Significance
Interleukin 4 (IL-4) has been shown to be highly protective against delayed type hypersensitivity and organ-specific autoimmune and autoinflammatory reactions in mice and humans, but its mode of action has remained controversial and has failed to be explained solely by redirection of TH1/TH17 toward a TH2-type immune response. Here we uncovered that IL-4 selectively suppresses IL-23 transcription and secretion by cells of the innate immune system. We further describe a previously unidentified therapeutic mode of action of IL-4 in TH17-mediated inflammation, and a physiologically highly relevant approach to selectively target IL-23/TH17-dependent inflammation while sparing IL-12 and TH1 immune responses.
Keywords: IL-4, TH17, IL-23, psoriasis, dendritic cells
Abstract
Interleukin 4 (IL-4) can suppress delayed-type hypersensitivity reactions (DTHRs), including organ-specific autoimmune diseases in mice and humans. Despite the broadly documented antiinflammatory effect of IL-4, the underlying mode of action remains incompletely understood, as IL-4 also promotes IL-12 production by dendritic cells (DCs) and IFN-γ–producing TH1 cells in vivo. Studying the impact of IL-4 on the polarization of human and mouse DCs, we found that IL-4 exerts opposing effects on the production of either IL-12 or IL-23. While promoting IL-12–producing capacity of DCs, IL-4 completely abrogates IL-23. Bone marrow chimeras proved that IL-4–mediated suppression of DTHRs relies on the signal transducer and activator of transcription 6 (STAT6)-dependent abrogation of IL-23 in antigen-presenting cells. Moreover, IL-4 therapy attenuated DTHRs by STAT6- and activating transcription factor 3 (ATF3)-dependent suppression of the IL-23/TH17 responses despite simultaneous enhancement of IL-12/TH1 responses. As IL-4 therapy also improves psoriasis in humans and suppresses IL-23/TH17 responses without blocking IL-12/TH1, selective IL-4–mediated IL-23/TH17 silencing is promising as treatment against harmful inflammation, while sparing the IL-12–dependent TH1 responses.
IL-4 is a canonical type 2 immune cytokine known for its capacity to induce IgE isotype switching in B cells and to initiate and sustain TH2 cell differentiation (1, 2). IL-4 provides protective immune responses to helminthes (3), and excessive endogenous IL-4 production is linked to TH2-dominated allergic asthma and atopic dermatitis (4) as well as to T-cell immunosuppression in leukemic cutaneous T-cell lymphoma (5). In vivo, IL-4 can suppress organ-specific autoimmune and delayed-type hypersensitivity reactions (DTHRs). Accordingly, IL-4 is absent in naturally occurring DTHRs, such as experimental autoimmune encephalomyelitis (EAE), multiple sclerosis (MS), rheumatoid arthritis (RA), inflammatory bowel disease (IBD), or psoriasis (6–10). Systemic IL-4 immunotherapy improves EAE (11), experimental colitis (12), nonobese diabetes (13), collagen-induced arthritis (14), and hapten-induced contact hypersensitivity (15) in mice and psoriasis in humans (16). The inhibitory effect of IL-4 on the autoimmune DTHRs, however, failed to be completely explained by the redirection of the TH1 immune responses toward IFN-γ–deficient type 2 immune responses. To the contrary, the number of peripheral IFN-γ+CD4+ TH1 cells or serum IFN-γ even increase after IL-4 administration in mice with EAE (11), hemophagocytic lymphohistiocytosis (17), or hapten-induced contact hypersensitivity (15), and in humans with psoriasis (16); mice with transgenic overexpression of IL-4 exhibit TH2-driven allergic-like inflammatory disease with elevated IFN-γ levels (18), and IL-4 can even instruct protective TH1 immunity in mice with Leishmania major infection (19, 20). Thus, although IL-4 might be an important natural inhibitor of many DTHRs, the mode of action by which IL-4 suppresses inflammatory autoimmune disease and DTHRs remains enigmatic. Functional and genetic data now revealed that a significant number of DTHRs that have long been associated with IFN-γ–producing TH1 cells and IL-12p70–producing antigen-presenting cells (APCs), are mediated by IL-17/IL-22–producing TH17 cells and IL-23–producing APCs, rather than by TH1/IL-12 responses (21–23). Consistently, recent reports have correlated the level of disease activity and the absence of IL-4 with the presence of IL-23–producing APCs and IL-23–dependent TH17 cells (24, 25).
We analyzed the impact of IL-4 on the regulation of IL-23 and TH17 in DTHRs in mice and in human psoriasis. Unexpectedly, IL-4 abolished the capacity of APCs to produce IL-23, while promoting IL-12p70. This selective inhibition impaired the induction and maintenance of pathogenic TH17 cells. Bone marrow chimeras with either signal transducer and activator of transcription 6 (STAT6)-deficient APCs or STAT6-deficient T cells proved that IL-4 suppressed TH17 cells by abrogating IL-23 production in APC. IL-4 therapy of psoriasis in humans also dose-dependently suppressed IL-23 production by APCs and TH17 cells, while preserving IL-12 and TH1 immunity. This may open an entirely new approach for a targeted abrogation of harmful IL-23/TH17 immune reactions without affecting potentially protective IL-12/TH1 immunity against intracellular parasites (19) and perhaps cancer (26).
Results
Strictly Opposing Effects of IL-4 on Either IL-12 or IL-23 Secretion by Dendritic Cells.
