Significance
Almost 20% of women and 40% of men in the United States abuse alcohol or have experienced alcohol dependence in their lifetime. Though accidents, traffic fatalities, and violent crimes account for the majority of alcohol-involved mortalities, excessive, chronic drinking also causes often irreversible heart and liver damage. We identify regulator of G protein signaling 6 (RGS6) as a novel drug target with substantive potential clinical utility in the treatment of alcoholism and amelioration of the resultant hepatic and cardiac toxicity. Mice lacking RGS6 exhibit a reduction in voluntary alcohol consumption, conditioned reward and withdrawal. In addition, RGS6−/− mice are largely protected from alcohol-induced cardiomyopathy, hepatic steatosis, gastrointestinal barrier dysfunction, and endotoxemia. Thus, targeting RGS6 could reduce alcohol cravings while simultaneously protecting the heart and liver from damage.
Keywords: RGS6, GPCRs, alcoholism, dopamine transporter, RGS proteins
Abstract
Alcohol is the most commonly abused drug worldwide, and chronic alcohol consumption is a major etiological factor in the development of multiple pathological sequelae, including alcoholic cardiomyopathy and hepatic cirrhosis. Here, we identify regulator of G protein signaling 6 (RGS6) as a critical regulator of both alcohol-seeking behaviors and the associated cardiac and hepatic morbidities through two mechanistically divergent signaling actions. RGS6−/− mice consume less alcohol when given free access and are less susceptible to alcohol-induced reward and withdrawal. Antagonism of GABAB receptors or dopamine D2 receptors partially reversed the reduction in alcohol consumption in RGS6−/− animals. Strikingly, dopamine transporter inhibition completely restored alcohol seeking in mice lacking RGS6. RGS6 deficiency was associated with alterations in the expression of genes controlling dopamine (DA) homeostasis and a reduction in DA levels in the striatum. Taken together, these data implicate RGS6 as an essential regulator of DA bioavailability. RGS6 deficiency also provided dramatic protection against cardiac hypertrophy and fibrosis, hepatic steatosis, and gastrointestinal barrier dysfunction and endotoxemia when mice were forced to consume alcohol. Although RGS proteins canonically function as G-protein regulators, RGS6-dependent, alcohol-mediated toxicity in the heart, liver, and gastrointestinal tract involves the ability of RGS6 to promote reactive oxygen species-dependent apoptosis, an action independent of its G-protein regulatory capacity. We propose that inhibition of RGS6 might represent a viable means to reduce alcohol cravings and withdrawal in human patients, while simultaneously protecting the heart and liver from further damage upon relapse.
Alcoholism imparts a large socioeconomic burden on the healthcare system in the United States, affecting an estimated 12% of the population at any given time, a prevalence greater than that for all other drugs of abuse combined. Despite decades of research, our understanding of the mechanisms underlying the acquisition of alcohol dependence remains limited. As a result, there are few currently approved therapeutics designed to reduce alcohol cravings or withdrawal symptomology, and abstinence remains the only effective way to prevent tissue damage that results from chronic alcohol abuse.
Alcohol dependence is a progressive, neurological disorder characterized by accumulating neuroadaptations resulting from chronic ethanol (EtOH) exposure. Unlike many drugs of abuse, no specific molecular target of EtOH has been identified. Instead, EtOH functions as a CNS depressant through its ability to simultaneously dampen excitatory neurotransmission mediated by NMDA subtype glutamate receptors and enhance inhibitory neurotransmission through ionotropic GABAA receptors (GABAARs) (1). Concomitant alterations in glutamatergic excitatory inputs and GABAergic inhibitory inputs, in addition to possible direct EtOH-mediated neuronal excitation, promotes acute neurotransmitter aberrations and chronic neural adaptations in the mesolimbic neuronal circuit, a major dopaminergic pathway in the brain implicated in drug addiction (2–5). This system includes the dopaminergic neurons of the ventral tegmental area (VTA), GABAergic neurons of the nucleus accumbens (NAc), and their efferent targets in the amygdala, hippocampus, and medial prefrontal cortex.
The neurotransmitters that facilitate neuronal communication in the mesolimbic pathway [e.g., dopamine (DA), GABA, opioids, and serotonin (5-HT)] bind to and activate a variety of G protein-coupled receptors (GPCRs) located on pre- and postsynaptic neurons. Indeed, genetic and epigenetic aberrations in dopamine D2 receptors (D2Rs), GABABRs, μ opioid receptors, and serotonin 1A receptors (5-HT1ARs), have been implicated in alcohol dependence in human alcoholics (6–10). Recently, preclinical evidence has indicated that the rewarding properties of multiple drugs of abuse can be ameliorated by activation of GABABRs in the VTA (7, 11). The GABABR agonist baclofen has been evaluated for clinical utility in alcoholics considered “at high risk” of continued drug abuse, to help with cravings and withdrawal symptoms, and is approved for use in Europe (12–14). The use of baclofen is controversial, however, because of potent sedative and muscle relaxant actions that are additive with alcohol and additional interactions with other drugs of abuse. Drugs targeting the opioid, DA, and 5-HT neurotransmitter systems have also been proposed as novel alcoholism therapies (15–17), but only one drug (naltrexone) is Food and Drug Administration approved. Clearly, novel mechanistic insight into the GPCR-regulated processes underlying alcohol dependence could facilitate the development of novel effective therapeutics.
By facilitating inactivation of heterotrimeric G-proteins, regulators of G protein signaling (RGS) proteins serve as gatekeepers of the cellular responses to extracellular signals acting through GPCRs (18–21). RGS6 belongs to the R7 subfamily, which share a characteristic three-domain structure. The RGS domain confers functional GTPase activating protein activity directed specifically toward Gαi/o (22). The N-terminal disheveled, EGL-10, pleckstrin homology domain is best known for mediating interaction between R7 family RGS proteins and the accessory protein R7 family binding protein (R7BP) required for shuttling of R7 family RGS proteins between the nucleus and plasma membrane (23). The Gγ subunit-like domain facilitates complex formation between R7 family members and the atypical Gβ subunit Gβ5 (24–26), an interaction required for stable expression of both proteins (27). RGS6 regulates Gαi/o-coupled GABABRs, 5-HT1ARs, and μ opioid receptors in the brain (28–30). Although some RGS proteins have been implicated in the pathophysiology of addiction, no studies have investigated the role of RGS proteins in alcohol dependence (31–33).
EtOH consumption is a major etiologic factor in the development of additional pathological sequelae, including nonischemic dilated cardiomyopathy and chronic liver disease that progresses from fatty liver to hepatitis, cirrhosis, and eventual organ failure. It is estimated that 1 in 10 deaths in working-age adults result from excess alcohol consumption. Although a majority of the mortality associated with alcohol drinking can be attributed to accidents and violent crimes, a substantive portion results from the long-term consequences of alcohol exposure, including heart and liver disease. Interestingly, the mechanisms underlying these distinct pathologies both involve the accumulation of reactive oxygen species (ROS) that contribute to cell death, inflammation, fibrotic remodeling, and loss of tissue functional integrity (34, 35). Although the exact pathogenic ROS source remains unclear, scavenging or inhibition of superoxide anion generation from activated NADPH oxidase (Nox) complexes protects against EtOH-induced tissue injury (36, 37). Although originally discovered as a G-protein regulator with the demonstrated capacity to modulate multiple GPCR-dependent physiological processes (28, 29, 38), our recently published studies have shown that RGS6 is also involved in ROS-dependent cardiomyopathy induced by doxorubicin (Dox) (39), and promotes ROS generation and ROS-mediated apoptosis in cancer cells through G protein-independent mechanisms (40). Both Dox- and EtOH-induced cardiomyopathies require Nox-dependent ROS generation (37, 41), leading us to the novel hypothesis that RGS6 also promotes EtOH-induced, ROS-mediated apoptosis, and subsequent pathology.
The dual G protein-dependent and -independent signaling actions of RGS6 afforded us the unique opportunity to interrogate the role of RGS6 in multiple aspects of alcohol pathology. Based on previous work, we hypothesized that RGS6 might promote alcohol-seeking behaviors through its ability to negatively regulate neuronal GPCR signaling, while simultaneously mediating ROS-dependent hepatic and cardiac toxicity. Here, we confirm that mice lacking the RGS6 gene are less susceptible to alcohol dependence and are largely protected from hepatic steatosis and alcoholic cardiomyopathy. This work identifies RGS6 as a novel therapeutic target in the treatment of human alcoholics, with the potential to reduce alcohol cravings and protect tissues from alcohol-induced damage.
Results
RGS6 Loss Ameliorates Alcohol Seeking, Conditioned Reward and Withdrawal in Mice Without Impacting EtOH-Induced Sedation and Ataxia.
