Significance
Butyrylcholinesterase (BChE), a common plasma enzyme, has been known for decades but its real physiological roles are just beginning to emerge. Although BChE eliminates the neurotransmitter acetylcholine, it is not vital for locomotion, cognition, or other cholinergic functions. Nevertheless, we now find that circulating BChE has a large impact on aggressive behavior in mice that is attributable to its ability to inactivate ghrelin, a peptide hormone involved in hunger, feeding, and stress. A key observation was decreased fighting among group-housed male mice overexpressing BChE after viral gene transfer. In contrast, BChE knockout mice exhibited increased fighting. These effects mirrored changes in plasma levels of active ghrelin. Controlling them might offer therapeutic potential for certain behavioral disorders.
Keywords: BChE, ghrelin, aggression, mice, viral vector
Abstract
Ongoing mouse studies of a proposed therapy for cocaine abuse based on viral gene transfer of butyrylcholinesterase (BChE) mutated for accelerated cocaine hydrolysis have yielded surprising effects on aggression. Further investigation has linked these effects to a reduction in circulating ghrelin, driven by BChE at levels ∼100-fold above normal. Tests with human BChE showed ready ghrelin hydrolysis at physiologic concentrations, and multiple low-mass molecular dynamics simulations revealed that ghrelin’s first five residues fit sterically and electrostatically into BChE’s active site. Consistent with in vitro results, male BALB/c mice with high plasma BChE after gene transfer exhibited sharply reduced plasma ghrelin. Unexpectedly, such animals fought less, both spontaneously and in a resident/intruder provocation model. One mutant BChE was found to be deficient in ghrelin hydrolysis. BALB/c mice transduced with this variant retained normal plasma ghrelin levels and did not differ from untreated controls in the aggression model. In contrast, C57BL/6 mice with BChE gene deletion exhibited increased ghrelin and fought more readily than wild-type animals. Collectively, these findings indicate that BChE-catalyzed ghrelin hydrolysis influences mouse aggression and social stress, with potential implications for humans.
Butyrylcholinesterase (BChE, EC 3.1.1.8) is ubiquitous in higher vertebrates, hydrolyzing a range of substrates such as acetylcholine and cocaine. It is widely viewed as a “backup enzyme” in cholinergic neurotransmission and a general-purpose metabolizer of bioactive esters in the diet (1). BChE was isolated 80 y ago (2) but was never tied definitively to a more specific physiological role. We now propose that one of its major functions is to hydrolyze ghrelin, a unique octanoyl peptide that stimulates hunger and feeding (3). In 2004, De Vriese et al. (4) reported that purified BChE releases ghrelin’s octanoyl group in vitro, converting it into a putatively inactive “desacyl” form. The physiological relevance of that effect was, apparently, never pursued.
Indications that BChE could be a physiological ghrelin regulator arose during our recent preclinical studies on gene transfer of enzyme mutated for enhanced cocaine hydrolysis (5). The intent of these studies was to assess the safety and efficacy of such a therapy for cocaine addiction. For that purpose a mouse BChE mutant (“mBChE-mut”) was delivered by viral vector. The experiments did not reveal cholinergic dysfunction, immunological reactions to enzyme, or liver damage from viral vector (6). They did confirm that gene transfer of cocaine-hydrolyzing BChE can suppress drug-seeking behavior by eliminating cocaine before it reaches the brain (7–9). Unexpectedly, the treatment also led to reduced fighting among mice that overexpressed BChE. In-cage fighting is common among male mice (10), but initially there was no obvious mechanism to explain why BChE should reduce such behavior. Further study, however, implicated enhanced metabolism of ghrelin, which is known for involvement in stress and anxiety (3, 11, 12).
Unlike humans, mice express a plasma carboxylase that efficiently hydrolyzes ghrelin (13), and mouse BChE is not essential (14). Nonetheless, our present data show that manipulating BChE in mice can improve or worsen social interactions. Positive effects occur when BChE is stably increased (i.e., gene transfer), and negative effects occur when it is lost (i.e., gene knockout). As will be shown, a plausible mechanism is the substantial difference in ghrelin levels between these two conditions.
The serendipitous findings on mouse fighting warranted careful re-examination of the potential links between BChE and ghrelin-mediated behavior. Here we provide evidence that BChE catalyzes ghrelin hydrolysis even at subnanomolar peptide concentrations, and we present a computational model that sheds some light on the structural basis of the reaction. Our data also demonstrate long-term control of ghrelin levels by viral gene transfer of native BChE or certain mutants (see Materials and Methods and Table 1 for list of variants used), and they indicate a strong impact of ghrelin manipulation on social behavior and aggression in mice.
Table 1.