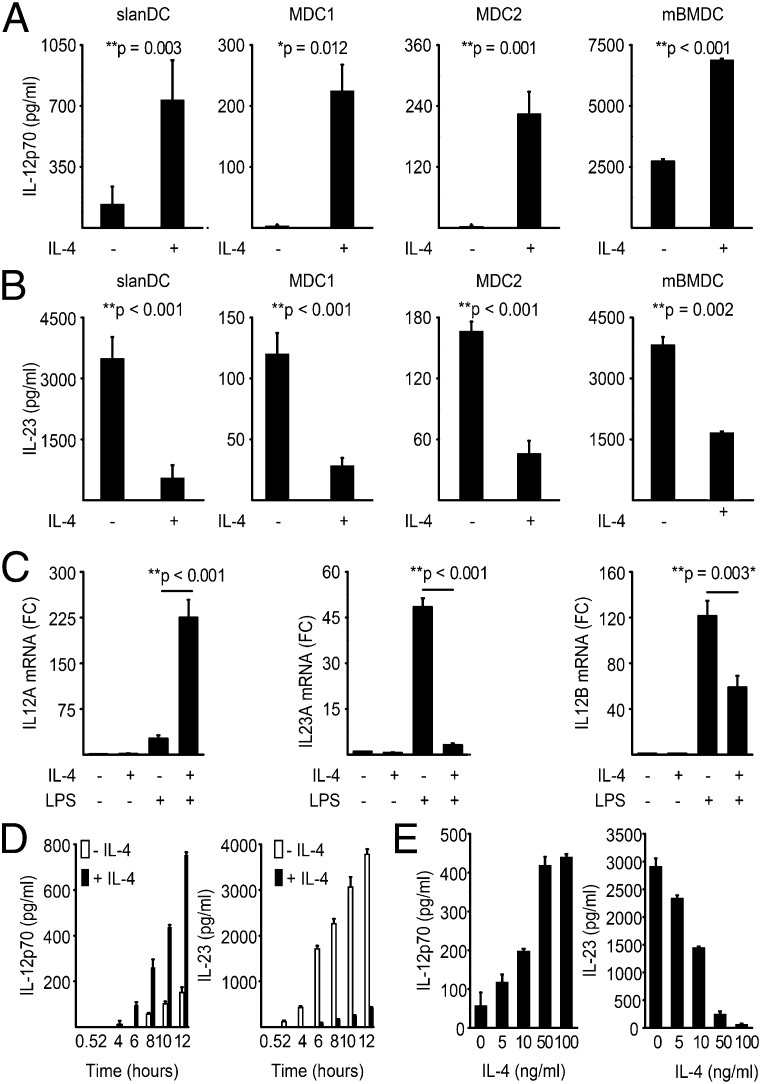
To dissect the pro- and antiinflammatory effects of IL-4 on dendritic cells (DCs), we stimulated, with toll-like receptor (TLR) ligands in the presence or absence of IL-4, four distinct DC populations: BDCA-1–expressing DCs (MDC1), BDCA-3–expressing DCs (MDC2), 6-sulfo-LacNAc–expressing DCs (slanDC), and murine bone-marrow derived DCs (mBMDC). IL-4 strongly and significantly induced IL-12p70 production in all four DC subsets, in human DCs 10- to 100-fold and in murine BMDCs about 3-fold (Fig. 1A). Surprisingly, IL-4 simultaneously and almost completely abrogated TLR-triggered IL-23 production in all human and mouse DC populations (Fig. 1B). High concentrations of IL-13, a cytokine that shares a common signaling pathway with IL-4, also suppressed IL-23 secretion in DCs (SI Appendix, Fig. S1), which was not the case for IL-5 (SI Appendix, Fig. S2A). The opposing effects of IL-4 on the production of either IL-12 or IL-23 were transcriptionally regulated. IL-4 significantly suppressed the TLR-driven induction of il23a mRNA (P = 0.001), while strongly inducing il12a mRNA expression (P < 0.001; Fig. 1C). IL-4 also suppressed TLR-induced expression of the common IL-12/23p40 (il12b) subunit in most APCs (P < 0.003; Fig. 1C). To determine whether IL-4 affects the dynamics of IL-12 or IL-23 induction rather than the total production, we performed time-course studies over 12 h in slanDCs. We observed that, following LPS stimulation, slanDCs started to produce IL-12 and IL-23 after 2–4 h, and IL-23 levels increased more than 100-fold during the first 12 h (Fig. 1D). IL-4 simultaneously suppressed IL-23 but enhanced IL-12p70 production over the entire study period, showing that IL-4 did not alter the dynamics of either IL-12 or IL-23 production. Moreover, the opposing effects of IL-4 were dose dependent and reached saturation at 100 ng IL-4/mL (Fig. 1E). IL-4 also reduced the secretion of other innate cytokines, such as IL-1β and IL-6 (SI Appendix, Fig. S2B).
Fig. 1.
Strictly opposing effects of IL-4 on either IL-12 or IL-23 secretion by DCs. (A and B) Different subsets of human myeloid DCs and mouse BMDCs were preincubated for 24 h with 100 ng/mL IL-4 and then stimulated with LPS. The IL-12 (A) and IL-23 (B) levels in the culture supernatants were determined by an ELISA. The data shown are from at least three independent experiments, and the results are expressed as the means ± SD. (C) The expression levels of transcripts encoding IL23A, IL12A, and IL12B were determined by quantitative real-time PCR in slanDCs treated as described in A. The values from three independent experiments were calculated relative to the expression levels of the housekeeping gene G6PD and were normalized to the unstimulated control. FC, fold change. (D and E) SlanDCs were treated as described in A, and IL-12p70 and IL-23 secretion was analyzed at the indicated time points (D) or as a function of IL-4 (E). The data are expressed as means ± SD of triplicates and are representative of five independent experiments.
IL-4 Selectively Abrogates the TH17 Cell-Inducing Capacity of DCs.