To evaluate the impact of RGS6 loss on alcohol consumption, WT and RGS6−/− mice were provided free access to two bottles of drinking water with and without EtOH [8% (vol/vol)] according to the schematic outlined in Fig. S1A. Remarkably, mice lacking RGS6 consume significantly less EtOH (Fig. 1A) and exhibit a reduction in EtOH preference (Fig. 1B). No differences were observed in water consumption or forced EtOH consumption (Fig. S1B). Mice of both genotypes were also subjected to a long-term model of binge drinking. Mice were given a free choice between tap water and an EtOH-containing solution, with the EtOH concentration increased weekly from 3% to 20% (vol/vol) for a total period of 1 mo. As shown in Fig. 1C, RGS6−/− mice were significantly less susceptible to alcohol-seeking behaviors compared with WT mice. We confirmed that these results were not secondary to alterations in taste preference in RGS6−/− mice for either bitter (quinine) or sweet (saccharin) solutions in experiments depicted in Fig. S1 C and D, respectively.
Fig. 1.
RGS6 deficiency reduces alcohol consumption, withdrawal, and reward without impacting sedation and ataxia. WT (n = 12) and RGS6−/− (n = 11) mice were tested in the short-term two-bottle free-choice alcohol consumption paradigm depicted in Fig. S1A. (A) EtOH consumption in free-choice and forced phases of the experiment normalized to body weight (gram of EtOH per kilogram body weight per day) and (B) EtOH preference are shown. (C) EtOH consumed by WT (n = 14) and RGS6−/− mice (n = 14) given free access to increasing concentrations of EtOH containing solutions [3%, 6%, 10%, 20% (vol/vol)] for 1 wk each. (D) Time spent by WT (n = 9) and RGS6−/− (n = 8) mice in the EtOH box during the testing phase of the conditioned place preference paradigm. EtOH withdrawal severity in WT (n = 9) and RGS6−/− mice (n = 9) as depicted by (E) hourly handling-induced convulsions score and (F) area under the curve from E. (G) Duration of the loss of righting reflex (LORR) in EtOH-treated WT (n = 8) and RGS6−/− (n = 9) mice. (H) Net impact of EtOH challenge on the rotarod speed at which WT (n = 8) and RGS6−/− (n = 8) mice fell from the rod. **P < 0.01; ***P < 0.001 vs. WT by Student’s t test (single variable) or ANOVA with the Bonferroni post hoc adjustment (multivariable). Data are presented as mean ± SEM.
Voluntary EtOH consumption and preference are known to correlate well with measures of EtOH reward (42). Consistent with the reduction in alcohol drinking, RGS6−/− mice were less susceptible to EtOH-mediated conditioned reward (Fig. 1D). Furthermore, mice lacking RGS6 experienced less-severe withdrawal symptomology and recovered faster from EtOH withdrawal compared with their WT counterparts (Fig. 1 E and F). In contrast, no differences were observed in EtOH-induced sedation (Fig. 1G), and although RGS6−/− mice exhibit a baseline ataxic phenotype as we previously reported (28), the net effect of EtOH on motor coordination was equivalent (Figs. S2 C–E) or reduced (Fig. 1H and Fig. S2 A and B) in mice lacking RGS6. This is an important advantage of RGS6 inhibition over drugs such as baclofen, which possess potent sedative and ataxic actions that are additive with alcohol.
RGS6-Mediated Regulation of Multiple GPCRs and DA Bioavailability Contributes to Alcohol-Seeking Behaviors in Mice.
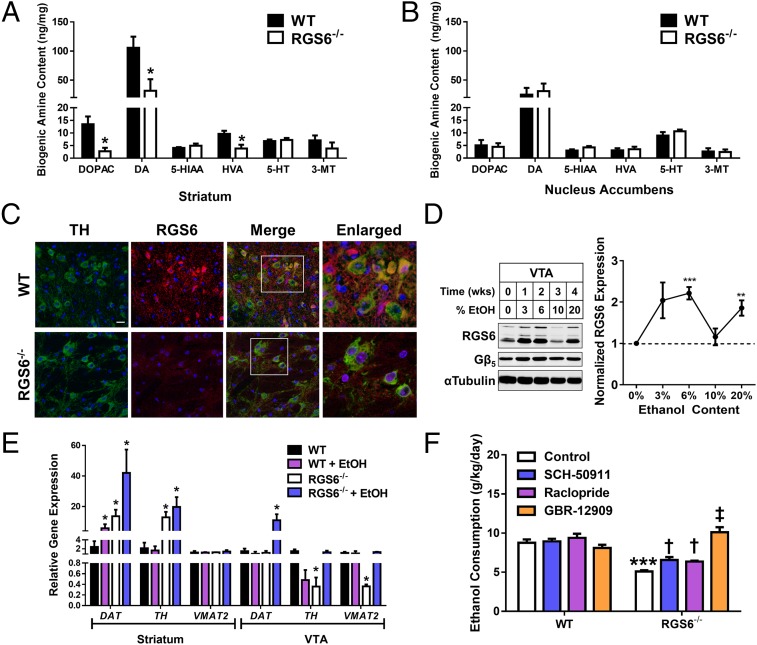
Alcohol’s hedonic value results from hijacking of the endogenous reward system in the brain, beginning with the release of DA from neurons in the VTA (2–5). Under control conditions, a dramatic reduction (∼70%) in DA content and the levels of DA metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid but not 3-methyoxytyramine were observed in the whole striatum of RGS6−/− mice (Fig. 2A). The steady-state content of serotonin and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were similar in WT and RGS6−/− mice (Fig. 2A). No changes in the tissue content of the various biogenic amines was observed in the NAc in the absence of a stimulus (Fig. 2B). Nevertheless, the observed alterations in steady-state DA levels in striatum identify RGS6 as an important regulator of DA homeostasis.
Fig. 2.
RGS6-mediated regulation of multiple GPCRs and DA bioavailability contributes to alcohol-seeking behaviors in mice. The content of the various biogenic amines including DA and serotonin (5-HT) and their metabolites were quantified from whole tissue in (A) the dorsal striatum and (B) NAc. (C) Immunohistochemical staining of RGS6 expression (red) in the VTA of WT and RGS6−/− mice. TH is used as a marker of dopaminergic neurons (green). DAPI (blue) was used to stain the nuclei. (Scale bar, 20 μm; white boxes, regions shown in enlarged images at 40× magnification.) (D) RGS6 and Gβ5 immunoblots in the VTA of WT mice given free access to graduated alcohol containing solutions [0–20% (vol/vol)] for increasing periods of time (Left). Densitometric quantification was performed with protein levels (n = 3) normalized to tubulin loading control and expressed relative to control conditions (Right). (E) DAT, TH, and VMAT2 mRNA expression in the striatum and VTA of WT (n = 3–6) and RGS6−/− mice (n = 3–6) fed on an isocaloric control or EtOH [5% (vol/vol)] containing Lieber deCarli liquid diet for 2 mo. (F) Both WT (n = 8–9) and RGS6−/− (n = 8–9) mice were tested in the short-term alcohol consumption paradigm with the addition of daily injections of SCH-50911 (12.5 mg/kg), raclopride (3 mg/kg), or GBR-12909 (10 mg/kg) to inhibit GABABR, D2R, and DAT, respectively. EtOH consumption in the free-choice phase of the experiment is depicted normalized to body weight (grams of EtOH per kilogram body weight per day). None of the drugs tested impacted water or forced EtOH consumption (data not shown). *P < 0.05; **P < 0.01; ***P < 0.001 vs. WT control and †P < 0.05; ‡P < 0.001 vs. RGS6−/− control by Student’s t test or ANOVA with the Bonferroni post hoc adjustment where appropriate. Data are presented as mean ± SEM.
A population of RGS6 and tyrosine hydroxylase (TH)-positive neurons were identified in the VTA of WT mice with RGS6 expression lost in RGS6−/− mice (Fig. 2C). The VTA, like cerebellum, expresses multiple RGS6 splice forms (28, 43), and although readily detectable in naïve animals, alcohol exposure caused a large increase in RGS6 expression in the VTA (Fig. 2D) but not the cerebellum, cortex, or striatum (Fig. S3). In fact, RGS6 is down-regulated in the cortex and striatum following a month of EtOH treatment (Fig. S3). Gβ5, an RGS6 binding partner required for RGS6 stability, followed an identical trend in VTA, although the fold-induction was lower in magnitude (Fig. 2D). Gβ5 expression was unchanged during prolonged exposure in non-VTA brain regions (Fig. S3). Changes in RGS6 expression appear to require prolonged EtOH exposure because a single dose of EtOH was insufficient to cause alterations in RGS6 expression in any tissue surveyed (Fig. S4).
The mRNA expression of the DA synthesizing enzyme (TH) and the protein responsible for transporting DA into vesicles for synaptic release, the vesicular monoamine transporter 2 (VMAT2), are reduced in the VTA of RGS6−/− mice (Fig. 2E). Interestingly, although no difference was observed under control conditions, we observed a dramatic increase in expression of the dopamine transporter (DAT) specifically in the VTA of RGS6−/− mice following chronic EtOH exposure (Fig. 2E). Increased DAT, combined with reduced expression of TH and VMAT2 in the VTA of RGS6-deficient mice, would be expected to limit the availability of synaptic DA. In contrast, striatal DAT and TH expression were increased under control conditions in RGS6−/− mice (Fig. 2E).