Plasma testosterone, ghrelin, and BChE activity levels in untreated control, BChE knockout mice (KO), and groups treated with separate viral vectors for luciferase, wild type (WT) or mutated (mut) mouse BChE or human BChE, ghrelin, and GOAT
| Treatment | Strain | n | BChE (U/mL) | Fasting ghrelin (pg/mL) | Testosterone (ng/mL) |
| Untreated | BALB/c | 10 | 1.3 ± 0.1 | 300 ± 27 | 360 ± 68 |
| AAV-luciferase | BALB/c | 8 | 1.2 ± 0.1 | 325 ± 29 | 406 ± 69 |
| AAV-mBChE WT | BALB/c | 7 | 890 ± 140** | 56 ± 20** | — |
| AAV-mBChE mut | BALB/c | 10 | 160 ± 16** | 69 ± 10** | 465 ± 75 |
| hd-AD-mBChE mut | BALB/c | 7 | 107 ± 12** | 38 ± 5** | 400 ± 62 |
| AAV-CocH-6 ΔT | BALB/c | 11 | 180 ± 25** | 285 ± 50 | 270 ± 57 |
| Untreated | C57BL/6 | 10 | 1.5 ± 0.1 | 590 ± 41 | 4.8 ± 1.0 |
| AAV-luciferase | C57BL/6 | 10 | 1.5 ± 0.1 | 600 ± 60 | — |
| AAV-mBChE mut | C57BL/6 | 10 | 200 ± 19** | 25 ± 17** | — |
| AAV-ghrelin + GOAT | C57BL/6 | 11 | 1.6 ± 0.1 | 915 ± 153** | — |
| AAV-ghrelin + GOAT + mBChE mut | C57BL/6 | 10 | 95 ± 8** | 535 ± 75 | — |
| AAV-hBChE WT | C57BL/6 | 5 | 1,460 ± 280** | 305 ± 55** | — |
| Untreated | C57BL/6-KO | 9 | 0 | 910 ± 65** | 3.4 ± 0.5 |
| AAV-mBChE mut | C57BL/6-KO | 10 | 27 ± 5** | 530 ± 43 | — |
Statistical significance vs. untreated controls and luciferase controls: **P < 0.01. Means ± SEM are shown. —, not determined.
Results
In-cage fighting is common among male BALB/c mice (10). The observational studies initiating this project consistently encountered spontaneous aggression among group-housed subjects at ages beyond 3 mo. Strikingly, such behavior came exclusively from animals that received only “irrelevant” luciferase vector (n = 10) or no treatment (n = 23). Each control mouse eventually required single caging after reaching full maturity (separation age, 4.7 ± 0.8 mo). In contrast, weekly cage-side observations detected no bite marks or torn ears in the mice given BChE gene transfer at 6 wk of age with helper-dependent adenoviral vector (hd-AD; 1012 particles; n = 6) or adeno-associated viral vector (AAV; 1013 particles; n = 29). These treated mice, with up to 100-fold increases in plasma BChE, never required isolation over the following 2 y.
Inspections after animal care alerts in the early stages of this work revealed superficial bite wounds on many control mice, whereas age-matched enzyme-vector groups retained clean, glossy fur and showed no scratches, bites, or stress. After separation, the bitten controls did heal, but at 16 mo they exhibited ragged coats. By contrast, the vector-treated animals remained lively and robust (Movie S1). At this point, serum was tested to determine if a virus-induced or BChE-related drop in male hormone could explain reduced mouse-on-mouse aggression. Testosterone levels, however, were equivalent in all treatment groups (Table 1).