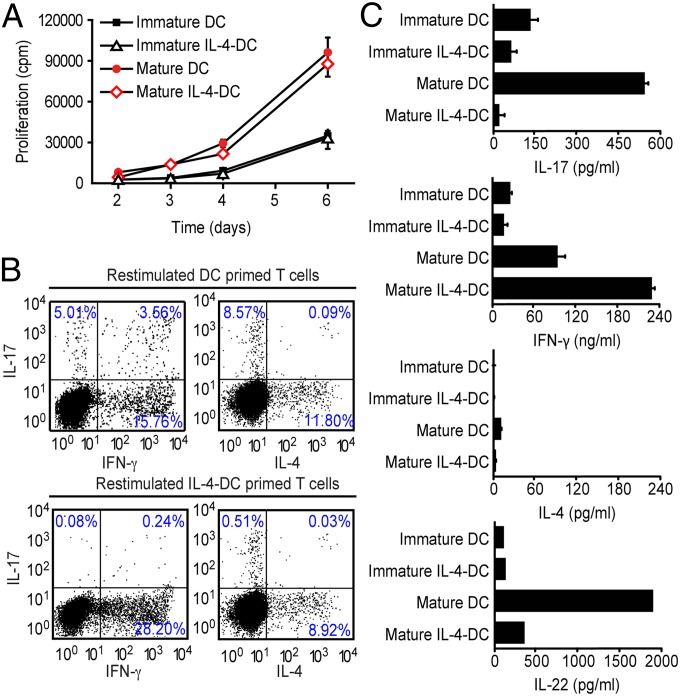
The hallmark of DC function is the ability to prime naïve T cells and steer TH cell differentiation into either TH1, TH17, or TH2 cells. To address whether IL-4 affects the capacity of DCs to drive proliferation of naïve T cells, we first used either immature or in vitro matured DCs to stimulate naïve autologous CD4+CD45RA+ T cells. As expected, immature DCs were less efficient than mature DCs in inducing the proliferation of naïve CD4+ T cells (Fig. 2A). IL-4 did not affect T-cell proliferation induced by either DC population (Fig. 2A). Based on our observation that IL-4 affected the cytokine pattern secreted by DCs, we tested whether IL-4 also affected their capacity to prime naïve T cells for either TH1, TH17, or TH2 differentiation. For this test, we matured DCs in the presence or absence of IL-4, used them to prime naïve CD4+ T cells, expanded the cells, and restimulated such primed CD4+ T cells for cytokine production. Maturation of DCs in the absence of exogenous IL-4 resulted in a DC phenotype that induced both TH1 and TH17 cells, which produced large amounts of either IL-17 and IL-22 or IFN-γ (Fig. 2B), as previously reported (27, 28). Maturation of DCs in the presence of IL-4 (IL-4–DC) resulted in a DC phenotype that failed to induce TH17 cells (≤0.5%) (Fig. 2B); instead the percentage of IFN-γ–producing TH1 cells increased (Fig. 2C). Moreover, IL-17+ cells within fully differentiated IL-17 secreting cells (SI Appendix, Fig. S3) and total CD4+ T cells (SI Appendix, Fig. S4) were significantly reduced upon coculture with IL-4–DC. This reduction could be restored by exogenous IL-23 but not by IL-1β and/or IL-6 (SI Appendix, Fig. S4).
Fig. 2.
IL-4 selectively abrogates the TH17 cell-inducing capacity of DCs. (A) DCs stimulated with LPS in the presence or absence of 100 ng/mL IL-4 or control DCs were cocultured with autologous naïve T cells in the presence of staphylococcal enterotoxin B (SEB), and proliferation was determined by 3H-thymidine uptake. (B) DCs stimulated with LPS in the presence or absence of 100 ng/mL IL-4 and control DCs were cocultured with autologous naïve T cells over 12 d. Cytokine production by CD4+ T cells was determined by flow cytometry following restimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. (C) T cells cocultured with DCs stimulated as indicated in B were reactivated on day 12 with anti-CD3/CD28 for an additional 48 h, and cytokines were analyzed by ELISA. The results are expressed as means ± SD, and the data shown represent independent experiments from three different donors.
IL-4–Induced Immune Suppression Strictly Depends on Direct Suppression of IL-23.
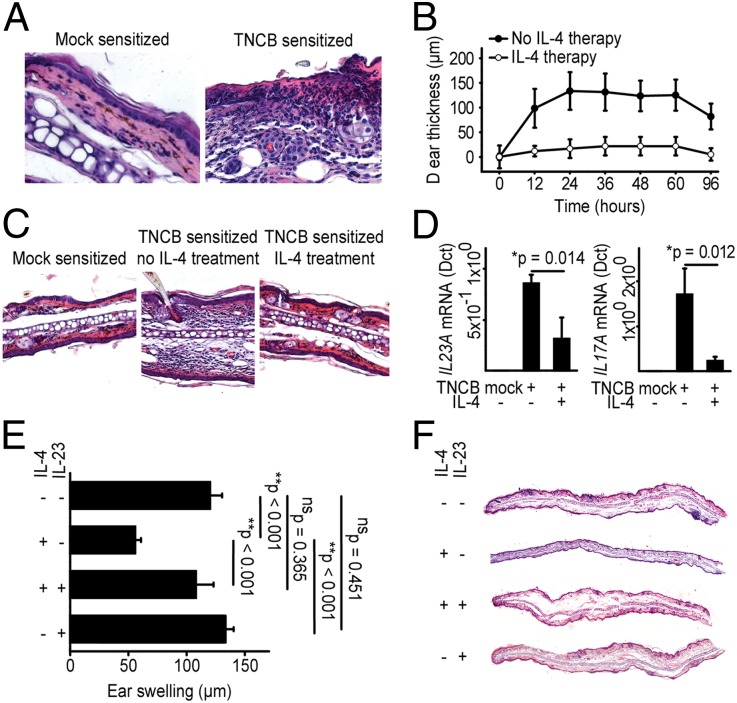
To analyze the biological relevance of this IL-4–mediated suppression of IL-23 and its effect on the subsequent maintenance of TH17 cells in vivo, we first studied IL-4–induced immune suppression in 2,4,6-trinitrochlorobenzene (TNCB)-induced DTHRs in C57BL/6 mice. Challenging sensitized mice with TNCB resulted in pronounced ear swelling and skin inflammation characterized by epidermal hyperplasia, subcorneal neutrophilic infiltrates, and angiogenesis (Fig. 3A). Systemic administration of IL-4 during the challenge significantly reduced the ear swelling in TNCB-sensitized mice (Fig. 3 B and C), abrogated the infiltration of polymorphonuclear (PMN) cells, and normalized skin morphology (Fig. 3C). The TNCB challenge caused a strong induction of il23a and il17a mRNA in ear tissues of mice challenged with TNCB (Fig. 3D), and IL-4 treatment during the TNCB challenge suppressed il23a and il17a mRNA (Fig. 3D). Upon TNCB challenge, IL-4 further induced il12a mRNA, whereas ifnγ mRNA was not significantly affected (SI Appendix, Fig. S5). To directly test whether IL-4–mediated suppression of IL-23 also suppressed inflammation, we treated sensitized mice with recombinant mouse (rm) IL-4 during the TNCB challenge. Subsequently, we aimed in half of the IL-4–treated mice to prevent suppression of inflammation via systemic administration of rmIL-23, rmIL-6, or PBS. Neither PBS nor rmIL-6 restored the rmIL-4–mediated suppression of IL-23 and DTHRs to almost background levels (SI Appendix, Fig. S6). In contrast, rmIL-23 fully rescued the cutaneous DTHRs, as determined by the ear swelling responses (Fig. 3 E and F).
