Abstract
The ability of a series of anions to inhibit [3H]strychnine binding to spinal cord synaptic membranes correlates closely with their neurophysiologic capacity to reverse inhibitory postsynaptic potentials in the mammalian spinal cord. Seven neurophysiologically active anions are also effective inhibitors of [3H]strychnine binding with mean effective doses ranging from 160 to 620 mM. Seven other anions that are ineffective neurophysiologically also fail to alter strychnine binding. Chloride inhibits strychnine binding in a noncompetitive fashion. Hill plots of the displacement of [3H]strychnine by chloride give coefficients of 2.3-2.7. The inhibition of strychnine binding by these anions suggests that strychnine binding is closely associated with the ionic conductance mechanism for chloride in the glycine receptor.
Keywords: ionophore, cooperativity, neurotransmitter, ion conductance, chloride
Full text
PDF
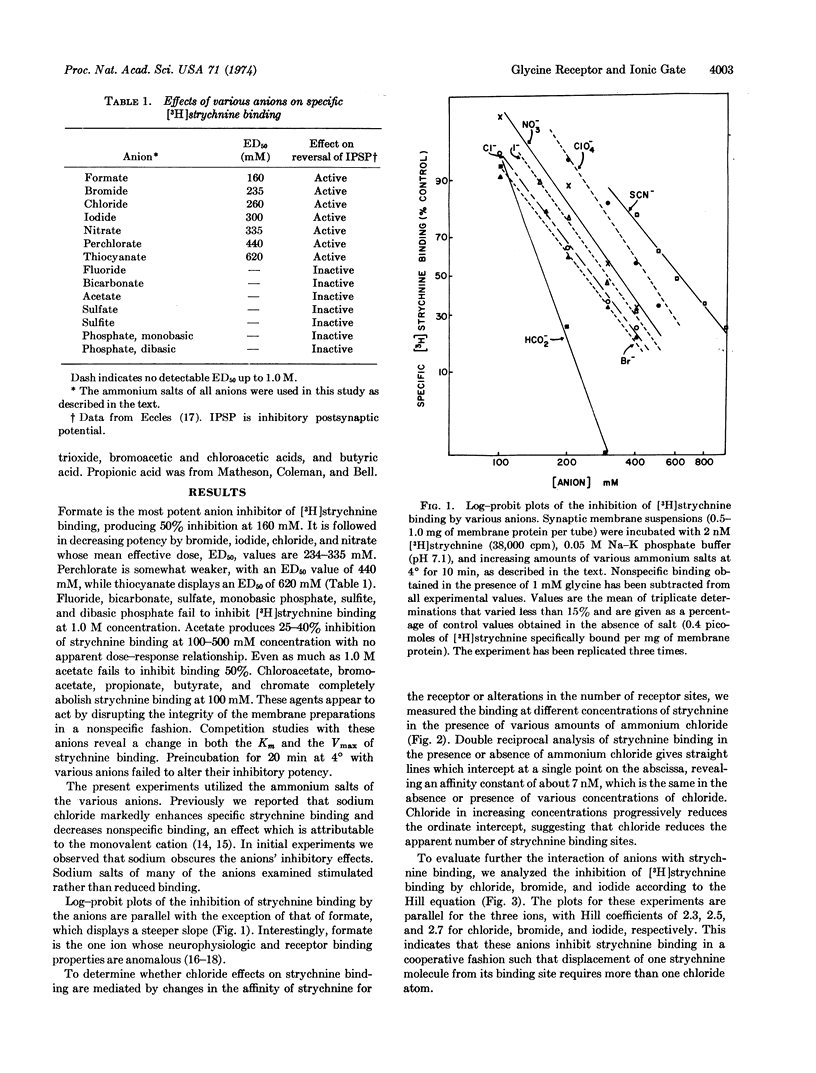
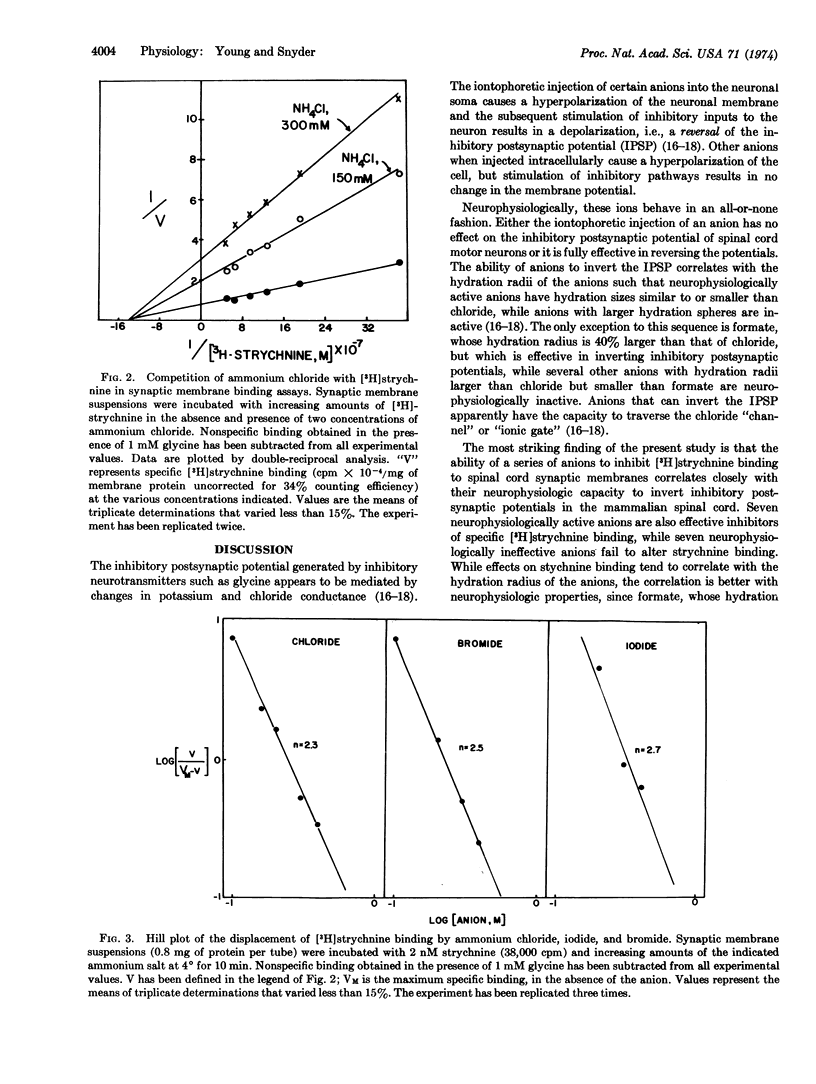

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprison M. H., Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965 Nov;4(21):2075–2083. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- BRADLEY K., EASTON D. M., ECCLES J. C. An investigation of primary or direct inhibition. J Physiol. 1953 Dec 29;122(3):474–488. doi: 10.1113/jphysiol.1953.sp005015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N. Werman R:The cooperativity of -aminobutyric action on the membrane of locust muscle fibers. Mol Pharmacol. 1973 Jul;9(4):571–579. [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res. 1971 Jun 29;12(5):547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Aprison M. H., Werman R. The effects of strychnine on the inhibition of interneurons by glycine and gamma-aminobutyric acid. Int J Neuropharmacol. 1969 Mar;8(2):191–194. doi: 10.1016/0028-3908(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. The ionic mechanisms of excitatory and inhibitory synaptic action. Ann N Y Acad Sci. 1966 Jul 14;137(2):473–494. doi: 10.1111/j.1749-6632.1966.tb50176.x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Light and electron microscopic autoradiography on spinal cord slices after incubation with labeled glycine. Brain Res. 1971 Sep 10;32(1):189–194. doi: 10.1016/0006-8993(71)90163-6. [DOI] [PubMed] [Google Scholar]