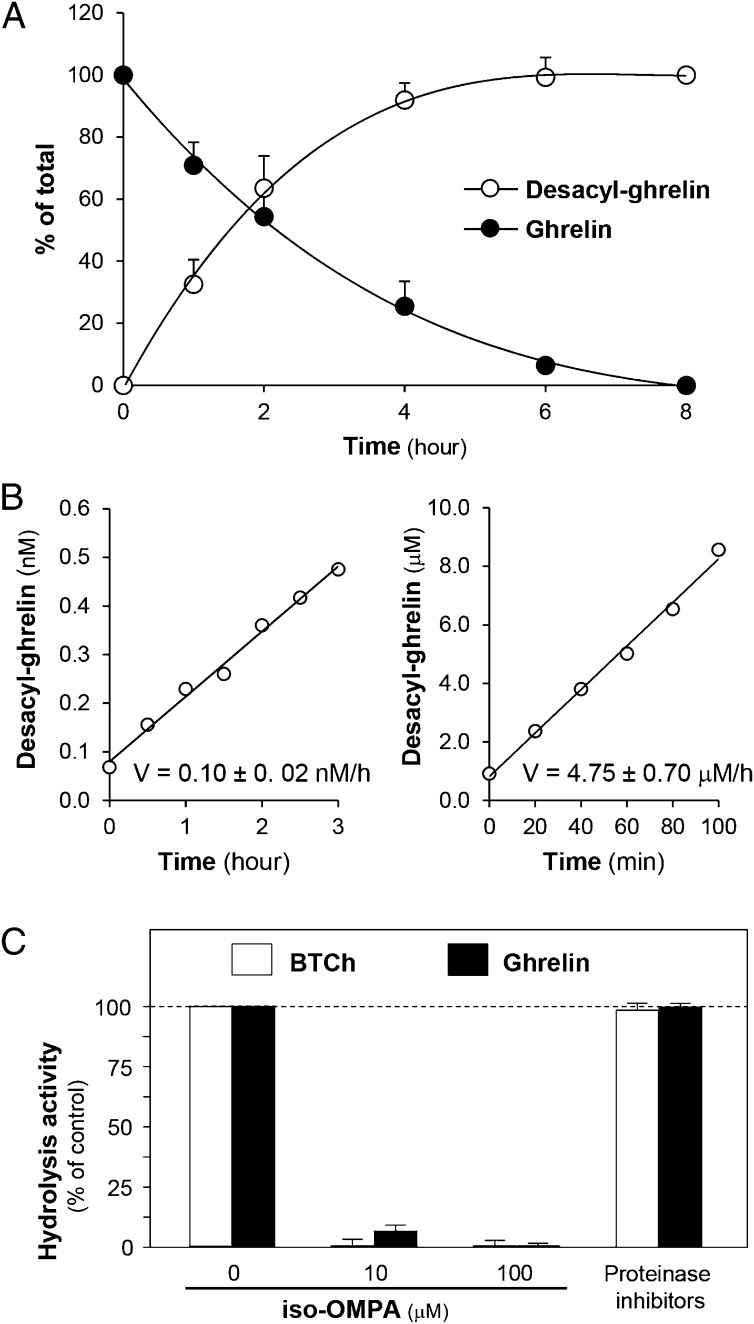
Among BChE substrates that might drive differential responses to chronic social stress, ghrelin was the only plausible candidate (15). As BChE’s catalytic activity with ghrelin had never been tested at physiological peptide concentrations (∼0.5 nM), we ran in vitro assays at that level and at 25 µM. ELISAs of ghrelin and desacyl-ghrelin in the reaction mixture (Materials and Methods) showed linear, mirror-image decreases and increases, respectively (Fig. 1 A and B). Both reactions were inhibited in parallel with butyrylthiocholine hydrolysis by the selective anti-BChE inhibitor iso-OMPA, but not by proteinase inhibitors (Fig. 1C).
Fig. 1.
In vitro enzymatic deacylation of human ghrelin by human BChE (125 nM). (A) Reciprocal changes in ghrelin and desacyl-ghrelin (0.5 nM initial substrate). (B) Generation of desacyl-ghrelin from ghrelin, 0.5 nM (Left) or 25 µM (Right). (C) Inhibitor sensitivity. BChE was incubated 10 min with iso-OMPA or proteinase inhibitors (1 µM aprotinin, 20 µM leupeptin, 15 mM pepstatin). Ghrelin was then added (0.5 nM). Hydrolysis activities compared with no-inhibitor controls were determined 1 h later by Ellman assay and desacyl-ghrelin immunoassay.
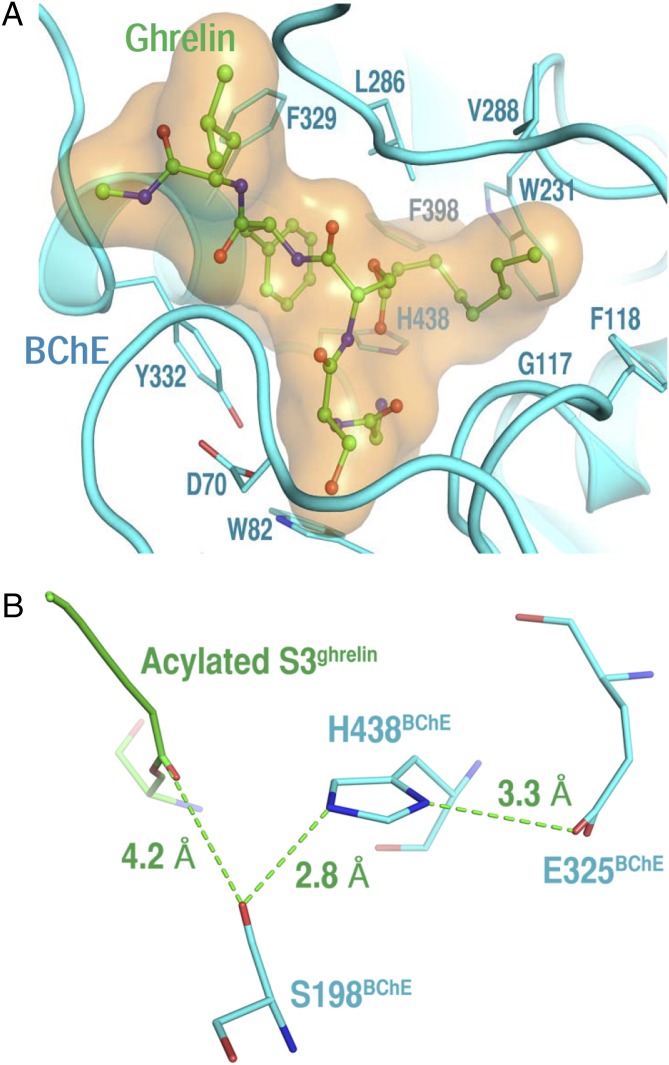
To investigate how BChE accommodates a peptide much bulkier than butyrylthiocholine, 100 low-mass molecular dynamics simulations were performed at 300 K (2.0 ns each, 1.0-fs time steps, and all atomic masses systemically reduced 10-fold to improve configurational sampling) (16). These simulations revealed that ghrelin’s first five residues fill the active site at a reversible complex state before hydrolysis (i.e., Michaelis–Menten state) in a complementary manner consistent with the hypothesis of BChE-catalyzed ghrelin hydrolysis (Fig. 2A). This was evident from the