Fig. 3.
IL-4–induced immune suppression strictly depends on direct suppression of IL-23. (A) Representative H&E stains (40×) from skin inflammation after challenge with TNCB in TNCB-sensitized C57BL/6 mice (B and C). Time course (B) of the ear swelling and representative H&E stains (20×) (C) after TNCB challenge in sensitized C57BL/6 mice, treated intraperitoneally with either PBS or IL-4 during challenge. The results in B are means ± SD. (D) Expression of transcripts encoding IL-23A and IL-17A in DTHR ear samples in mice treated with IL-4 as described in B. Quantitative real-time PCR was performed, and the data are expressed in relative units (Δct) compared with the housekeeping gene. The results are the means ± SD. (E and F) Ear swelling (E) and representative H&E stains (F) 24 h after challenge with TNCB in sensitized C57BL/6 mice. Mice were treated intraperitoneally with either PBS or IL-4 as in B. Additionally, IL-23 was applied to the groups where indicated. The data are expressed as means ± SD.
IL-4–Responsive APCs Orchestrate IL-4–Induced Suppression of TH17 Responses.
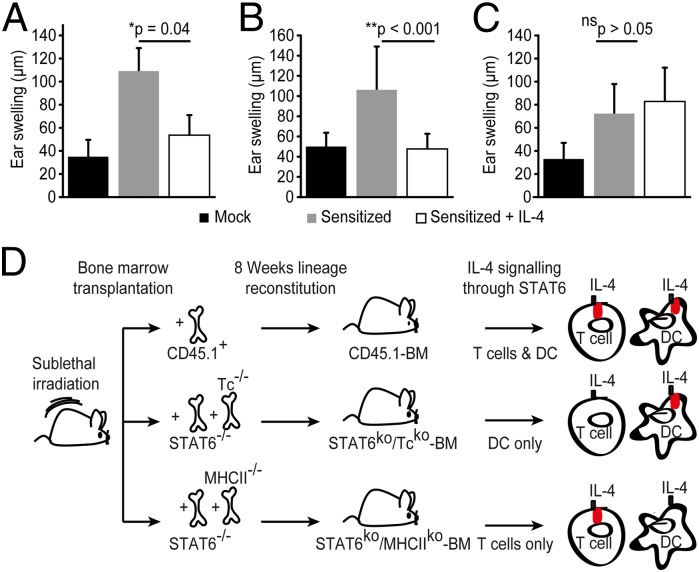
Administration of IL-4 induces IL-4–producing TH2 cells when acting directly on T cells (1) and restimulation of these Th2 cells may further provide IL-4 necessary to modulate DTHRs during the effector phase, possibly by affecting APCs (11, 15, 16). We next set up experiments to analyze the role of IL-4 during the effector phase of DTHRs and whether IL-4 suppresses T-cell–mediated inflammation not only by its action on CD4+ T cells but also through the suppression of IL-23 production of APCs in vivo. Therefore, we first generated bone marrow chimeric (BMC) mice with CD45.1+ hematopoiesis on a CD45.2+ background (CD45.1+ → CD45.2+ mice). Eight weeks after transplantation, engraftment efficiency and lineage reconstitution were >90%, and residual host CD45.2+ BMC was <3% (SI Appendix, Figs. S7 and S8). The chimeric mice developed a typical DTHR showing that the BMC mice could be sensitized normally; in addition, rmIL-4 suppressed cutaneous DTHRs in such CD45.1+ → CD45.2+ mice as in previous experiments (Fig. 4A). To distinguish the effects of IL-4 on either APCs or T cells, we selectively blocked IL-4 signaling in either T cells or APCs of the BMC mice. This was achieved first by generating BMC mice deficient for STAT6 in the T-cell lineage (STAT6−/−/Tc−/− → WT mice). In detail, by transplanting BM of STAT6−/− mice into lethally irradiated recipient mice, the T-cell repertoire of STAT6−/− mice was established in BMC mice. By cotransplantation of BM devoid of any T cells from Tc−/− mice into these BMC mice, those chimeric mice had normal IL-4 sensitive STAT6-expressing APCs from the Tc−/− donor organism, but only harbored STAT6neg T cells unresponsive to IL-4 therapy from the STAT6−/− mice. A TNCB challenge in sensitized STAT6−/−/Tc−/− → WT chimeric mice resulted in a similar ear swelling response compared with the control chimeric mice (CD45.1+ → WT CD45.2+ mice) (Fig. 4 A and B). Of note, IL-4 significantly reduced ear swelling and abrogated cutaneous inflammation in these sensitized STAT6−/−/Tc−/− → WT chimeric mice (Fig. 4B), highlighting a key role for APCs in mediating the beneficial effect of IL-4–induced immune deviation in the effector phase of DTHRs. Next, to further study the role of APCs in this process, we generated BMC mice with a mixed STAT6−/−/MHCII−/− hematopoiesis on a wild-type background (STAT6−/−/MHC II−/− → WT mice), in which IL-4 signaling was completely abrogated in functional MHCIIpos APCs. TNCB challenge after sensitization of those chimeric mice resulted in increased ear swelling comparable to what we observed in the other two chimeric mouse models (Fig. 4 A–C). However, when we treated the STAT6−/−/MHC II−/− → WT mice with IL-4, cutaneous inflammation failed to improve, and the ear swelling was not reduced but remained comparable to that of STAT6−/−/MHC II−/− → WT mice not treated with IL-4 (Fig. 4C). This demonstrates the indispensable role for APCs in regulating the antiinflammatory therapeutic effect of IL-4 during DTHR elicitation. Fig. 4D presents schematically the experimental approach for the generation of the BMC mice. In vitro coculture assays of DCs and T cells from either WT or STAT6−/− mice, provided additional evidence for the mode of action of IL-4 on DCs. Both WT and STAT6−/− T cells secreted less IL-17 upon coculture with IL-4–exposed WT DCs, but not when cultured with STAT6−/− DCs (SI Appendix, Fig. S9).
