Abstract
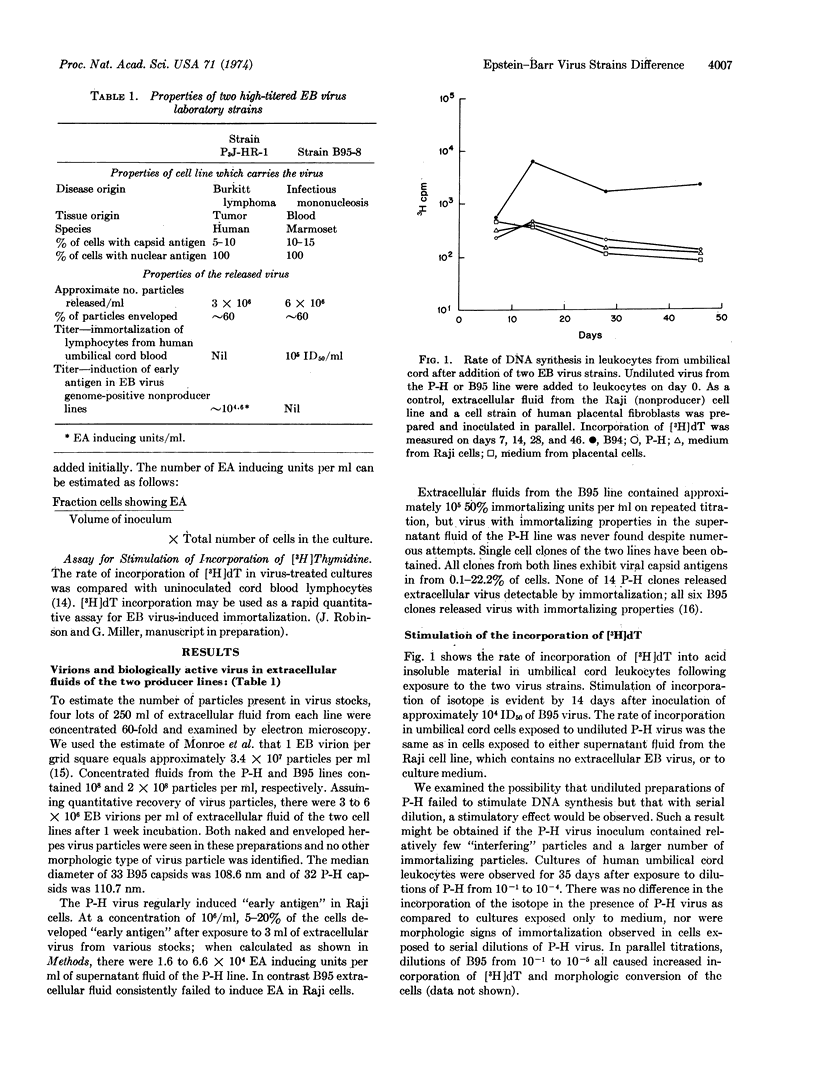
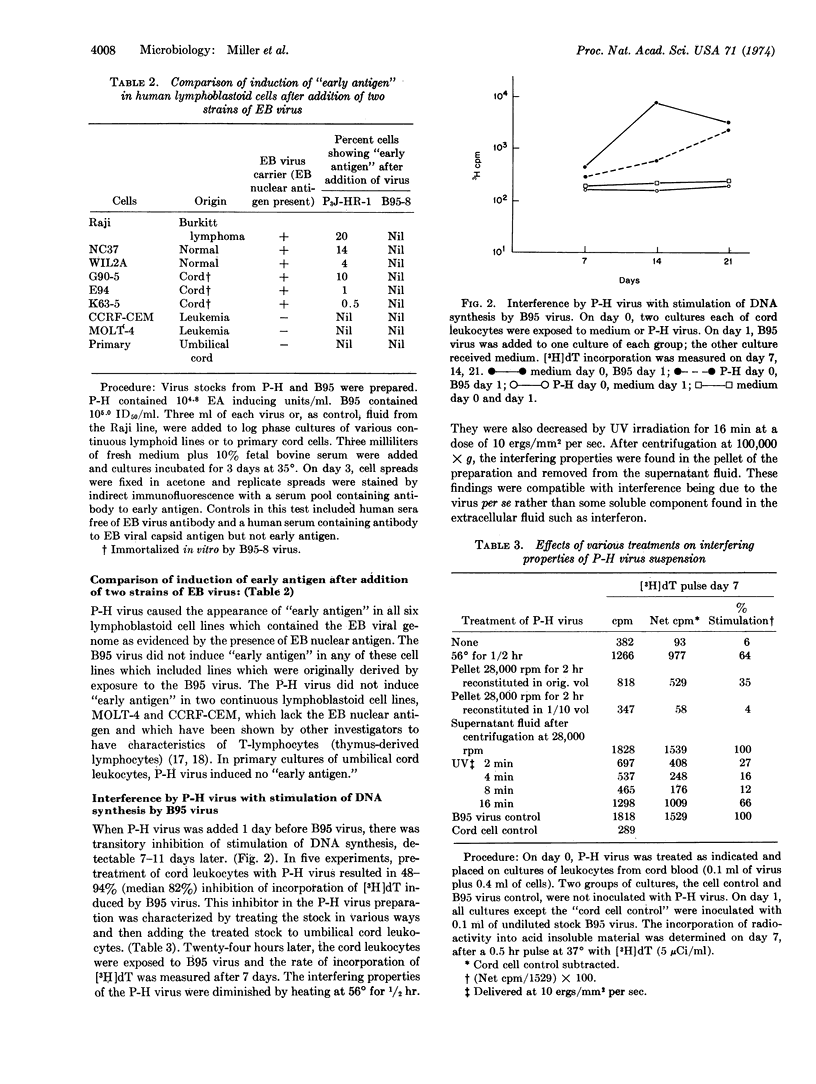
Biologic activities of extracellular Epstein-Barr virus (EB virus) from two laboratory strains, namely, P3J-HR-1 (P-H) from Burkitt lymphoma and B95-8 (B95) from infectious mononucleosis, were compared. Virus stocks from both sources contained approximately the same number of virions. Virus from the P-H line induced “early antigen” in six nonproducer EB virus genome carrier cell lines; virus from B95 did not induce “early antigen.” Extracellular virus from B95 regularly caused lymphocytes from human umbilical cords to form continuous lines (immortalization); P-H virus did not cause primary cultures of human lymphocytes to grow continuously. B95 virus stimulated DNA synthesis as determined by rate of incorporation of [3H]thymidine into acid-insoluble material; P-H virus did not stimulate DNA synthesis. Pretreatment of lymphocytes with undiluted P-H virus inhibited immortalization and stimulation of DNA synthesis by B95 virus. The inhibitory properties of the P-H virus were sedimented at 100,000 × g and inactivated by heat and UV irradiation; interference by the P-H virus was neutralized by human serum with antibody to EB virus and not by antibody-negative human serum.
The hypothesis most consistent with these results is that the P-H virus is defective in gene(s) needed for initiation of immortalization. We speculate that the absence of this gene allows early antigen to be expressed upon super-infection of nonproducer cell lines. The availability of two laboratory strains of EB virus which differ in biologic behavior provides starting material for analysis of the mechanism of lymphocyte immortalization by EB virus and of virus structural differences which affect immortalization.
Keywords: human lymphocytes, viral transformation, viral interference, extra-cellular virus