cation–pi interaction of ghrelin’s N-terminal ammonium group with Trp82BChE; the hydrogen bond between Ser3ghrelin and Asp70BChE; the van der Waals interactions between ghrelin’s acyl chain and Gly117BChE, Phe118BChE, Trp231BChE, Leu286BChE, Val288BChE, and Phe398BChE; and the pi–pi interactions of Phe4ghrelin with F329BChE, Tyr332BChE, and Phe398BChE. In addition, the ghrelin-bound BChE model derived from the simulation is nearly identical to the crystal structure of BChE liganded with benzoic acid (Protein Data Bank ID: 3O9M). Such an outcome indicates that complexation with ghrelin does not cause a large conformational rearrangement at the BChE active site, thus providing a structural basis for ghrelin hydrolysis. Furthermore, the distance between the catalytic serine hydroxyl oxygen atom and the carbonyl carbon atom of the octanoyl chain is 4.2 Å in the ghrelin-bound BChE model at the Michaelis–Menten state, indicating that the octanoyl group is in the proximity of the catalytic serine (Fig. 2B). That distance is shorter than the corresponding ones seen in cocaine–BChE simulations (5.9 Å for the natural cocaine and 6.6 Å for the unnatural cocaine) (17) and in HI-6⋅sarin-acetylcholinesterase (5.0 Å) (18). This suggests that a smaller degree of conformational rearrangement is needed for BChE-catalyzed ghrelin hydrolysis than for BChE-catalyzed cocaine hydrolysis and for reactivation of sarin-inactivated acetylcholinesterase by HI-6.
Fig. 2.
Ghrelin-bound BChE model. (A) Acylated ghrelin bound in the active site of human BChE at a reversible complex state before hydrolysis obtained from 100 2.0-ns low-mass molecular dynamics simulations. Ghrelin is in ball-and-stick model; BChE is in cartoon model with key amino acids indicated. (B) Close-up of ghrelin’s acyl group in relation to catalytic triad of human BChE.