Gαi/o-coupled D2Rs expressed presynaptically on dopaminergic neurons act as autoreceptors blocking vesicular DA release and activating DAT through multiple mechanisms (44–46). In addition, stimulation of GABABRs, which also signal through Gαi/o, in the VTA reduces alcohol consumption (2, 7, 47). RGS6 functions to impede the ataxic (28) and sedative actions of GABABR agonists (Fig. S5A) and RGS6 loss prolongs the time course of GABABR-induced activation and deactivation of the MAPK signaling cascade (Fig. S5B). However, no studies have evaluated the impact of RGS6 loss on D2R-dependent behaviors. To directly test the involvement of GABABR and D2R signaling in the reduction in alcohol-seeking behavior seen in RGS6−/− mice, we repeated the short-term free-choice EtOH consumption paradigm with the addition of daily GABABR (SCH-50911) or D2R (raclopride) antagonist treatments. Treatment with either SCH-50911 or raclopride partially reversed the reduction in alcohol consumption observed in RGS6−/− mice (Fig. 2F), indicating that potentiation of GABABR or D2R signaling underlies, in part, the reduction in voluntary alcohol consumption observed in mice lacking RGS6. Clearly, both GABABRs and D2Rs are involved in the aberrant alcohol-seeking behaviors in RGS6−/−mice, but it remained unclear whether these receptors were acting through a similar mechanism in the VTA to achieve this end. Strikingly, we found that singular inhibition of DAT with the inhibitor GBR-12909 completely restored EtOH consumption in RGS6−/− animals to levels observed in WT mice (Fig. 2F). These data indicate that, although at least two GPCRs contribute to alcohol indifference in mice lacking RGS6, increased DAT activity is the predominant mechanism underlying this phenomenon. Thus, RGS6 is a critical novel regulator of DAT expression and activity, actions that would be expected to limit the pool of synaptic DA capable of propagating the response to rewarding stimuli.
Mice Lacking RGS6 Are Protected Against Alcohol-Induced Cardiomyopathy.
Having established a critical role for RGS6 in promoting alcohol-seeking behaviors, we next sought to investigate the involvement of RGS6 in the pathogenesis of cardiac damage induced by EtOH. We reasoned that, given the ability of RGS6 to promote ROS-dependent apoptosis in the Dox-treated myocardium (39) and studies implicating ROS in the development of alcoholic cardiomyopathy (37), RGS6 might mediate oxidative stress-induced cytotoxicity in alcohol-exposed hearts. To test this hypothesis, we used a “forced” EtOH feeding paradigm, which eliminates genotype effects on EtOH seeking and reward behaviors and has been shown to cause detectable loss of cardiac contractility in addition to compromising liver function after 2 mo (36, 37). During the course of treatment, both WT and RGS6−/− mice lost weight (Fig. S6A). However, although the weight loss was accelerated in mice lacking RGS6, they consistently drank more of the alcohol-containing food compared with their WT counterparts, with their consumption more closely resembling that of mice consuming the control diet (Fig. S6B).
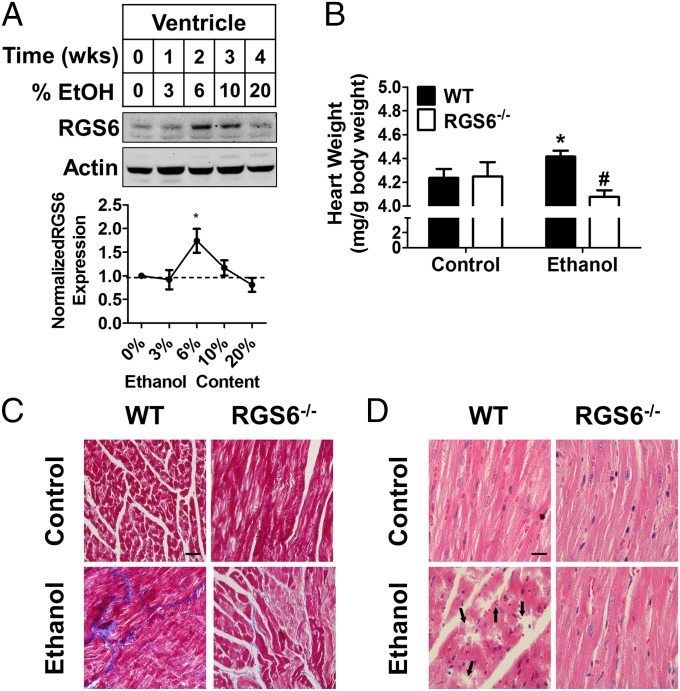
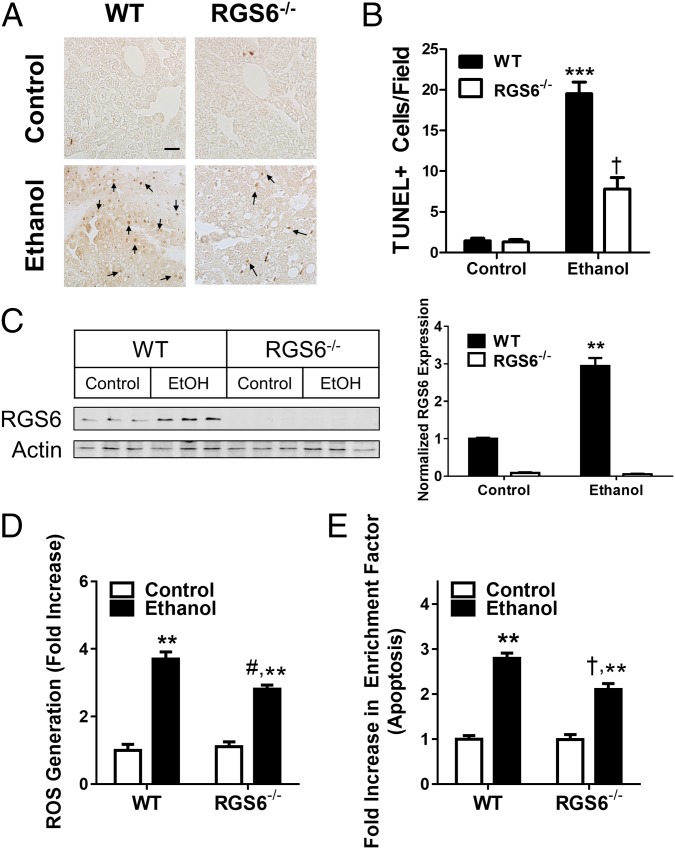
We have previously shown that cardiotoxic stimuli can induce up-regulation of RGS6 in heart (39). A transient rise in RGS6 protein levels was observed in the heart after 2 wk of EtOH exposure, which returned to baseline by 1 mo (Fig. 3A). Consistent with previous reports, chronic EtOH treatment resulted in cardiac hypertrophy (Fig. 3B), fibrosis (Fig. 3C), and myofilament disarray (Fig. 3D). However, mice lacking RGS6 were significantly protected from these pathogenic aberrations (Figs. 3 B–D), despite our observation that they in fact consumed more of the EtOH-containing diet (Fig. S6B). Importantly, the differences in the cytotoxic actions of RGS6 in the heart were not a result of differential EtOH absorption and metabolism, as blood alcohol concentration following oral EtOH administration was equivalent in WT and RGS6−/− mice (Fig. S6C). These results provide intriguing new evidence implicating RGS6 as a critical component of the signaling cascades contributing to alcoholic cardiomyopathy.
Fig. 3.
RGS6 deficiency protects mice from alcoholic cardiomyopathy. (A) RGS6 expression in the ventricle of WT mice given free access to graduated alcohol containing solutions [0–20% (vol/vol)] for increasing periods of time (Top). Densitometric quantification was performed with protein levels (n = 3) normalized to actin loading control and expressed relative to control conditions (Bottom). Animals (WT, n = 8–10 ; RGS6−/−, n = 8–10) were fed on 5% (vol/vol) EtOH containing or isocaloric maltose dextrin Lieber deCarli liquid diet for 2 mo. At the end of the treatment regimen (B) heart weight was measured (WT, n = 8–10; RGS6−/−, n = 7–10), and (C) Masson trichrome (blue, fibrotic remodeling) and (D) H&E staining were performed in the right ventricle. Black arrows indicate areas of microfilament disarray in the H&E-stained sections. Images are representative of at least three independent experiments. (Scale bars, 100 μm.) *P < 0.05 vs. WT control and #P < 0.01 vs. WT EtOH-treated samples by ANOVA with the Bonferroni post hoc adjustment. Data are presented as mean ± SEM.
RGS6 Promotes Apoptosis in Alcohol-Treated Ventricular Cardiac Myocytes via a Nox-Dependent Mechanism.