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Gerber P., Hoyer B. H. Induction of cellular DNA synthesis in human leukocytes by Epstein-Barr virus. Nature. 1971 May 7;231(5297):46–47. doi: 10.1038/231046a0. [DOI] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. Appearance of Epstein-Barr virus-associated antigens in infected Raji cells. Virology. 1971 Jul;45(1):10–21. doi: 10.1016/0042-6822(71)90107-3. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Comparison of the yield of infectious virus from clones of human and simian lymphoblastoid lines transformed by Epstein-Barr virus. J Exp Med. 1973 Dec 1;138(6):1398–1412. doi: 10.1084/jem.138.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lisco H., Kohn H. I., Stitt D., Enders J. F. Establishment of cell lines from normal adult human blood leukocytes by exposure to Epstein-Barr virus and neutralization by human sera with Epstein-Barr virus antibody. Proc Soc Exp Biol Med. 1971 Sep;137(4):1459–1465. doi: 10.3181/00379727-137-35810. [DOI] [PubMed] [Google Scholar]
- Miller G., Niederman J. C., Andrews L. L. Prolonged oropharyngeal excretion of Epstein-Barr virus after infectious mononucleosis. N Engl J Med. 1973 Feb 1;288(5):229–232. doi: 10.1056/NEJM197302012880503. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Identification of the filtrable leukocyte-transforming factor of QIMR-WIL cells as herpes-like virus. Int J Cancer. 1969 May 15;4(3):255–260. doi: 10.1002/ijc.2910040302. [DOI] [PubMed] [Google Scholar]
- Rocchi G., Hewetson J., Henle W., Henle G. Antigen expression and colony formation of lymphoblastoid cell lines after superinfection with Epstein-Barr virus. J Natl Cancer Inst. 1973 Feb;50(2):307–314. doi: 10.1093/jnci/50.2.307. [DOI] [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Strauch B., Andrews L. L., Siegel N., Miller G. Oropharyngeal excretion of Epstein-Barr virus by renal transplant recipients and other patients treated with immunosuppressive drugs. Lancet. 1974 Feb 16;1(7851):234–237. doi: 10.1016/s0140-6736(74)92546-x. [DOI] [PubMed] [Google Scholar]
- zur Hansen H., Diehl V., Wolf H., Schulte-Holthausen H., Schneider U. Occurrence of Epstein-Barr virus genomes in human lymphoblastoid cell lines. Nat New Biol. 1972 Jun 7;237(75):189–190. doi: 10.1038/newbio237189a0. [DOI] [PubMed] [Google Scholar]