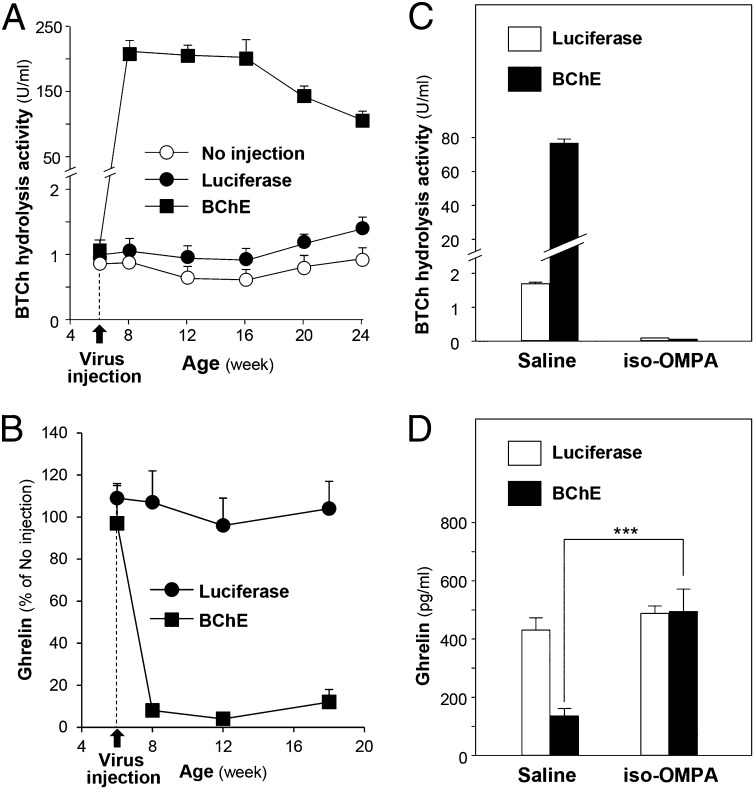
BChE’s catalytic activity with ghrelin implied that BChE gene transfer would reduce plasma levels of the acylated peptide. To test that prediction, AAV mouse BChE mutant vector (Materials and Methods) was given to 3-mo-old C57BL/6 mice (1013 particles). In 2 wk, ghrelin plasma levels fell by 95 ± 5% whereas desacyl-ghrelin levels rose by 1.8- ± 0.2-fold (P < 0.001). This effect persisted in parallel with the sustained elevation of plasma BChE activity in treated mice (Fig. 3 A and B). To confirm dependence on enzyme activity, four mice were given the selective BChE inhibitor iso-OMPA, which restored the balance of ghrelin forms to control levels within 6 h under fasting conditions, whereas ghrelin remained low in mice given no drug (Fig. 3 C and D).
Fig. 3.
BChE and ghrelin levels after gene transfer in C57BL/6 mice. (A) Plasma BChE activity with butyrylthiocholine in mice given 1013 particles of AAV-luciferase or AAV-mBChE mutant vector. Mean values ± SEM (n = 8 per group). (B) Ghrelin levels after AAV-luciferase or AAV-mBChE mutant vector treatment as percentage of untreated control. (C and D) BChE activity and ghrelin levels under fasting conditions, 6 h after treatment with iso-OMPA, 50 mg/kg, i.p., or saline. Values are means ± SEM (n = 4); ***P < 0.001 compared with saline group.
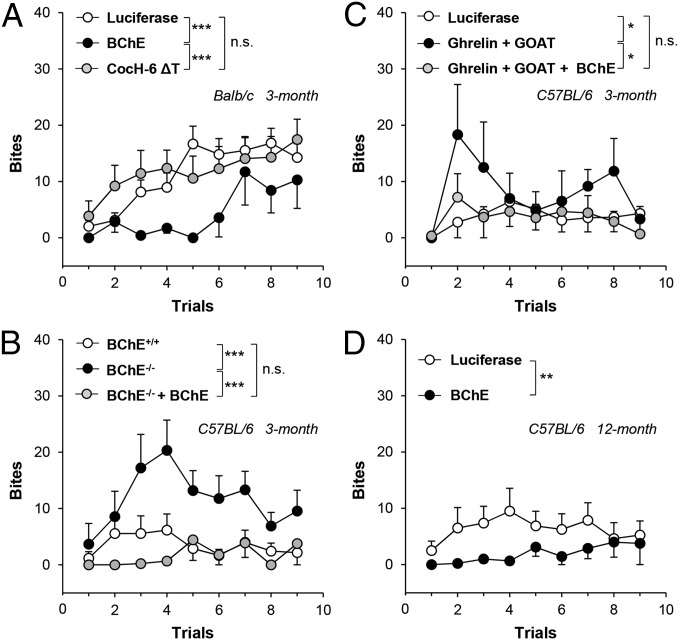
Next, aggressive tendencies were evaluated with the classic, quantitative, resident/intruder paradigm (19). In this provocation test, a female mouse is paired with a “resident” male for 2 wk and is then removed. Subsequently, an “intruder male” of the same strain is repeatedly introduced for specific brief intervals while attacks and bites by the resident are video-recorded for off-line quantitation and aggression scoring (Materials and Methods). The tests yielded a clear-cut difference between 3-mo-old BALB/c controls and same-age mice treated with BChE vector (1013 particles) with much lower ghrelin levels (Table 1). The latter were significantly slower to initiate fighting (P < 0.001, Fig. 4A). In contrast, vector encoding CocH-6 ΔT (1013 particles), a mutant BChE that was efficient with cocaine but did not reduce ghrelin levels (Table 1), did not affect bite scores in the resident-intruder protocol. This negative outcome strongly supported a link between ghrelin hydrolysis and BChE effects on aggression.
Fig. 4.