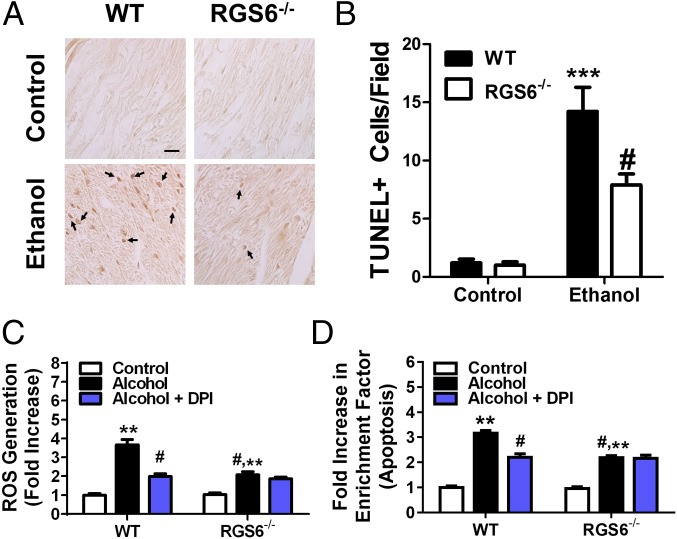
Alcohol induces apoptosis in isolated ventricular cardiac myocytes (VCM) by triggering Nox-dependent ROS generation (37). We observed a striking reduction in apoptotic nuclei in the myocardium of mice lacking RGS6 (Fig. 4 A and B). In keeping with the known requirement for ROS generation in the cytotoxic actions of alcohol, EtOH-induced ROS production was reduced in RGS6−/− VCM (Fig. 4C). To determine the enzymatic source of RGS6-dependent ROS production, we treated VCM with the Nox complex inhibitor diphenyleneiodonium (DPI). Nox-dependent ROS generation in response to alcohol was completely RGS6-dependent as DPI significantly reduced ROS generation in WT but not RGS6−/− VCM (Fig. 4C). The alcohol-induced apoptotic response was also dependent on Nox-mediated ROS generation in WT, but not RGS6−/− VCM (Fig. 4D). These results provide new evidence that RGS6 has a critical role as an upstream activator of Nox-derived ROS required for alcohol-induced VCM apoptosis.
Fig. 4.
RGS6 promotes cardiomyocyte apoptosis by facilitating Nox-dependent ROS generation. Animals (WT, n = 8–10; RGS6−/−, n = 8–10) were fed on 5% (vol/vol) EtOH containing or isocaloric maltose dextrin Lieber deCarli liquid diet for 2 mo. At the end of the treatment regimen (A) TUNEL staining was performed in the right ventricle. Black arrows indicate apoptotic cells in the TUNEL-stained sections. Images are representative of at least three independent experiments. (Scale bar, 100 μm.) TUNEL+ cells were quantified from 10 random microscope fields in B. (C) ROS generation (CM-H2-DCFDA fluorescence) was measured in neonatal VCM isolated from WT and RGS6−/− mice (n = 4) and treated with alcohol following pretreatment with DPI to inhibit Nox. (D) VCM were treated as in B (n = 3) and apoptosis measured.*P < 0.05; **P < 0.01; ***P < 0.001 vs. WT controls and #P < 0.01 vs. WT EtOH-treated samples by ANOVA with the Bonferroni post hoc adjustment. Data are presented as mean ± SEM.
RGS6 Deficiency Ameliorates Alcoholic Hepatic Steatosis and Apoptosis.
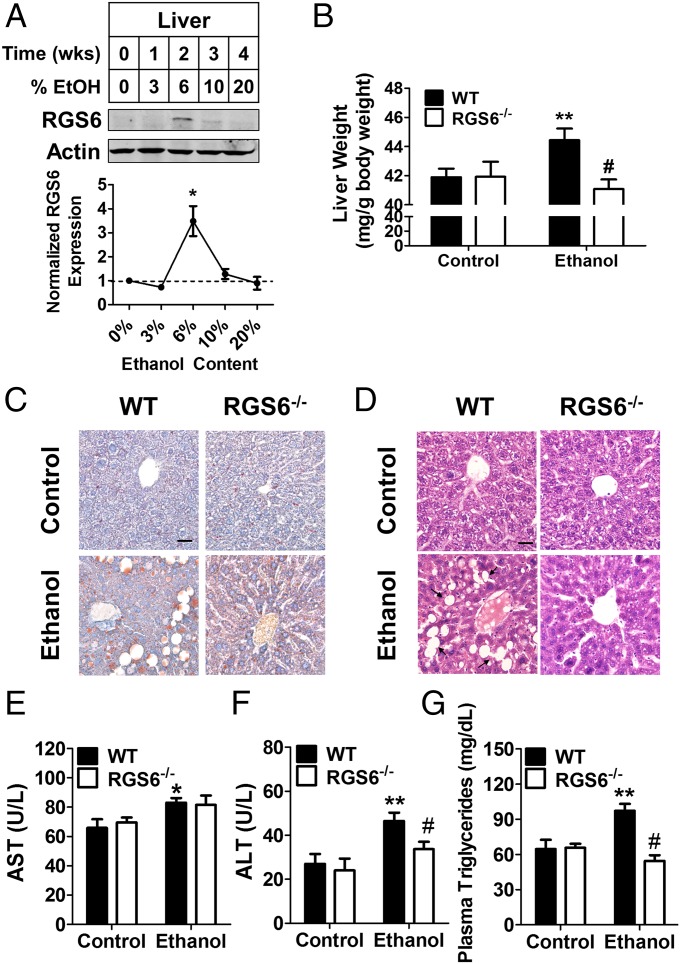
The most common long-term health complication associated with chronic alcohol abuse is hepatic cirrhosis. Like alcoholic cardiomyopathy, removal of Nox-derived ROS ameliorates alcoholic hepatic steatosis (36, 48). Very little RGS6 is detectable in the liver under basal conditions, but 2 wk of chronic alcohol consumption is sufficient to up-regulate RGS6 by several-fold (Fig. 5A). Strikingly, mice lacking RGS6 were substantially protected against alcohol-induced liver hypertrophy (Fig. 5B), fatty acid accumulation (Fig. 5C), and macrovesicular hepatic steatosis (Fig. 5D). Liver function tests revealed an alcohol-dependent increase in plasma aspartate transaminase (AST) (Fig. 5E), alanine transaminase (ALT) (Fig. 5F), and triglycerides (Fig. 5G). Although mice lacking RGS6 exhibited a similar increase in AST compared with their WT counterparts, RGS6−/− mice were protected against alcohol-induced increases in circulating ALT and triglyceride levels (Fig. 5 E–G). To provide some mechanistic insight into the role of RGS6 in fatty acid metabolism in the liver, we measured the cellular content of various genes involved in fatty acid synthesis and oxidation in the liver. mRNA levels of the nuclear receptors peroxisome proliferator-activated receptors-α (PPARα) and -γ (PPARγ) were lower in the livers of RGS6−/− mice irrespective of EtOH treatment (Fig. S7). Because PPARγ is involved in fatty acid synthesis, this reduction would be expected to protect mice against pathological fatty acid accumulation. Similarly, although expression of fatty acid synthase (FASN) was increased in the livers of alcohol-treated WT mice, no such increase was observed in RGS6−/− mice, which also exhibit a baseline reduction in FASN and acetyl-CoA carboxylase (ACACA) (Fig. S7). Together, these data indicate that RGS6 modulates baseline expression of multiple genes impinging upon fatty acid homeostasis in the liver.
Fig. 5.
RGS6−/− mice are protected against alcoholic hepatic steatosis. (A) RGS6 expression in the liver of WT mice given free access to graduated alcohol containing solutions [0–20% (vol/vol)] for increasing periods of time (Top). Densitometric quantification was performed with protein levels (n = 3) normalized to actin loading control and expressed relative to control conditions (Bottom). Animals (WT, n = 8–10 ; RGS6−/−, n = 8–10) were fed on 5% (vol/vol) EtOH-containing or isocaloric maltose dextrin Lieber deCarli liquid diet for 2 mo. At the end of the treatment regimen (B) liver weight (WT, n = 8–10; RGS6−/−, n = 7–10) was measured and (C) Oil Red O (red, fatty acid deposition) and (D) H&E were performed. Black arrows indicate areas of macrovesicular hepatic steatosis in the H&E-stained sections. Images are representative of at least three independent experiments. (Scale bars, 100 μm.) Plasma (E) AST, (F) ALT, and (G) triglycerides were measured in WT (n = 6) and RGS6−/− mice (n = 6–10) following the 2 mo alcohol feeding regimen. *P < 0.05; **P < 0.01 vs. WT controls and #P < 0.01 vs. WT EtOH-treated samples by ANOVA with the Bonferroni post hoc adjustment. Data are presented as mean ± SEM.
Finally, we wished to determine whether RGS6 deficiency protects against hepatic ROS generation and apoptosis in alcohol-treated cells via a hepatocyte-intrinsic mechanism. Similar to results observed in heart, the number of apoptotic cells in the liver of RGS6−/− mice was dramatically reduced (Fig. 6 A and B). Interestingly, as we observed in vivo, alcohol exposure triggered up-regulation of RGS6 in isolated hepatocytes (Fig. 6C). Furthermore, similar to trends we observed in isolated VCM, the ability of alcohol to trigger increases in ROS levels (Fig. 6D) and cellular apoptosis (Fig. 6E) in hepatocytes was compromised in cells lacking RGS6. These results indicate that RGS6 protects hepatocytes from alcohol-induced damage via, at least in part, hepatic parenchymal actions of RGS6.
