emission spectra were recorded at either 25 °C or 42 °C as described (20). To determine bis-ANS binding as a function of temperature, the protein:bis-ANS mixtures were heated from 25 to 70 °C (at a rate of 1 °C/min), and changes in bis-ANS fluorescence were monitored continuously at 500 nm. To determine temperature-mediated changes in the secondary structure of the mTXNPx, far-UV CD spectra of 0.1 mg/mL mTXNPxred or mTXNPxox were recorded in 10 mM sodium phosphate buffer (pH 8) at 25 °C and 42 °C using a Jasco-J810 spectropolarimeter. To monitor the thermostability of mTXNPx, the CD signal at 197 nm was followed from 20 to 80 °C. The temperature was increased at a rate of 1 °C/min and was controlled by a Jasco Peltier device. All spectra were buffer-corrected.
Protein Aggregation in Vivo.
Aggregated proteins from L. infantum promastigotes exposed to either 25 °C or 37 °C were prepared as previously described (44). Briefly, 107 promastigote cells were collected, washed with lysis buffer [50 mM potassium phosphate buffer (pH 7), 1 mM EDTA, 5% (vol/vol) glycerol] and stored at −80 °C. Cell lysis was achieved by thawing samples at 37 °C and subsequent sonication. Upon removal of intact cells by centrifugation (775 × g, 10 min, 4 °C), total cell extracts were centrifuged again (11,385 × g, 20 min, 4 °C), and pellets were resuspended in lysis buffer. Membrane proteins then were solubilized by addition of 20% (vol/vol) Nonidet P-40 and were separated from aggregates by centrifugation (11,385 × g, 20 min, 4 °C). Pellets containing aggregated proteins were analyzed by SDS/PAGE.
Parasite Culture and Genetic Manipulation.
L. infantum promastigotes (MHOM MA67ITMAP263) were cultured at 25 °C in RPMI medium 1640 with GlutaMAX-I (GIBCO) supplemented with 10% (vol/vol) inactivated FBS (FBSi; GIBCO), 50 U/mL penicillin (GIBCO), 50 mg/mL streptomycin (GIBCO), and 25 mM Hepes sodium salt, pH 7.4 (Sigma). For growth-rate determination, L. infantum promastigotes, previously synchronized by three or four daily passages at 106 cells/mL, were seeded at 106 cells/mL, and their density was examined over time in Neubauer counting chambers. Transfection of promastigotes was performed as described (45). Isolated clones were transferred from agar plates containing selective drug [17.5 mg/mL phleomycin (Sigma)] into liquid medium.
Generation of mtxnpx−/MTS.His.THR-mTXNPx Mutants.
The pSSU-PHLEO-infantum-MTS.His.THR-mTXNPx fragment to complement mtxnpx− L. infantum promastigotes was assembled according to the scheme in SI Appendix, Fig. S3A. To generate this construct, a DNA fragment corresponding to His.THR-mTXNPx was PCR amplified with primers P1 and P2 (SI Appendix, Table S1) from pET28c-mTXNPx (13), which harbors the mTXNPx ORF in frame with an N-terminal His/thrombin tag. The resulting PCR product was cloned into the SacII and XbaI sites of pSSU-PHLEO-infantum-mTXNPx (13). Before this cloning step, the pSSU-PHLEO-infantum-mTXNPx (13) vector was manipulated by site-directed mutagenesis with primers P3 and P4 to eliminate the SacII restriction site located in the 5′ small subunit 18S rRNA (SSU) fragment of the plasmid. The resulting pSSU-PHLEO-infantum-MTS.His.THR-mTXNPx construct was further subjected to site-directed mutagenesis with primers P5 and P6 to remove an undesired NdeI restriction site in the His.THR-mTXNPx ORF. Before transfection of mtxnpxˉ promastigotes, the construct was linearized by digestion with NdeI-PmeI and purified from agarose gel by electroelution. Expression of His.THR-mTXNPx in the resulting mtxnpx−/+MTS.His.THR-mTXNPx clones was confirmed by Western blot and indirect immunofluorescence according to previously described procedures (13, 45).