Fig. 4.
IL-4 responsive APCs orchestrate IL-4–induced suppression of TH17 responses. (A–C) Ear swelling after TNCB challenge in sensitized C57BL/6 mice bone marrow (BM) chimeric mice. IL-4 treatment during challenge was administered in some of the groups as indicated. Data from control CD45.1+ BM chimeric mice on wild-type nonhematopoietic background are presented in A. Data from STAT6−/−/Tc−/− BM chimeric mice are presented in B, and data from STAT6−/−/MHCII−/− BM chimeric mice are presented in C. Data are expressed as mean ± SD and represent two independent experiments. At least six mice per group have been analyzed. (D) Schematic presentation of the experimental approach for the generation of bone marrow chimeric mice.
IL-4–Mediated Suppression of IL-23 Is Partly Mediated Through ATF3.
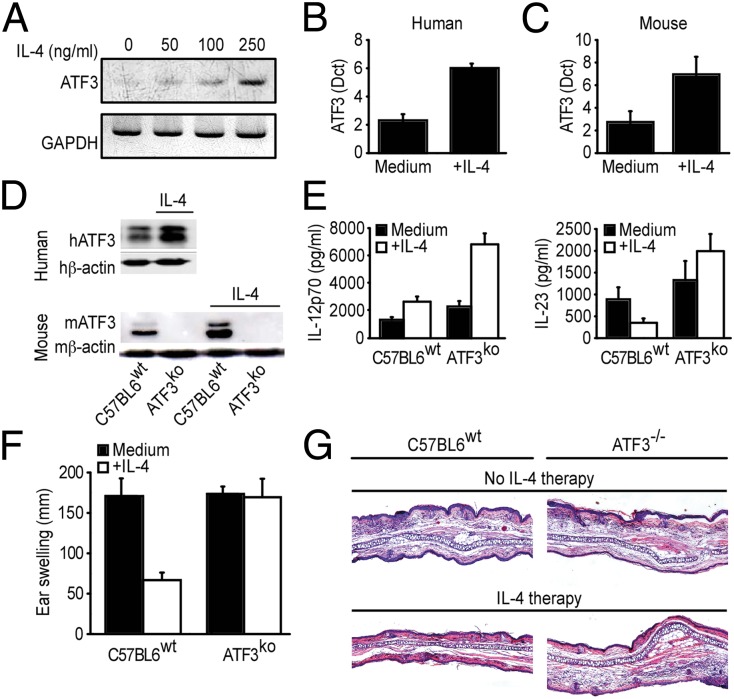
ATF3 is a repressor of il6, but also tnf and il23b transcription in TLR4-stimulated macrophages (29, 30). ATF3 blocks il23b transcription by binding to repressive promotor elements near the genes coding for the il23b subunit in macrophages and possibly other APCs (29, 31). Because IL-4 significantly suppresses il23b transcription (Fig. 1C), and because both IL-23 and ATF3 could be modulated by cyclic adenosine monophosphate (32–34), we assessed whether the effects of IL-4 could be mediated through ATF3. Indeed, IL-4 markedly up-regulated ATF3 mRNA expression and protein production in murine and human DCs, and in murine RAW264.7 cells (Fig. 5 A–D). To determine the functional relevance of ATF3 on IL-23 production, we stimulated DCs from either WT or ATF3−/− mice with LPS and assessed mRNA expression and production of IL-23. Even in the absence of IL-4, ATF3-deficient DCs produced higher amounts of IL-23 than WT DCs, and IL-4 suppressed transcription and production of IL-23 only in the ATF3-competent, but not in the ATF3-deficient DCs (Fig. 5E). Accordingly, IL-4 reduced the TNCB-mediated DTHR (Fig. 5 F and G) and il17 mRNA (SI Appendix, Fig. S10) in WT mice but not in ATF3−/− mice.
Fig. 5.
IL-4–mediated suppression of IL-23 is partly mediated through activating transcription factor 3 (ATF3). (A) Representative data from a semiquantitative PCR for ATF3 mRNA expression in murine RAW264.7 cells, analyzed as a function of IL-4. (B and C) Quantification of ATF3 mRNA expression in LPS-stimulated human (B) and murine (C) DCs. Data are expressed in relative units (Δct) compared with the housekeeping gene. The results are the means ± SD. (D) Human DCs and BMDCs from either C57BL6 WT mice or ATF3−/− mice were preincubated with IL-4 and then stimulated with LPS. ATF3 protein was analyzed semiquantitatively by Western blotting. Representative data from three independent experiments are shown. (E) BMDCs from either C57BL6 WT mice or ATF3−/− mice were treated as in D. IL-12 and IL-23 levels in the culture supernatants were determined by ELISA. The data shown are from three independent experiments, and the results are expressed as the means ± SD. (F and G) Ear swelling (F) and representative H&E stains (G) 48 h after challenge with TNCB in sensitized C57BL/6 and ATF3−/− mice. Mice were treated intraperitoneally with either PBS or IL-4 as in Fig. 3B. The data are expressed as means ± SD.
IL-4 Therapy of Psoriasis Abrogates Intralesional IL-23 and IL-17 in Human Skin.