- ITO M., KOSTYUK P. G., OSHIMA T. Further study on anion permeability of inhibitory post-synaptic membrane of cat motoneurones. J Physiol. 1962 Oct;164:150–156. doi: 10.1113/jphysiol.1962.sp007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Bloom F. E. Studies of the uptake of 3 H-gaba and ( 3 H)glycine in slices and homogenates of rat brain and spinal cord by electron microscopic autoradiography. Brain Res. 1972 Jun 8;41(1):131–143. doi: 10.1016/0006-8993(72)90621-x. [DOI] [PubMed] [Google Scholar]
- Larson M. D. An analysis of the action of strychnine on the recurrent IPSP and amino acid induced inhibitions in the cat spinal cord. Brain Res. 1969 Sep;15(1):185–200. doi: 10.1016/0006-8993(69)90318-7. [DOI] [PubMed] [Google Scholar]
- Matus A. I., Dennison M. E. Autoradiographic localisation of tritiated glycine at 'flat-vesicle' synapses in spinal cord. Brain Res. 1971 Sep 10;32(1):195–197. doi: 10.1016/0006-8993(71)90164-8. [DOI] [PubMed] [Google Scholar]
- Owen A. G., Sherrington C. S. Observations on strychnine reversal. J Physiol. 1911 Nov 20;43(3-4):232–241. doi: 10.1113/jphysiol.1911.sp001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Logan W. J., Bennett J. P., Arregui A. Amino acids as central nervous transmitters: biochemical studies. Neurosci Res (N Y) 1973;5(0):131–157. doi: 10.1016/b978-0-12-512505-5.50013-6. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Young A. B., Bennett J. P., Mulder A. H. Synaptic biochemistry of amino acids. Fed Proc. 1973 Oct;32(10):2039–2047. [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Zukin S. R., Snyder S. H. Interaction of benzodiazepines with central nervous glycine receptors: possible mechanism of action. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2246–2250. doi: 10.1073/pnas.71.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]