Animals and Ethics Statement.
BALB/c and National Marine Research Institute (NMRI) mice were purchased from the animal facility house at Instituto de Biologia Molecular e Celular (IBMC). All mice used in this study were raised in specific pathogen-free conditions. Mice were euthanized in a 20% isofluorane atmosphere. The experimental animal procedures were approved by the local Animal Ethics Committee of IBMC, University of Porto, Portugal and were licensed by the General Directory of Veterinary (DGV), Ministry of Agriculture, Rural Development and Fishing, of the Portuguese government, on May 18, 2006 (reference 520/000/000/2006). All animals were handled in strict accordance with good animal practice as defined by national authorities (DGV, Law nu1005/92 October 23) and European legislation (EEC/86/609.)
Determination of Parasite Burden by Limiting Dilution Assay.
Parasite lines were passaged through NMRI mice before the infection experiment. BALB/c male mice then were inoculated i.p. with 108 L. infantum stationary-phase promastigotes. Experimental groups were randomized as to mouse age, cage, and cage location. Mice were euthanized 2 and 8 wk after infection. The spleens were excised, weighed, and homogenized in Schneider’s medium (Sigma) supplemented with 10% (vol/vol) FBSi, 100 U/mL penicillin, 100 mg/mL streptomycin, 5 mM Hepes (pH 7.4), 5 mg/mL phenol-red (Sigma), and 2% (vol/vol) sterile human urine. The number of parasites per gram of organ (parasite burden) was calculated as described in ref. 46. Briefly, homogenates were diluted to 10 mg/mL and subsequently titrated in quadruplicate across a 96-well plate in serial fourfold dilutions (four titrations per spleen). After 2 wk of growth at 25 °C, the last dilution containing promastigotes was recorded, and the parasite burden was calculated.
Supplementary Material
Acknowledgments
We thank Frederico Silva for help with size-exclusion chromatography experiments, and Ana G. Gomes-Alves and Ricardo Silva for constructing the pSSU-PHLEO-infantum-MTS.His.THR-mTXNPx plasmid. This work was supported by National Institutes of Health Grant GM065318 (to U.J.) and Project “NORTE-07-0124-FEDER-000002-Host-Pathogen Interactions” cofunded by Programa Operacional Regional do Norte under the Quadro de Referência Estratégico Nacional, through Fundo Europeu de Desenvolvimento Regional, and by the Portuguese Foundation for Science and Technology (FCT) (A.M.T.). F.T. and H.C. were supported by Portuguese FCT Fellowships SFRH/BD/70438/2010 and SFRH/BPD/80836/2011, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419682112/-/DCSupplemental.