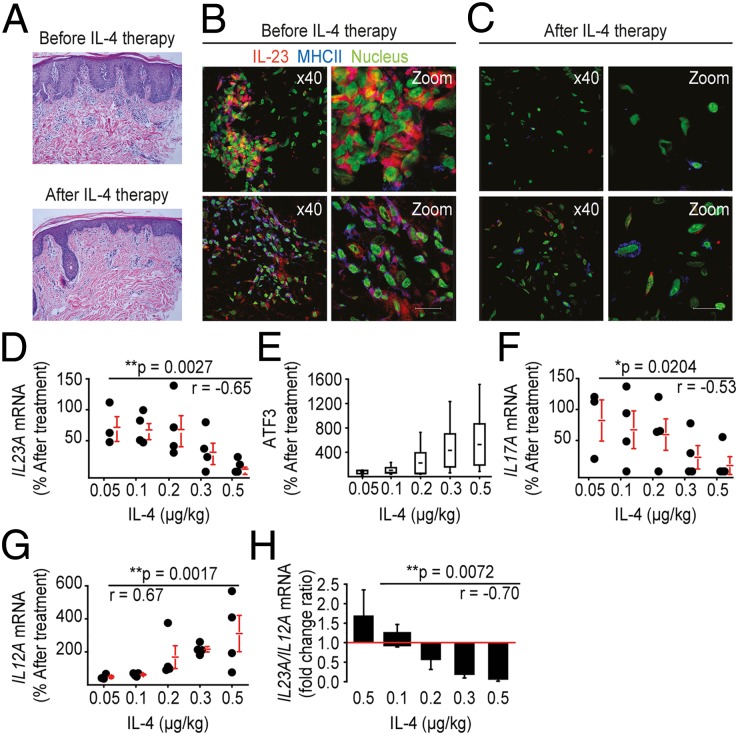
IL-4 suppresses IL-23 production in mouse and human DCs and abrogates their capacity to induce/maintain TH17 responses. Moreover, rmIL-4 suppresses DTHRs by suppressing IL-23 and downstream IL-17 during contact hypersensitivity in mice. We therefore asked whether this mode of immune suppression also translates to human autoimmune diseases, namely psoriasis, which is a disease strongly improved by IL-4 therapy or the mAb-mediated blockade of either IL-17 or IL-23 (35). To this end, we studied a unique population of patients with psoriasis who had successfully been treated with increasing doses of systemically administered IL-4. Consistent with recent data (36), il23a and il17a mRNA were both increased in psoriasis skin lesions (SI Appendix, Fig. S11A). Confocal laser scanning microscopy colocalized the abundant IL-23 protein with HLA-DR–expressing APCs, and the IL-17 protein with CD3+ T cells (SI Appendix, Figs. S11B and S12) in psoriasis plaques, but not in healthy skin (SI Appendix, Figs. S13 and S14). In addition to TH1 and TH17 cells, the psoriasis plaques contained numerous polymorphonuclear cells and shared many similarities with the TNCB-induced DTHR. Because IL-4 therapy improves psoriasis without suppressing IFN-γ–expressing T cells in the peripheral blood (16), we asked whether effective IL-4 therapy improved psoriasis by suppressing IL-23 and IL-17 in lesional skin. We examined the effect of IL-4 therapy on the expression and production of IL-17/IL-23 in a cohort of 22 patients with psoriasis (i.e., 19 patients, 3 drop-outs). The study was designed as a dose-escalation study, where patients were treated for psoriasis systemically with increasing doses of IL-4 over 6 wk. The therapy was initiated with either 0.05, 0.1, 0.2, 0.3, or 0.5 μg/kg of IL-4 and increased to the next level after 3 wk, except in the last group (16). Systemic IL-4 therapy significantly improved psoriasis in a dose-dependent manner and normalized the skin morphology (16) (see also Fig. 6A). Cryopreserved tissue sections of these study patients revealed that untreated psoriasis plaques contained abundant IL-23 that colocalized with HLA-DR–expressing APCs and abundant IL-17 protein that colocalized with CD3+ T cells (Fig. 6B and SI Appendix, Fig. S15A). After 6 wk of IL-4 therapy, both IL-23 and IL-17 protein were almost undetectable (Fig. 6C and SI Appendix, Fig. S15B), suggesting that IL-4 therapy suppressed IL-23 and IL-17 production also in human skin. We further analyzed the tissue samples from the study patients for the expression of il17a, atf3, il23a, or il12a mRNA. The dose-escalation design of the study allowed us to correlate local mRNA changes for each of the three cytokines (i.e., IL17a, IL23a, and IL12a) and of the transcription factor atf3 with the IL-4 treatment dose. IL-4 therapy suppressed il23a mRNA expression in a dose-dependent manner, with 20% suppression at 0.05 μg/kg IL-4 and almost 90% suppression at 0.5 μg/kg of IL-4 (Fig. 6D). Similarly, we detected a dose-dependent up-regulation of atf3 expression in the analyzed tissue (Fig. 6E). Consistent with the il23a mRNA suppression, IL-4 therapy dose-dependently suppressed il17a mRNA expression (Fig. 6F). As predicted by the in vitro and animal data shown above, IL-4 therapy increased il12a mRNA expression in human skin during the 6 wk of IL-4 therapy (Fig. 6G). Finally, at low concentrations, IL-4 induced an IL23A/IL12A ratio of >1 (1.7 at 0.05 µg/kg IL-4), but at high IL-4 concentrations, IL-4 therapy induced a very low IL23A/IL12A ratio (0.05 at 0.5 µg/kg IL-4), a finding that could be important for the design of future IL-4 treatment regimens in humans (Fig. 6H).
Fig. 6.
IL-4 therapy of psoriasis abrogates intralesional IL-23 and IL-17 in human skin. (A) Representative H&E stains from colocalized biopsies of psoriatic skin before (A) and after systemic IL-4 treatment. (B and C) Visualization of colocalized IL-23 (red) and MHC II (blue) and IL-17 (red) and CD3 (blue) in human psoriatic skin lesions before (B) and after (C) IL-4 therapy. The nuclei are stained with green-fluorescent YO-PRO-1 stain. For colorblind-accessible images, please refer to SI Appendix, Fig. S9. (D–G) RT-PCR expression of transcripts encoding IL-23A, ATF3, IL-17A, and IL-12A in psoriatic skin samples before and after different doses of IL-4 therapy. The expression of the target gene within psoriasis tissue before treatment (relative to the housekeeping gene G6PD) was set to 100%, and the expression after treatment is presented as a percentage of this value. Each dot represents one pair of specimens from a single study patient; the horizontal bars indicate the means ± SEM *P < 0.05, **P < 0.01. (H) Ratio of IL23A (percent after treatment) to IL12A (percent after treatment) as detected by quantitative real-time PCR in the skin samples from the study patients treated with different doses of IL-4. The data are expressed as the means ± SEM *P < 0.05, **P < 0.01. r = Pearson correlation coefficient.