Bite scores in confrontations between a resident male mouse and a male intruder. (A) Three-month-old BALB/c mice with AAV-luciferase vector (n = 18) vs. mBChE mutant vector-treated mice (n = 7) and AAV-CocH-6 ΔT-treated mice (n = 14). (B) Untreated 3-mo-old C57BL/6 wild-type mice (n = 18) vs. same-age BChE knockouts (n = 9) and vector-treated BChE knockouts (n = 9). (C) AAV-luciferase–treated 3-mo-old C57BL/6 wild-type (n = 9) vs. same-age C57BL/6 treated simultaneously with AAV vectors encoding cDNA for ghrelin and GOAT (n = 6) and same-age mice treated triply with vectors for ghrelin, GOAT, and mBChE mutant (n = 9). (D) C57BL/6 mice given AAV-luciferase (n = 9) or mBChE mutant vector (n = 9) at 6 wk and tested for aggression at 12 mo. Data were analyzed by two-way ANOVA with a Holm–Sidak multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
As animals with high BChE and low ghrelin proved less aggressive than controls, the impact of BChE deficiency was also investigated (Fig. 4B). These experiments compared 3-mo-old wild-type C57BL/6 mice with BChE knockouts of the same age and strain lacking all enzyme activity. Both groups were seriously testosterone-deficient (Table 1), a recognized C57BL trait (20). Ghrelin in the knockout plasma was 54% higher than in controls (P < 0.007). Desacyl-ghrelin levels were also elevated (3,400 ± 180 pg/mL vs. 2,750 ± 180 pg/mL, P < 0.015). In the resident-intruder tests, young wild-type C57BL/6 mice proved less willing to fight than BALB/c mice. By contrast, the knockouts were decidedly aggressive, but BChE gene transfer reversed this behavior.
Next, we attempted to raise ghrelin levels in C57BL/6 mice by simultaneous gene transfer of cDNA for ghrelin (5 × 1011 particles) and its activator, ghrelin octanoyl acyl transferase (GOAT, 2.5 × 1011 particles). Provocation tests of these doubly treated mice showed a modest but statistically significant increase in bites per session, which was prevented in a third group that received AAV vector for mouse BChE (1013 particles, Fig. 4C). Finally, at 1 y of age, wild-type C57BL/6 mice that received BChE vector treatment at 6 wk (retaining higher plasma BChE and lower ghrelin) were compared with mice of the same strain given luciferase vector. In provocation tests, the BChE-treated mice were decidedly less aggressive than the same-age controls (Fig. 4D).
Discussion
Our collected functional and structural observations are consistent with the hypothesis that BChE, over time, experienced evolutionary pressure toward a capability for ghrelin hydrolysis. We propose that this capability is not an accidental curiosity, but an important physiological function for this enzyme. Of special interest is the contrast between low but significant hydrolysis at typical plasma ghrelin concentrations and greatly enhanced hydrolysis at higher concentrations. One could speculate that such properties might have evolved to influence ghrelin levels appropriately in different settings, e.g., peptide circulating in the blood stream at nanomolar levels vs. abundant ghrelin at brain synapses or in the stomach.
The present results indicate that elevation of BChE reduces mouse aggression by hydrolyzing ghrelin and moderating emotional states that predispose to fighting. Conversely, they show that loss of BChE generates a rise in ghrelin and increased propensity to fight among young adult males. That finding is all the more surprising because BChE−/− mice retain plasma carboxylesterase, which also deacylates ghrelin (21). Actually, Lockridge et al., who developed the knockouts, reported 50% less ghrelin in the C57/BL-BChE−/− line than in their wild-type counterparts (22). A key difference in the prior study was that the knockout mice had eaten a high-fat diet for a full year and were obese, a condition typically associated with low ghrelin (23). In contrast, our mice with elevated acyl- and total ghrelin ate regular laboratory chow and were not obese.
A fuller picture of the mechanisms behind these effects must also incorporate additional factors, particularly leptin. The current literature on ghrelin–leptin interactions does not present a clear consensus, and results vary according to the models used, but there is some evidence for a negative feedback loop. Thus, ghrelin delivery is known to cause leptin release from cultured rat adipocytes by a mechanism dependent on the growth hormone secretagogue receptor (GHSR1a) (24). On the other hand, leptin suppresses ghrelin release from rat stomach (25, 26). Definitive studies have yet to clarify whether and how such reciprocal interactions change when ghrelin deacylation is chronically perturbed. However, our observations of elevated total ghrelin in BChE knockouts indicate that a sustained reduction of peptide deacylation does not elicit a compensating reduction of peptide production. Going forward, BChE gene transfer, BChE knockouts, and irreversible inhibition may be helpful in exploring mechanisms determining in vivo levels and turnover of ghrelin and leptin.