References
- 1.Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS J. 2009;276(9):2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae HZ, Chung SJ, Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269(44):27670–27678. [PubMed] [Google Scholar]
- 3.Hofmann B, Hecht HJ, Flohé L. Peroxiredoxins. Biol Chem. 2002;383(3-4):347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signaling. 2011;15(3):781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 5.Poole LB, Hall A, Nelson KJ. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol. 2011;49:7.9. doi: 10.1002/0471140856.tx0709s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang HH, et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117(5):625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Jang HH, et al. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differentially regulate its peroxidase and molecular chaperone functions. FEBS Lett. 2006;580(1):351–355. doi: 10.1016/j.febslet.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Saccoccia F, et al. Moonlighting by different stressors: Crystal structure of the chaperone species of a 2-Cys peroxiredoxin. Structure. 2012;20(3):429–439. doi: 10.1016/j.str.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelucci F, et al. Switching between the alternative structures and functions of a 2-Cys peroxiredoxin, by site-directed mutagenesis. J Mol Biol. 2013;425(22):4556–4568. doi: 10.1016/j.jmb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Castro H, et al. Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic Biol Med. 2002;33(11):1552–1562. doi: 10.1016/s0891-5849(02)01089-4. [DOI] [PubMed] [Google Scholar]
- 11.Castro H, Tomás AM. Peroxidases of trypanosomatids. Antioxid Redox Signal. 2008;10(9):1593–1606. doi: 10.1089/ars.2008.2050. [DOI] [PubMed] [Google Scholar]
- 12.Gretes MC, Poole LB, Karplus PA. Peroxiredoxins in parasites. Antioxid Redox Signal. 2012;17(4):608–633. doi: 10.1089/ars.2011.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro H, et al. Leishmania mitochondrial peroxiredoxin plays a crucial peroxidase-unrelated role during infection: Insight into its novel chaperone activity. PLoS Pathog. 2011;7(10):e1002325. doi: 10.1371/journal.ppat.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barranco-Medina S, Lázaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583(12):1809–1816. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Barranco-Medina S, Dietz KJ. Thermodynamics of 2-Cys peroxiredoxin assembly determined by isothermal titration calorimetry. Methods Enzymol. 2009;466:409–430. doi: 10.1016/S0076-6879(09)66017-1. [DOI] [PubMed] [Google Scholar]
- 16.Barranco-Medina S, Kakorin S, Lázaro JJ, Dietz KJ. Thermodynamics of the dimer-decamer transition of reduced human and plant 2-cys peroxiredoxin. Biochemistry. 2008;47(27):7196–7204. doi: 10.1021/bi8002956. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann JH, Linke K, Graf PC, Lilie H, Jakob U. Identification of a redox-regulated chaperone network. EMBO J. 2004;23(1):160–168. doi: 10.1038/sj.emboj.7600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amir-Shapira D, Leustek T, Dalie B, Weissbach H, Brot N. Hsp70 proteins, similar to Escherichia coli DnaK, in chloroplasts and mitochondria of Euglena gracilis. Proc Natl Acad Sci USA. 1990;87(5):1749–1752. doi: 10.1073/pnas.87.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leustek T, Dalie B, Amir-Shapira D, Brot N, Weissbach H. A member of the Hsp70 family is localized in mitochondria and resembles Escherichia coli DnaK. Proc Natl Acad Sci USA. 1989;86(20):7805–7808. doi: 10.1073/pnas.86.20.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graf PC, et al. Activation of the redox-regulated chaperone Hsp33 by domain unfolding. J Biol Chem. 2004;279(19):20529–20538. doi: 10.1074/jbc.M401764200. [DOI] [PubMed] [Google Scholar]
- 21.Foit L, George JS, Zhang BW, Brooks CL, 3rd, Bardwell JC. Chaperone activation by unfolding. Proc Natl Acad Sci USA. 2013;110(14):E1254–E1262. doi: 10.1073/pnas.1222458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardwell JC, Jakob U. Conditional disorder in chaperone action. Trends Biochem Sci. 2012;37(12):517–525. doi: 10.1016/j.tibs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musci G, Metz GD, Tsunematsu H, Berliner LJ. 4,4′-Bis[8-(phenylamino)naphthalene-1-sulfonate] binding to human thrombins: A sensitive exo site fluorescent affinity probe. Biochemistry. 1985;24(8):2034–2039. doi: 10.1021/bi00329a035. [DOI] [PubMed] [Google Scholar]