Fig. 6.
Alcohol-induced hepatic ROS generation and apoptosis is reduced in RGS6−/− hepatocytes. Animals (WT, n = 8–10 ; RGS6−/−, n = 8–10) were fed on 5% (vol/vol) EtOH-containing or isocaloric maltose dextrin Lieber deCarli liquid diet for 2 mo. (A) At the end of the treatment regimen, TUNEL staining was performed. Black arrows indicate apoptotic cells in the TUNEL-stained sections. Images are representative of at least three independent experiments. (Scale bar, 100 μm.) TUNEL+ cells were quantified from 11 random microscope fields in B. (C) RGS6 expression in the hepatocytes of alcohol treated WT and RGS6−/− hepatocytes (Left). Densitometric quantification was performed with protein levels (n = 3) normalized to actin loading control and expressed relative to control conditions (Right). (D) ROS generation (CM-H2-DCFDA fluorescence) was measured in hepatocytes isolated from WT and RGS6−/− mice (n = 3–4) and treated with alcohol. (E) Apoptosis was measured in lysates from WT and RGS6−/− hepatocytes (n = 3–4) treated with alcohol and expressed as the fold-increase in cytoplasmic histone-associated DNA fragments (enrichment factor). **P < 0.01 and ***P < 0.001 vs. WT alcohol treated cells and #P < 0.05; †P < 0.01 vs. WT control samples by ANOVA with the Bonferroni post hoc adjustment. Data are presented as mean ± SEM.
RGS6−/− Mice Exhibit a Reduction in Alcohol-Induced Gastrointestinal Apoptosis and Endotoxemia.
Although the aberrations in gene expression and a loss of alcohol-induced, proapoptotic ROS generation in VCM and hepatocytes likely contributes substantially to the protective effect of RGS6 loss on alcoholic cardiomyopathy and hepatic steatosis, further experiments revealed an additional endocrine mechanism that likely also plays a role in these processes. Acute EtOH exposure causes damage to the gastrointestinal mucosa, leading to an increase in the permeability of the gut mucosa to intragastric macromolecules, such as bacterial-derived endotoxin. The resultant endotoxemia triggers the release of ROS and proinflammatory cytokines (e.g., TNF-α), which act in an autocrine, paracrine, and endocrine manner to cause tissue damage (49). We now show that RGS6 is expressed in appreciable levels in the epithelium of the stomach and small and large intestines (Fig. S8A). Based on this observation, we sought to evaluate the impact of RGS6 loss on alcohol-induced gastric barrier dysfunction.
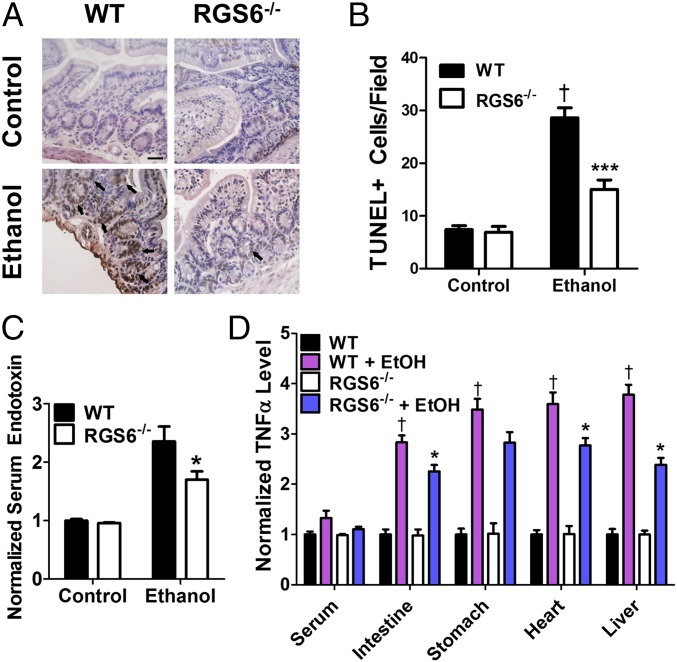
Because a robust increase in gastrointestinal epithelium apoptosis and leakage of endotoxin into the blood is detectable rapidly after alcohol exposure (50), we used an acute three-dose alcohol treatment regimen for these studies (51). We observed no up-regulation of RGS6 in the gut following this short treatment regimen (Fig. S8B), and histological analysis revealed similar intestinal crypt architecture in mice of both genotypes (Fig. S8C). However, whereas large clusters of apoptotic cells were evident in intestinal sections from WT mice, more diffuse apoptosis was seen in the intestinal epithelium of RGS6−/− mice (Fig. 7 A and B). Mice lacking RGS6 were also protected from alcohol-induced gastric hemorrhage (Fig. S8D). Consistent with our histopathological analyses, levels of serum endotoxin were significantly lower in RGS6−/− mice (Fig. 7C). Furthermore, although we were unable to detect a difference in serum and stomach TNF-α levels in mice of the two genotypes, TNF-α content was lower in the intestine, liver, and heart of RGS6−/− mice exposed to alcohol compared with their WT counterparts (Fig. 7D). Taken together, these results demonstrate that RGS6 deficiency allows for maintenance of the intestinal barrier, prevents leakage of endotoxin into the circulation, and reduces endotoxin-stimulated release of proinflammatory cytokines in multiple susceptible tissues.
Fig. 7.
RGS6 deficiency ameliorates alcohol-induced gastrointestinal dysfunction. Animals (WT, n = 8; RGS6−/−, n = 8) were treated with EtOH according to a three-dose acute protocol. At the end of the treatment regimen (A) TUNEL staining was performed in intestine. (Scale bar, 50 μm.) Black arrows indicate large clusters of apoptotic cells in TUNEL-stained sections. Images are representative of at least three independent experiments. TUNEL+ cells were quantified from 10 random microscope fields in B. (C) Serum endotoxin levels (WT, n = 3–8; RGS6−/−, n = 3–8) and (D) serum, liver, stomach, intestine, and heart TNF-α levels (WT, n = 3; RGS6−/−, n = 3) were measured in WT and RGS6−/− mice following 1 mo of EtOH exposure [5% (vol/vol) Lieber deCarli liquid diet]. *P < 0.05; ***P < 0.001 vs. EtOH-treated WT samples by ANOVA with the Bonferroni post hoc adjustment; #P < 0.05 vs. WT control via Student’s t test; and †P < 0.001 vs. WT control by ANOVA with the Bonferroni post hoc adjustment. Data are presented as mean ± SEM.
Discussion
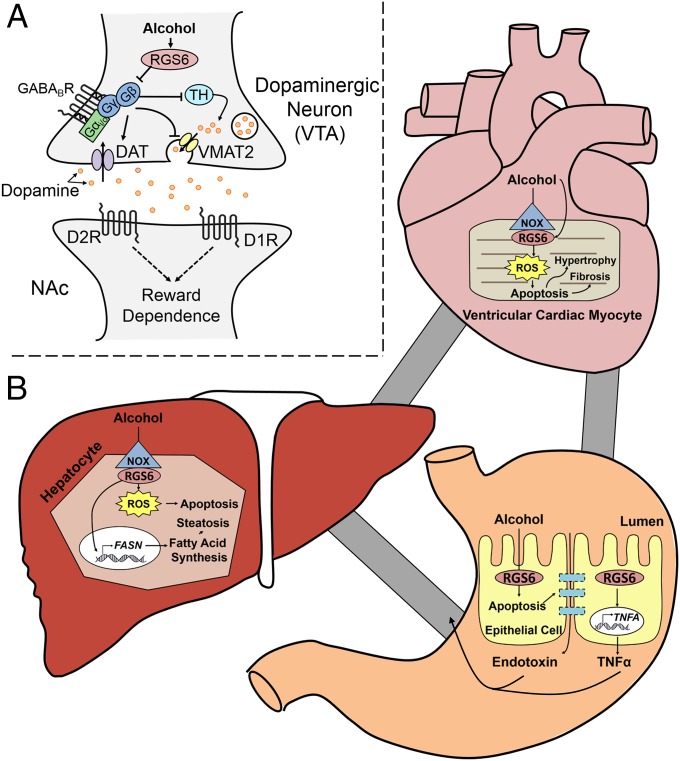
The results depicted herein describe a unique and multifarious role for RGS6 in the pathogenesis of alcoholism and alcohol-induced cardiac, gastrointestinal, and hepatic damage. To our knowledge, RGS6 is the only gene with a demonstrated ability to promote alcohol-seeking behaviors while simultaneously exacerbating the pathological impact of alcohol consumption on the heart, stomach, intestine, and liver. Of particular note, the ability of RGS6 to regulate these processes involves very distinct cellular mechanisms. In the CNS, the canonical function of RGS6 as a G-protein regulator affords it the capacity to inhibit G protein-coupled GABABR and D2R signaling disrupting DA synthesis, release, and reuptake (Fig. 8A). Conversely, in the gastrointestinal epithelium, cardiac myocytes and hepatocytes RGS6 promotes alcohol-induced apoptosis in part via its ability to promote Nox-derived ROS generation (Fig. 8B). Our previous studies have demonstrated that the ability of RGS6 to facilitate ROS generation is independent of its ability to regulate G proteins (40). These dichotomous actions of RGS6 in alcohol pathology highlight its distinctive utility as a potential therapeutic target, not only to reduce alcohol cravings in alcoholics but also to ameliorate the pathologic effects of chronic alcohol consumption in multiple susceptible tissues.