As for behavioral data, our aggression findings appear to be broadly in line with recent literature showing that ghrelin not only is crucial in feeding and caloric homeostasis (3, 27), but also has manifold roles in stress and anxiety (11, 12, 28). However, current literature does not fully agree on the nature of these roles. Some studies report that ghrelin relieves anxiety according to measures such as time spent in the open arm of an elevated plus maze (11, 12, 28). Others conclude that ghrelin acts in the amygdala to enhance fear and anxiety in rat models of posttraumatic stress disorder (15). The latter result supports our finding that chronic reduction of ghrelin promotes lower aggression, whereas chronic elevation has opposite effects. This is not necessarily a paradox because the brain circuitries for aggression and anxiety are not entirely congruent. Interpretation of varying outcomes with aggression and anxiety must take into account inherent differences in the underlying neural pathways, limitations in animal models of emotional states, and the variety of interventions. A hypothesis worth pursuing, in our view, is the following: Phasic ghrelin pulses exert antistress actions through GHSR1a, but these receptors are readily desensitized. Hence, they may be stronger when “background” levels of ghrelin are low and weaker when they are high. In view of the often-conflicting findings in this important arena, thorough dose–response studies to sort out the net effects of various steady-state levels of ghrelin on GHSR sensitivity should be a priority.
Experimental design is exceptionally important in ghrelin-mediated behavior because of the complex interconversion of acylated and deacylated peptide and the ready desensitization of its receptor target. Prior studies used gene deletions of ghrelin (27); of GOAT, its activating enzyme (27); and of GHSR1a, the receptor for ghrelin (12). In contrast, we manipulated BChE expression both upward and downward in ways that produced graded effects. It is now worthwhile to examine aggression alongside other behaviors linked to anxiety and stress after multiple different interventions. Key questions are whether GHSR knockout will reduce or enhance aggression in the resident-intruder model and whether BChE gene transfer will enhance or alleviate signs of anxiety in the elevated plus maze. A systematic comparison of the brain circuitry and interconnections involved is also needed, with special focus on the medial amygdala, a center with clear roles in both anxiety and aggression (11, 29, 30).
There may be clinical relevance, in view of the widely recognized genetic diversity of BChE in the human population, with several-fold differences in expression levels and activity for common substrates, including important medications and, most probably, ghrelin. Recent population-based studies have tied this natural and partly age-related enzymatic variation to clinical conditions that carry risk of major adverse cardiovascular events and premature death in patients with low levels of circulating BChE (31–34). We postulate that genetic or degenerative processes that reduce BChE-driven ghrelin deacylation may be driving these sorts of pathology and, hence, represent promising targets for intervention.
Materials and Methods
Animal Subjects and Ethics Statement.
Adult male mice (BALB/c) were obtained from Harlan Laboratories under protocol A27713 approved by the Mayo Clinic Institutional Animal Care and Use Committee. Adult male C57BL/6 wild-type and BChE−/− mice were obtained from Jackson Labs. All experiments were conducted in accord with the Guide for Care and Use of Laboratory animals (35) in a facility accredited by the American Association for the Accreditation of Laboratory Animal Care. Mice were kept four to five per cage until signs of fighting arose (4–5 mo) and then promptly moved to single-cage housing. Ghrelin-overexpressing mice and their controls were fed chow mixed with 5% (wt/wt) glyceryl trioctanoate (Sigma) to provide the fatty acid chain for the newly synthesized ghrelin (36). To determine the fasting ghrelin levels in plasma, mice were fasted for 20 h (1400 hours to 1000 hours) with water ad libitum, and all blood samples were collected within 1 h.
Plasma Samples.
Blood (0.2–0.3 mL) was taken by cheek puncture using a mouse-bleeding lancet, and sterile gauze was applied to stop bleeding. BChE samples were centrifuged for 15 min at 8,000 × g and stored at −80 °C. Ghrelin samples were collected in cooled EDTA-treated tubes with protease inhibitors (1 mM p-hydroxymercuribenzoic acid, Sigma-Aldrich; 1.5 µM aprotinin, Roche). Centrifuged plasma was immediately treated with 0.1 volume of 1 N HCl to prevent ghrelin deacylation, and supernatants were stored at −80 °C.
Enzyme Assays.
Quantities of highly purified native human BChE were supplied by O. Lockridge, University of Nebraska Medical Center, Omaha, NE. Basic BChE activity was assayed after preincubation with selective acetylcholinesterase inhibitor, 10 µM 1,5-bis (4-allyldimethylammoniumphenyl) pentan-3-one dibromide (BW284c51). Reaction mixtures contained 0.5 mM butyrylthiocholine iodide and 0.5 mM 5,5′-dithiobis-2-nitrobenzoic acid in 100 mM sodium phosphate, pH 7.4. Absorbance was read at 405 nm every 15 s for 10 min. Units of enzymatic activity were micromoles of product∙min−1∙ml−1 calculated using the Ellman reagent’s standard extinction coefficient (14,150 M−1⋅cm−1). All assay reagents were from Sigma Chemical.