Discussion
IL-4 reverts both TH1 and TH17 cell-mediated pathology and this effect is associated with the induction of IL-4–producing TH2 cells in mice and humans (13–16, 37, 38). The underlying mechanism was attributed to inhibition and replacement of pathologic TH1 and TH17 cells and their respective cytokines by TH2 cells and IL-4. However, this concept fails to completely explain the therapeutic effects observed, because IL-4 exerts opposing regulatory effects on T cells and DCs; IL-4 abrogates IFN-γ induction upon direct interaction with T cells (1). In contrast, IL-4 promotes IL-12 production by DCs, thus indirectly promoting IFN-γ in mice and humans (11, 19, 39–41). These effects are not exclusive; IL-4– and IL-4–producing TH2 cells efficiently improve established TH1/TH17 mediated inflammation in mice and humans, while enhancing both IL-12 and IFN-γ (15, 16). These phenomena are highly suggestive of a regulatory mechanism whereby IL-4 selectively prevents TH17 immunity, while sparing IL-12/TH1 immunity.
We addressed this question by analyzing in detail the effect of IL-4 on the regulation of IL-23 and TH17 cells. Starting with human DC subsets, we found that IL-4 had exactly opposing effects on IL-12 and IL-23. Whereas IL-4 induced IL-12, it abolished the induction of IL-23 and abrogated the capacity of DCs to maintain TH17 but not TH1 cells. Our observations are in agreement with reports suggesting different roles of IL-4 on DC-derived IL-12 and IL-23 (42–45), but go far beyond the former studies. We confirmed the biological relevance of this regulation in an in vivo experimental setting and demonstrated that IL-4 therapy could abrogate cutaneous inflammation in the elicitation phase of DTHRs. In extensive bone marrow reconstitution experiments, we elucidated the effects of IL-4 on the different immune cells and could demonstrate a previously unidentified in vivo mode of action of IL-4 on APCs. This is important because our data show for the first time to our knowledge that antigen-presenting innate immune cells are indispensable for the immunosuppressive effect of IL-4 therapy. Activation of APCs in an IL-4–deprived or IL-4–dominated inflammatory milieu dictated their capacity to orchestrate TH17 induction, which supports previous data suggesting that the amount of IL-4 ultimately determines whether immune responses promote or attenuate inflammatory autoimmune diseases (19, 39, 46, 47).
Psoriasis is characterized by the absence of IL-4, and both TH1 and TH17 cells prevail in the skin (6). However, the exact roles of either TH1 or TH17 cells remain to be defined. Our data have identified a sequence of immunological events, triggered by IL-4 that selectively impaired the IL-23/IL-17 axis and relieved TH17-mediated pathology, while promoting IL-12 and TH1 cytokines. This is relevant because IL-23, but not IL-12, also mediates inflammation in the absence of T cells (10), at least under experimental conditions, and via intracutaneous injection induces a psoriasis-like skin disease in mice (48). It is well accepted that IL-4 can suppress TH1 and TH17 and promote TH2 when directly acting on T cells (1, 49). Our data further showed that IL-4 also indirectly prevented the maintenance of IL-17–producing TH17 cells by abrogating the expression and production of the TH17 cell-associated cytokine IL-23 in APCs. Together with our data, the high degree of efficacy of the anti–IL-12/IL-23p40 monoclonal antibody in the treatment of psoriasis further emphasizes the crucial role for IL-23 in disease progression (50). These findings do not exclude a role for IL-12, TH1 cells, or TH1 cytokines in psoriasis but rather confirm that the therapeutic silencing of IL-23 (for example by IL-4 or newly engineered IL-4 superkines, currently under investigation) (51) is promising for psoriasis and other TH17/IL-23–associated autoimmune diseases.
Materials and Methods
Institutional Review Board Approval.
All human studies were approved by the ethics committees of the Medical School of Ludwig Maximilian University of Munich and of the Eberhard Karls University of Tübingen (110/2009B02; 116/2005; 383/2007B02; 418/2009B02), and written informed consent was obtained. The animal experiments were in compliance with both European Union and German law and were approved by local authorities (Regierungspräsidium Tübingen, HT5/10; HT1/13).
Reconstitution Experiments.
Tcrb−/−Tcrd−/− (Tc−/−) mice, STAT6−/− mice, and CD45.2+C57BL/6 mice were purchased from The Jackson Laboratory. MHCII−/−mice were a gift from Ludger Klein (Institute of Immunology, Ludwig Maximillian University, Munich). Recipient mice were lethally irradiated at 7.0 Gy and bone marrow cells (106 cells per recipient) of donor mice were i.v. injected into recipient mice. Donor hematopoietic cells were either bone marrow cells from CD45.1+ mice, a 1:1 mixture of bone marrow cells from STAT6−/− and Tc−/− mice, or a 1:1 mixture of STAT6−/− and MHCII−/− mice. To confirm the chimerism of mice, flow cytometry was made for analysis of CD45.2+ (recipient mice) and CD45.1+ (donor mice). TNCB sensitization experiments were performed 8 wk after irradiation.
A detailed description of all other experimental procedures and the statistical analysis is given in SI Appendix.