Fig. 8.
Schematic outlining the role of RGS6 in alcohol seeking behaviors and the resultant cardiac, hepatic, and gastrointestinal damage. (A) Our results indicated that, by inhibiting GPCR signaling and DAT, RGS6 promotes alcohol seeking behaviors. As a result, deletion of RGS6, normally up-regulated by EtOH in the VTA, ameliorates alcohol reward and withdrawal by impacting DA bioavailability. (B) At the same time, RGS6 normally functions to promote the cytotoxic actions of EtOH in the heart and liver through multiple mechanisms. First, in VCM, RGS6 promotes Nox-dependent ROS generation to facilitate alcohol-induced cell loss and also hypertrophy and fibrosis. In hepatocytes, RGS6 increases apoptosis and ROS generation and also increases the expression of genes involved in fatty acid synthesis leading to hepatic steatosis and compromised liver function after EtOH exposure. Finally, RGS6 also promotes apoptosis in the gut epithelium that contributes to intestinal leakage leading to the release of endotoxin and the inflammatory cytokine TNF-α into the peripheral circulation.
Our results provide striking new evidence that RGS6 functions as a critical mediator of alcohol-seeking behaviors in mice. Genetic ablation of RGS6 resulted in reduced alcohol consumption in both acute and chronic EtOH free-choice feeding paradigms. In the brain, alcohol produces a comparatively rapid and robust RGS6 up-regulation that appears to be unique to the VTA, a region of the brain heavily implicated in addiction. Elevations in DA release from the VTA are known to mediate the initial stages in the acquisition of alcohol dependence (2, 52). In this brain region, Gαi/o-coupled GPCRs block vesicular DA release and activate DAT, which reduces synaptic DA bioavailability (44). Of note, although inhibition of either GABABRs or D2Rs partially rescued EtOH drinking in RGS6−/− mice, blockade of DAT completely restored alcohol consumption to levels comparable to WT mice. Thus, the predominant mechanism contributing to RGS6-mediated alcohol-seeking behaviors appears to occur via suppression of DA reuptake. Although RGS6 may also impact vesicular DA release via inhibition of D2Rs, these actions appear to be dispensable for the ability of RGS6 to promote alcohol consumption in an acute alcohol-drinking paradigm.
Canonically, D2 autoreceptors increase DAT activity through a direct protein–protein interaction that facilitates recruitment of intracellular DAT to the plasma membrane enhancing DA clearance (44–46). If RGS6-dependent suppression of DAT occurred solely via inhibition of D2R signaling, the behavioral effects of D2R antagonists and DAT inhibitors should be equivalent. Instead, D2R inhibition was only able to partially restore alcohol consumption in mice lacking RGS6, whereas DAT blockade resulted in full phenotype reversal. Although this observation may be simply a result of differences in potency of the pharmacological agents used, it could also reflect the involvement of additional GPCRs capable of modulating DAT (e.g., GABABRs) or RGS6-regulated, GPCR-independent mechanisms contributing to DAT expression or function. In line with this supposition, there is no evidence that D2R signaling effects DAT transcription, whereas RGS6 deficiency results in a marked increase in DAT mRNA in both dorsal striatum and the VTA following EtOH exposure. Future work will likely focus on identifying the specific mechanisms whereby RGS6 modulates DAT activity, which clearly has a profound impact on alcohol-seeking behaviors.
Mice lacking Gβ5, a protein responsible for stabilizing R7 subfamily RGS6 proteins including RGS6, exhibit a reduction in basal DA levels in the dorsal striatum, indicating that RGS protein–Gβ5 complexes are important determinants of DA bioavailability (53). No changes were observed in serotonin levels in the NAc or striatum of RGS6−/− mice, consistent with the lack of 5-HT1A autoreceptor regulation by RGS6 (29). However, we now report DA depletion in the striatum of mice lacking RGS6, indicating that the biochemical phenotype identified in Gβ5−/− mice likely results from destabilization of RGS6. These results are consistent with regulation of D2 autoreceptors by RGS6 in the substantia nigra pars compacta (SNc), the population of dopaminergic neurons projecting to the dorsal striatum.
There is evidence that the magnitude of DA release in response to addictive substances differs between the VTA and SNc, with DA release onto the NAc dominating (54). However, we saw no difference in baseline DA levels in the NAc. Although this observation may appear to go against our assertion that RGS6 regulates D2R expressed on VTA neurons projecting to the NAc, it is in fact consistent with our biochemical and pharmacological data. We propose a mechanism whereby RGS6 induction in the VTA prevents EtOH-induced DAT up-regulation and impedes rapid DA clearance from the synapse. In the absence of RGS6, the differential DAT activity is only unmasked in the presence of an EtOH challenge. There are two observations to support this hypothesis. First, EtOH-induced RGS6 up-regulation is unique to the VTA and striatal RGS6 levels remain unchanged in response to EtOH. Second, DAT up-regulation in the VTA of RGS6-null mice, a primary contributor to suppression of alcohol drinking in the absence of RGS6 (Fig. 2F), requires EtOH treatment (Fig. 2E). In addition, the methodology used in this work does not differentiate between intracellular and extracellular DA levels and, thus, cannot specifically measure released DA or DA sequestered in vesicles or the intracellular space, which may also be impacted by RGS6 gene deletion because TH and VMAT2 expression are altered in the VTA of RGS6−/− mice. Additional experiments are necessary to identify the exact impact of RGS6 on DA synthesis, packaging into synaptic vesicles, release, and clearance. Based on the data reported herein, it is possible that RGS6 deletion perturbs multiple aspects of DA homeostasis, which culminates in loss of DA-mediated reward.
The functional consequence of the increased DAT expression observed in the striatum of RGS6−/− mice remains unclear because this brain region consists primarily of GABAergic medium spiny neurons expressing D2R heteroreceptors, but not DAT. However, there is evidence that D2 heteroreceptors contribute to DA release specifically from dopaminergic afferents projecting to the dorsal striatum (55). Thus, this DAT up-regulation could result from loss of RGS6-mediated suppression of D2 heteroreceptor signaling in the dorsal striatum, which also expresses a large quantity of RGS6 (Fig. S4). It is important to note that mice lacking RGS9-2, another R7 family RGS protein, develop dyskinesias resulting from dysregulation in D2R signaling. However, RGS9-2 expression is restricted to GABAergic neurons in the NAc and dorsal striatum, and as a result, RGS9-2 is thought to only regulate D2R heteroreceptor populations (31, 56, 57). Investigations into the impact of RGS6 on DA homeostasis in each region may aid in the identification of divergent mechanisms responsible for controlling D2R signaling and dopaminergic neuron function in both the mesolimbic and nigrostriatal circuits.
Studies in rodents have identified Nox-derived ROS as critical determinants of the apoptotic and fibrotic response to long-term EtOH exposure in heart (37). We now show that RGS6−/− mice are protected against alcoholic cardiomyopathy, including heart hypertrophy, fibrosis, microfilament disarray, and apoptosis. RGS6 mediates Nox-derived ROS generation and Nox-dependent apoptosis in alcohol-treated VCM. Hypertrophic and profibrotic factors are released from dying and damaged myocytes to promote repair, maintain the functional integrity of the heart, and ensure proper cardiac output (58). Because VCM in the myocardium of RGS6−/− mice fail to undergo apoptosis to the extent observed in WT tissue, the lack of heart hypertrophy and amelioration of fibrosis likely result from a lack of alcohol-induced VCM toxicity. Thus, we have identified RGS6, transiently induced by EtOH, as a crucial upstream factor required for Nox-mediated cardiac dysfunction following chronic alcohol exposure (Fig. 8B).
The accumulation of ROS in hepatocytes is one proposed mechanism leading to hepatic dysfunction in alcoholics (34). Nox complexes, activated by diverse stimuli, including TNF-α and endotoxin (59), appear to represent the source of EtOH-induced ROS as it has long been known that mice lacking the p47phox subunit of Nox1/2 are protected from alcoholic liver disease (36). Similar to results obtained in isolated VCM, RGS6−/− hepatocytes were also protected against alcohol-induced ROS accumulation and cell death. In vivo, livers isolated from RGS6-deficient animals following chronic alcohol treatment exhibited a dramatic reduction in apoptosis as well as macrovesicular hepatic steatosis. Unsurprisingly, plasma ALT and triglycerides were consistently lower in RGS6−/− mice treated with alcohol compared with their WT counterparts. Although ROS accumulation can influence fatty acid metabolism in the liver (34), RGS6 also appears to influence a number of genes involved in fatty acid synthesis, including PPARγ and FASN. Alcohol-induced up-regulation of RGS6 in the liver clearly plays a critical role in mediating both the cell death and accumulation of fatty acids in the liver of alcohol-treated mice (Fig. 8B), a process known to precede the development of liver cirrhosis in human patients.