- 24.Meissner U, Schröder E, Scheffler D, Martin AG, Harris JR. Formation, TEM study and 3D reconstruction of the human erythrocyte peroxiredoxin-2 dodecahedral higher-order assembly. Micron. 2007;38(1):29–39. doi: 10.1016/j.micron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Harris JR, et al. Comparison of the decameric structure of peroxiredoxin-II by transmission electron microscopy and X-ray crystallography. Biochim Biophys Acta. 2001;1547(2):221–234. doi: 10.1016/s0167-4838(01)00184-4. [DOI] [PubMed] [Google Scholar]
- 26.Choi HJ, Kang SW, Yang CH, Rhee SG, Ryu SE. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat Struct Biol. 1998;5(5):400–406. doi: 10.1038/nsb0598-400. [DOI] [PubMed] [Google Scholar]
- 27.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 28.Piñeyro MD, et al. Crystal structure of the tryparedoxin peroxidase from the human parasite Trypanosoma cruzi. J Struct Biol. 2005;150(1):11–22. doi: 10.1016/j.jsb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Castro H, et al. Specificity and kinetics of a mitochondrial peroxiredoxin of Leishmania infantum. Free Radic Biol Med. 2002;33(11):1563–1574. doi: 10.1016/s0891-5849(02)01088-2. [DOI] [PubMed] [Google Scholar]
- 30.Lim JC, et al. Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J Biol Chem. 2008;283(43):28873–28880. doi: 10.1074/jbc.M804087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Y, Jin JH, Yu Y, Wang J. Significant enhancement of hPrx1 chaperone activity through lysine acetylation. ChemBioChem. 2014;15(12):1773–1776. doi: 10.1002/cbic.201402164. [DOI] [PubMed] [Google Scholar]
- 32.Konig J, et al. The conformational bases for the two functionalities of 2-cysteine peroxiredoxins as peroxidase and chaperone. Journal of experimental botany. 2013;64(11):3483–3497. doi: 10.1093/jxb/ert184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDiarmid CW, et al. Peroxiredoxin chaperone activity is critical for protein homeostasis in zinc-deficient yeast. J Biol Chem. 2013;288(43):31313–31327. doi: 10.1074/jbc.M113.512384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voth W, et al. The protein targeting factor Get3 functions as ATP-independent chaperone under oxidative stress conditions. Mol Cell. 2014;56(1):116–127. doi: 10.1016/j.molcel.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saibil HR, Fenton WA, Clare DK, Horwich AL. Structure and allostery of the chaperonin GroEL. J Mol Biol. 2013;425(9):1476–1487. doi: 10.1016/j.jmb.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Dekker C, Willison KR, Taylor WR. On the evolutionary origin of the chaperonins. Proteins. 2011;79(4):1172–1192. doi: 10.1002/prot.22952. [DOI] [PubMed] [Google Scholar]
- 37.Castro H, et al. Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Mol Biochem Parasitol. 2004;136(2):137–147. doi: 10.1016/j.molbiopara.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Buchberger A, Schröder H, Büttner M, Valencia A, Bukau B. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat Struct Biol. 1994;1(2):95–101. doi: 10.1038/nsb0294-95. [DOI] [PubMed] [Google Scholar]
- 39.Herbst R, Schäfer U, Seckler R. Equilibrium intermediates in the reversible unfolding of firefly (Photinus pyralis) luciferase. J Biol Chem. 1997;272(11):7099–7105. doi: 10.1074/jbc.272.11.7099. [DOI] [PubMed] [Google Scholar]
- 40.Gray MJ, et al. Polyphosphate is a primordial chaperone. Mol Cell. 2014;53(5):689–699. doi: 10.1016/j.molcel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohi M, Li Y, Cheng Y, Walz T. Negative Staining and Image Classification - Powerful Tools in Modern Electron Microscopy. Biol Proced Online. 2004;6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 43.Frank J, et al. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116(1):190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 44.Tomoyasu T, Mogk A, Langen H, Goloubinoff P, Bukau B. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol Microbiol. 2001;40(2):397–413. doi: 10.1046/j.1365-2958.2001.02383.x. [DOI] [PubMed] [Google Scholar]
- 45.Sousa AF, et al. Genetic and chemical analyses reveal that trypanothione synthetase but not glutathionylspermidine synthetase is essential for Leishmania infantum. Free Radic Biol Med. 2014;73:229–238. doi: 10.1016/j.freeradbiomed.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Buffet PA, Sulahian A, Garin YJ, Nassar N, Derouin F. Culture microtitration: A sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother. 1995;39(9):2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.