Supplementary Material
Acknowledgments
We thank B. Fehrenbacher, R. Nordin, H. Bischof, H. Möller, M. Haberbosch, N. Hamdi, and C. Reitmeier for their excellent technical support. This work was supported by the Deutsche Forschungsgemeinschaft (GU1271/2-1, Bi 696/3-3; Bi 696/5-2; Bi 696/10-1; Ro 764/14-1; SFB 685 A6 and C1; SFB 773 Z2), Baden Württemberg Stiftung (P-LS-AL/17, P-LS-AL/15), Wilhelm Sander Stiftung (2012.056.1 and 2), Deutsche Krebshilfe (107114), the Bundesministerium für Bildung und Forschung (FKZ 0315079), the Schering-Plough Research Institute, the Fortüne Program of the University of Tuebingen (F1261193, F126810, and F1261150), as well as by the Swiss National Science Foundation (PMPDP3_151326), the Forschungskredit of the University of Zurich (FK-14-032), the Louis Widmer Foundation, Sciex-NMSch 14.111, and the Helmut Horten Foundation. Additionally, this work has been partially supported by the National Research Foundation of Korea, the Ministry of Education, Science and Technology through the Creative Research Initiative Program (R16-2004-001-01001-0), the Global Research Laboratory Program (2011-0021874), and the Global Core Research Center Program (2012-0001187). The human studies were partially supported by the Schering-Plough Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
3Deceased November 8, 2010.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416922112/-/DCSupplemental.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168(3):853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200(4):507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang HE, et al. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13(1):58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guenova E, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19(14):3755–3763. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199(1):125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 8.Nair RP, et al. Collaborative Association Study of Psoriasis Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonel G, et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J Immunol. 2010;185(10):5688–5691. doi: 10.4049/jimmunol.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlig HH, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Racke MK, et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180(5):1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh SZ, et al. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186(9):5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184(3):1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarner IH, et al. Retroviral gene therapy of collagen-induced arthritis by local delivery of IL-4. Clin Immunol. 2002;105(3):304–314. doi: 10.1006/clim.2002.5299. [DOI] [PubMed] [Google Scholar]
- 15.Biedermann T, et al. Reversal of established delayed type hypersensitivity reactions following therapy with IL-4 or antigen-specific Th2 cells. Eur J Immunol. 2001;31(5):1582–1591. doi: 10.1002/1521-4141(200105)31:5<1582::AID-IMMU1582>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Ghoreschi K, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9(1):40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 17.Milner JD, et al. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116(14):2476–2483. doi: 10.1182/blood-2009-11-255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tepper RI, et al. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62(3):457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 19.Biedermann T, et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2(11):1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 20.Hurdayal R, Brombacher F. The role of IL-4 and IL-13 in cutaneous Leishmaniasis. Immunol Lett. 2014;161(2):179–183. doi: 10.1016/j.imlet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 22.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang GX, et al. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170(4):2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elder JT. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. 2009;10(3):201–209. doi: 10.1038/gene.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braumüller H, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494(7437):361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 27.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 28.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilchrist M, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441(7090):173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 30.Hoetzenecker W, et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat Med. 2012;18(1):128–134. doi: 10.1038/nm.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitmore MM, et al. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007;179(6):3622–3630. doi: 10.4049/jimmunol.179.6.3622. [DOI] [PubMed] [Google Scholar]
- 32.Hertz AL, et al. Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte-derived macrophages. Proc Natl Acad Sci USA. 2009;106(51):21978–21983. doi: 10.1073/pnas.0911684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira CJ, et al. Deconstructing tick saliva: Non-protein molecules with potent immunomodulatory properties. J Biol Chem. 2011;286(13):10960–10969. doi: 10.1074/jbc.M110.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Cao Y, Steiner DF. Regulation of proglucagon transcription by activated transcription factor (ATF) 3 and a novel isoform, ATF3b, through the cAMP-response element/ATF site of the proglucagon gene promoter. J Biol Chem. 2003;278(35):32899–32904. doi: 10.1074/jbc.M305456200. [DOI] [PubMed] [Google Scholar]
- 35.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 36.Lowes MA, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 37.Gomez MR, et al. Basophils control T-cell responses and limit disease activity in experimental murine colitis. Mucosal Immunol. 2014;7(1):188–199. doi: 10.1038/mi.2013.38. [DOI] [PubMed] [Google Scholar]
- 38.Coffman RL. The origin of Th2 responses. Science. 2010;328(5982):1116–1117. doi: 10.1126/science.1192009. [DOI] [PubMed] [Google Scholar]
- 39.Guenova E, et al. IL-4-mediated fine tuning of IL-12p70 production by human DC. Eur J Immunol. 2008;38(11):3138–3149. doi: 10.1002/eji.200838463. [DOI] [PubMed] [Google Scholar]
- 40.Hochrein H, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192(6):823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201(12):1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morita Y, Gupta R, Seidl KM, McDonagh KT, Fox DA. Cytokine production by dendritic cells genetically engineered to express IL-4: Induction of Th2 responses and differential regulation of IL-12 and IL-23 synthesis. J Gene Med. 2005;7(7):869–877. doi: 10.1002/jgm.730. [DOI] [PubMed] [Google Scholar]
- 43.Uemura Y, et al. Cytokine-dependent modification of IL-12p70 and IL-23 balance in dendritic cells by ligand activation of Valpha24 invariant NKT cells. J Immunol. 2009;183(1):201–208. doi: 10.4049/jimmunol.0900873. [DOI] [PubMed] [Google Scholar]
- 44.Bullens DM, Kasran A, Thielemans K, Bakkus M, Ceuppens JL. CD40L-induced IL-12 production is further enhanced by the Th2 cytokines IL-4 and IL-13. Scand J Immunol. 2001;53(5):455–463. doi: 10.1046/j.1365-3083.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar S, Tesmer LA, Hindnavis V, Endres JL, Fox DA. Interleukin-17 as a molecular target in immune-mediated arthritis: Immunoregulatory properties of genetically modified murine dendritic cells that secrete interleukin-4. Arthritis Rheum. 2007;56(1):89–100. doi: 10.1002/art.22311. [DOI] [PubMed] [Google Scholar]
- 46.Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol. 2011;187(9):4440–4450. doi: 10.4049/jimmunol.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghoreschi K, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208(11):2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fallon PG, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17(1):7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 50.Krueger GG, et al. CNTO 1275 Psoriasis Study Group A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 51.Junttila IS, et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat Chem Biol. 2012;8(12):990–998. doi: 10.1038/nchembio.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.