Further experiments revealed an additional endocrine mechanism that likely also contributes to RGS6-mediated, alcohol-induced cardiac and hepatic toxicity. Acute EtOH exposure causes damage to the gastrointestinal lining leading to an increase in the permeability of the gut mucosa to intragastric macromolecules, including bacterial-derived endotoxin. The resultant endotoxemia triggers the release of ROS and proinflammatory cytokines in both liver and heart, which act in an autocrine, paracrine, and endocrine manner to exacerbate tissue damage (49). RGS6 is expressed in the epithelium of the stomach and small and large intestines. Furthermore, the gastrointestinal epithelium of mice lacking RGS6 exhibited a dramatic reduction in EtOH-induced apoptotic cell death. Because of maintenance of the gastrointestinal mucosa, the leakage of endotoxin into the circulation was also reduced in RGS6−/− mice. In addition, levels of the proinflammatory cytokine TNF-α were lower in RGS6−/− mice exposed to alcohol compared with their WT counterparts. Thus, it is likely that the lack of intestinal barrier dysfunction observed in alcohol treated RGS6−/− mice also serves to protect the heart and liver from further damage (Fig. 8B).
Thus far, the ability of RGS6 to regulate G protein-independent signaling cascades is unique among the members of the R7 family. In peripheral tissue such as heart, this is likely because of the fact that RGS6 is the predominant R7 family RGS protein expressed (38, 60). However, despite robust expression of additional R7 family RGS protein in the brain regions mentioned herein, they appear to be unable to compensate fully for singular loss of RGS6. Although any attempt to explain the apparent individual functions of proteins sharing significant sequence homology is purely speculative at this point in time, this could result from differences in effector or receptor modulation, expression in subcellular compartments or neuronal populations, or drug-specific regulation, all of which have been demonstrated for various R7 family members. Future studies using the newly developed RGS7−/− and RGS11−/− mouse lines will likely shed additional light on the participation of these proteins in addictive behaviors.
Few therapeutics have proven effective in treating alcoholism in human patients. Currently, acamprosate is first-line pharmacotherapy aimed at reducing cravings and withdrawal in alcoholics. Although generally well tolerated, acamprosate is most effective when combined with psychotherapy and abstinence from alcohol (61). There are currently no drugs marketed for the express purpose of reducing alcohol-mediated damage in the heart and liver. Given the prevalence of alcohol abuse worldwide, there is a clear need for more effective therapeutics. We propose that RGS6 inhibition could represent a novel means to counteract alcohol dependence by opposing alcohol-induced DA elevations via potentiation Gαi-coupled GPCR-mediated DAT activation in the VTA. At the same time, loss of RGS6 function would ameliorate the cytotoxic actions of alcohol in the heart and liver through simultaneous reduction of alcohol-induced, ROS-mediated proapoptotic signaling in hepatocytes and VCM and prevention of gastrointestinal barrier dysfunction. Although allosteric inhibitors of various RGS proteins have been developed, these drugs have been screened for their ability to specifically impair the G protein-dependent actions of RGS proteins (62). Obviously, such an agent would alleviate RGS6-mediated alcohol-seeking behaviors without impacting its proapoptotic actions in peripheral tissues. Similarly, although R7BP binding is important for membrane targeting and function of R7 family RGS proteins in neurons, it is not expressed outside of the nervous system (23, 63, 64). As a result, interfering with RGS6–R7BP complex formation may impact alcohol-seeking behaviors, but would not be expected to effect RGS6-mediated apoptotic signaling in the heart, liver, and gastrointestinal tract. Unlike R7BP, Gβ5 can be detected in nonneuronal tissues where other R7 family RGS proteins are expressed including heart, adrenal gland, pancreas, and breast (38, 60, 65, 66). Targeting the interaction between RGS6 and Gβ5 would destabilize both proteins (27), leading to loss of RGS6 expression in the CNS, heart, liver, and gastrointestinal tract with the desired functional impact of reducing alcohol dependence and ameliorating alcohol-induced cytotoxicity.
Materials and Methods
Mice.
RGS6−/− mice were generated as described previously (38). Experiments were performed using age-matched (10- to 12-wk-old) WT and RGS6−/− littermates. Mice were housed on a 12-h light/dark cycle and behavioral experiments performed during the light cycle. Animals of both genders were used for experiments as we observed no sex-specific differences in mouse performance. Animals naïve to each paradigm were used for all behavioral experiments. Drugs were administered 30 min before behavioral testing via intraperitoneal injection unless otherwise noted. Experiments were performed in agreement with the Guide for the Use and Care of Laboratory Animals (67). Protocols describing the behavioral paradigms used in this work can be found in SI Materials and Methods.
Chronic EtOH Treatment.
To assess the impact of RGS6 loss on EtOH-induced hepatic and cardiac toxicity, WT (n = 8–10) and RGS6−/− (n = 8–10) mice were fed on a Lieber-DeCarli control or isocaloric 5% (vol/vol) EtOH containing liquid diet (Bio-Serv) for 2 mo. During the treatment period, body weight changes and total EtOH consumption were recorded every 3 d (Figs. S6 A and B). At the end of the experiment serum was collected to measure plasma ALT, AST, and triglycerides (University of Iowa Hospitals). Liver and heart tissues were weighed and divided for immunoblotting, RT-PCR, and histological analyses. Tissue sections were processed for H&E, Oil Red O (liver), Masson trichrome (heart), and TUNEL staining at the University of Iowa Central Microscopy Core to detect general histology, liver lipid accumulation, cardiac fibrosis, and apoptotic cells, respectively.
Ventricular Cardiomyocyte Isolation and Culture.
Primary neonatal VCM were isolated from 2- to 3-d-old WT and RGS6−/− mice according to our previously published protocol (39). Twenty-four hours after isolation and plating, cells were treated with 200 mM EtOH (24 h) in the presence or absence of DPI (1 μM, 1.5-h pretreatment) where indicated. Apoptosis was measured from cell lysates using the Cell Death Detection ELISA kit (Roche). Intracellular ROS generation was estimated using the cell-permeable oxidation-sensitive probe, CM-H2DCFDA (Sigma), as described previously (40).
Hepatocyte Isolation.
Primary adult hepatocytes were isolated from 2-mo-old WT and RGS6−/− mice according to a standard collagenase perfusion protocol. Cells were suspended in Krebs–Henseleit bicarbonate buffer following isolation and maintained at 37 °C in a humidified cell culture incubator (5% CO2). Cells were treated with EtOH (200 mM) for 2 h. ROS accumulation and apoptosis were measured as described above.
Gastrointestinal Toxicity.
The impact of RGS6 loss on EtOH-induced gastrointestinal dysfunction was evaluated following an acute treatment protocol as previously described (51). Briefly, WT and RGS6−/− mice were exposed to three doses of EtOH (6 g/kg, oral gavage) or dextrose (control) at 12 h intervals. One hour following the final EtOH dose, blood, intestine, and stomach were harvested for histological (H&E) and biochemical analyses. Serum endotoxin levels were measured using the ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript) according to the manufacturer’s instructions. Serum and tissue TNF-α levels were detected with the Mouse TNF-α ELISA Ready-SET-Go! (eBioscience) following the manufacturer’s protocol.
Statistical Analyses.
Data were analyzed by Student’s t test or two-way ANOVA with the Bonferroni post hoc adjustment as appropriate. Statistical analyses were performed using Prism software (GraphPad Software). Results were considered significantly different at P < 0.05. Values are expressed as means ± SEM.
Additional information regarding materials and methods used in this work can be found in SI Materials and Methods. See Table S1 for PCR primer sequences.
Supplementary Material
Acknowledgments
We thank Dr. Matthew Potthoff (University of Iowa) for providing invaluable assistance with the mouse hepatocyte isolation. Funding for the Zeiss microscope (LSM710) was provided by NIH Grant 1 S10 RR025439-01 to the University of Iowa Central Microscopy Facility. This work was supported by NIH Grant CA161882, American Heart Association Grant 14GRNT20460208, and a University of Iowa Office of the Vice President for Research and Economic Development Award (to R.A.F.); a Pre-Doctoral Fellowship in Pharmacology/Toxicology from the PhRMA Foundation and a Presidential Fellowship from the University of Iowa Graduate College (to A.S.); and Post-doctoral Fellowship 14POST20490039 from the American Heart Association (to B.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418795112/-/DCSupplemental.
References
- 1.Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol Rev. 1997;77(1):1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23(11):1848–1852. [PubMed] [Google Scholar]
- 3.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109(1-2):92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 4.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32(6):1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao C, et al. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34(2):307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccioni P, Colombo G. Role of the GABA(B) receptor in alcohol-seeking and drinking behavior. Alcohol. 2009;43(7):555–558. doi: 10.1016/j.alcohol.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Moore EM, Boehm SL., 2nd Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123(3):555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson PM, Cruz DA, Olukotun DY, Delgado PL. Serotonin receptor, SERT mRNA and correlations with symptoms in males with alcohol dependence and suicide. Acta Psychiatr Scand. 2012;126(3):165–174. doi: 10.1111/j.1600-0447.2011.01816.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, et al. Hypermethylation of OPRM1 promoter region in European Americans with alcohol dependence. J Hum Genet. 2012;57(10):670–675. doi: 10.1038/jhg.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Simen A, Arias A, Lu QW, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Hum Genet. 2013;132(3):347–358. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerasimov MR, et al. Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse. 1999;34(1):11–19. doi: 10.1002/(SICI)1098-2396(199910)34:1<11::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Addolorato G, et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med. 2002;112(3):226–229. doi: 10.1016/s0002-9343(01)01088-9. [DOI] [PubMed] [Google Scholar]
- 13.Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16(19):2113–2117. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- 14.de Beaurepaire R. Suppression of alcohol dependence using baclofen: A 2-year observational study of 100 patients. Front Psychiatry. 2012;3:103. doi: 10.3389/fpsyt.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sari Y, Johnson VR, Weedman JM. Role of the serotonergic system in alcohol dependence: From animal models to clinics. Prog Mol Biol Transl Sci. 2011;98:401–443. doi: 10.1016/B978-0-12-385506-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swift R. Medications acting on the dopaminergic system in the treatment of alcoholic patients. Curr Pharm Des. 2010;16(19):2136–2140. doi: 10.2174/138161210791516323. [DOI] [PubMed] [Google Scholar]
- 17.Oslin DW, Berrettini WH, O’Brien CP. Targeting treatments for alcohol dependence: The pharmacogenetics of naltrexone. Addict Biol. 2006;11(3-4):397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 18.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86(3):445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 19.Dohlman HG, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J Biol Chem. 1997;272(7):3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 20.Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS4 and GAIP are GTPase-activating proteins for Gq alpha and block activation of phospholipase C beta by gamma-thio-GTP-Gq alpha. Proc Natl Acad Sci USA. 1997;94(2):428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 22.Hooks SB, et al. RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J Biol Chem. 2003;278(12):10087–10093. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
- 23.Drenan RM, et al. R7BP augments the function of RGS7*Gbeta5 complexes by a plasma membrane-targeting mechanism. J Biol Chem. 2006;281(38):28222–28231. doi: 10.1074/jbc.M604428200. [DOI] [PubMed] [Google Scholar]
- 24.Witherow DS, et al. Complexes of the G protein subunit gbeta 5 with the regulators of G protein signaling RGS7 and RGS9. Characterization in native tissues and in transfected cells. J Biol Chem. 2000;275(32):24872–24880. doi: 10.1074/jbc.M001535200. [DOI] [PubMed] [Google Scholar]
- 25.Snow BE, Betts L, Mangion J, Sondek J, Siderovski DP. Fidelity of G protein beta-subunit association by the G protein gamma-subunit-like domains of RGS6, RGS7, and RGS11. Proc Natl Acad Sci USA. 1999;96(11):6489–6494. doi: 10.1073/pnas.96.11.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posner BA, Gilman AG, Harris BA. Regulators of G protein signaling 6 and 7. Purification of complexes with gbeta5 and assessment of their effects on G protein-mediated signaling pathways. J Biol Chem. 1999;274(43):31087–31093. doi: 10.1074/jbc.274.43.31087. [DOI] [PubMed] [Google Scholar]
- 27.Chen CK, et al. Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5. Proc Natl Acad Sci USA. 2003;100(11):6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maity B, et al. Regulator of G protein signaling 6 (RGS6) protein ensures coordination of motor movement by modulating GABAB receptor signaling. J Biol Chem. 2012;287(7):4972–4981. doi: 10.1074/jbc.M111.297218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart A, et al. Regulator of G-protein signaling 6 (RGS6) promotes anxiety and depression by attenuating serotonin-mediated activation of the 5-HT(1A) receptor-adenylyl cyclase axis. FASEB J. 2014;28(4):1735–1744. doi: 10.1096/fj.13-235648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garzón J, López-Fando A, Sánchez-Blázquez P. The R7 subfamily of RGS proteins assists tachyphylaxis and acute tolerance at mu-opioid receptors. Neuropsychopharmacology. 2003;28(11):1983–1990. doi: 10.1038/sj.npp.1300263. [DOI] [PubMed] [Google Scholar]
- 31.Rahman Z, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38(6):941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- 32.Zachariou V, et al. Essential role for RGS9 in opiate action. Proc Natl Acad Sci USA. 2003;100(23):13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labouèbe G, et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10(12):1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: Updated experimental and clinical evidence. J Dig Dis. 2012;13(3):133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11(5):453–462. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- 36.Kono H, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106(7):867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan Y, et al. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol. 2012;59(16):1477–1486. doi: 10.1016/j.jacc.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, et al. RGS6, a modulator of parasympathetic activation in heart. Circ Res. 2010;107(11):1345–1349. doi: 10.1161/CIRCRESAHA.110.224220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, et al. G-protein inactivator RGS6 mediates myocardial cell apoptosis and cardiomyopathy caused by doxorubicin. Cancer Res. 2013;73(6):1662–1667. doi: 10.1158/0008-5472.CAN-12-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maity B, et al. Regulator of G protein signaling 6 (RGS6) induces apoptosis via a mitochondrial-dependent pathway not involving its GTPase-activating protein activity. J Biol Chem. 2011;286(2):1409–1419. doi: 10.1074/jbc.M110.186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 2010;70(22):9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green AS, Grahame NJ. Ethanol drinking in rodents: Is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42(1):1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee TK, Liu Z, Fisher RA. Human RGS6 gene structure, complex alternative splicing, and role of N terminus and G protein gamma-subunit-like (GGL) domain in subcellular localization of RGS6 splice variants. J Biol Chem. 2003;278(32):30261–30271. doi: 10.1074/jbc.M212687200. [DOI] [PubMed] [Google Scholar]
- 44.Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28(4):415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Dickinson SD, et al. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72(1):148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen R, et al. Protein kinase Cβ is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem. 2013;125(5):663–672. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119(1):213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- 48.Levin I, Petrasek J, Szabo G. The presence of p47phox in liver parenchymal cells is a key mediator in the pathogenesis of alcoholic liver steatosis. Alcohol Clin Exp Res. 2012;36(8):1397–1406. doi: 10.1111/j.1530-0277.2012.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: Effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29(11, Suppl):166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 50.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS ONE. 2014;9(5):e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdelmegeed MA, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- 53.Xie K, et al. Gβ5-RGS complexes are gatekeepers of hyperactivity involved in control of multiple neurotransmitter systems. Psychopharmacology (Berl) 2012;219(3):823–834. doi: 10.1007/s00213-011-2409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J Neurophysiol. 2007;98(6):3388–3396. doi: 10.1152/jn.00760.2007. [DOI] [PubMed] [Google Scholar]
- 55.Anzalone A, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gold SJ, et al. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci. 2007;27(52):14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovoor A, et al. D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25(8):2157–2165. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106(1):47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63(1):218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 60.Posokhova E, Wydeven N, Allen KL, Wickman K, Martemyanov KA. RGS6/Gβ5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ Res. 2010;107(11):1350–1354. doi: 10.1161/CIRCRESAHA.110.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mason BJ, Heyser CJ. The neurobiology, clinical efficacy and safety of acamprosate in the treatment of alcohol dependence. Expert Opin Drug Saf. 2010;9(1):177–188. doi: 10.1517/14740330903512943. [DOI] [PubMed] [Google Scholar]
- 62.Turner EM, Blazer LL, Neubig RR, Husbands SM. Small molecule inhibitors of regulator of G protein signalling (RGS) proteins. ACS Med Chem Lett. 2012;3(2):146–150. doi: 10.1021/ml200263y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grabowska D, et al. Postnatal induction and localization of R7BP, a membrane-anchoring protein for regulator of G protein signaling 7 family-Gbeta5 complexes in brain. Neuroscience. 2008;151(4):969–982. doi: 10.1016/j.neuroscience.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7BP, a novel neuronal protein interacting with RGS proteins of the R7 family. J Biol Chem. 2005;280(7):5133–5136. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- 65.Maity B, et al. Regulator of G protein signaling 6 is a novel suppressor of breast tumor initiation and progression. Carcinogenesis. 2013;34(8):1747–1755. doi: 10.1093/carcin/bgt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, et al. Targeted deletion of one or two copies of the G protein β subunit Gβ5 gene has distinct effects on body weight and behavior in mice. FASEB J. 2011;25(11):3949–3957. doi: 10.1096/fj.11-190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.