Ghrelin Determinations.
Octanoylghrelin was obtained from Bio Vision. Peptide quantities were measured by EIA immunoassay kits 10006307 and 10008953, for ghrelin and desacyl-ghrelin (Cayman Chemical). Rates of peptide hydrolysis were determined at 37 °C in 50 mM Tris⋅Cl buffer containing 0.1% BSA, pH 7.4. Enzymatic reactions were stopped by freezing in dry-ice/acetone. All samples were pretreated with 100 µM tetraisopropyl pyrophosphoramide (iso-OMPA, Sigma) before immunoassays. Duplicate assays of product and residual substrate were performed at each time point with different substrate concentrations under conditions generating values linear with assay duration. Absolute values were calculated from desacyl ghrelin production matched against a linear standard curve. To avoid data bias, all group comparisons were based on side-by-side observations from simultaneous assays.
Theoretical Model of Human Ghrelin⋅BChE Complex.
The initial model of GSSoctanoylFL⋅hBChE was generated by manually docking ghrelin residues 1–5, with N-methyl substituted at the C terminus in an extended backbone conformation, into the hBChE active site taken from the crystal structure of full-length recombinant hBChE (Protein Data Bank ID: 3O9M). This docking placed (i) ghrelin’s ammonium group atop Trp82BChE and (ii) Phe4Ghrelin close to Phe329BChE and Tyr332BChE. Forcefield parameters for Ser with n-octanoylated hydroxyl (Soctanoly) were generated by a published procedure (37, 38). The energy-minimized complex was neutralized with 11 chlorides, solvated with 13,050 TIP3P water molecules (39), and containing 36 NaCl molecules. The resulting system was refined with 100 2-ns molecular dynamics simulations, each of which used a unique seed number for initial velocities and a 1.0-fs time step, at 300 K and atmospheric pressure using the PMEMD module of the AMBER 11 program. Further details and model coordinates are available in SI Materials and Methods and Dataset S1, respectively.
Viral Gene Transfer.
cDNAs encoding the following peptides and proteins were subcloned into AAV backbone vector: luciferase, mouse BChE wild type, mouse BChE mutant (A199S/S227A/S287G/ A328W/Y332G), human BChE wild type, human BChE mutant (A199S/S227A/S287G/A328W/Y332G), CocH-6 ΔT (human BChE E1-V529 with A199S/F227A/S287G/A328W/Y332G/E441D), mouse GOAT, and mouse ghrelin. The resulting transfer vectors were cotransfected into HEK293T cells with the helper vectors pHELP (Applied Viromics) and pAAV 2/8 (Department of Pathology and Laboratory Medicine, University of Pennsylvania) (8). Viruses in cell lysates were isolated by ultracentrifugation, and viral particles were determined by real-time PCR. Other experiments used hd-AD with mouse BChE mutant cDNA (same as above) under regulation by a human ApoE hepatic control region (40) as described (41). Helper virus contamination was ∼0.2%. Vector (200 µL) was given i.v. followed by 200 µL of 0.9% sterile NaCl solution.
Aggression Tests.
A resident/intruder paradigm (19) was followed, starting in the last hour of the light cycle (1700 hours). Resident males were first paired in their home cage with a same-strain female for two continuous weeks. The female was then swapped with a small male for sessions that terminated in 5 min in the absence of fighting or else 5 min after the first fight, three times per week. Behavior was video-recorded with a Canon ELPH 320 HS for a treatment-blind observer to count fighting episodes and bites (the primary data for group comparisons).
Data Analysis.
Primary dependent measures were BChE enzyme activity, plasma testosterone, ghrelin and desacyl ghrelin, fights, and bites. Statistical analyses, including enzyme kinetics, were performed using Sigma Stat (Sistat Software). Aggression data were analyzed by two-factor mixed analyses of variance (ANOVA) with treatment group (vector, control) as the between-subjects factor and blocks of testing trials as the repeated measure. After significant interactions, Holm–Sidak post hoc tests were performed (P < 0.05 was considered significant).
Supplementary Material
Acknowledgments
We thank the Baltimore Laboratory at the California Institute of Technology, Pasadena CA, for providing AAV vector backbone. This work was supported by the Mayo Foundation for Medical Education and Research, by an Avant-Garde Award from the National Institute on Drug Abuse (DP1 DA31340), and by a grant from the Minnesota Partnership for Biotechnology and Medical Genomics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421536112/-/DCSupplemental.
References
- 1.Silver A. The Biology of Cholinesterases. North-Holland; Oxford: 1974. [Google Scholar]
- 2.Stedman E, Stedman E. The relative choline-esterase activities of serum and corpuscles from the blood of certain species. Biochem J. 1935;29(9):2107–2111. doi: 10.1042/bj0292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 4.De Vriese C, et al. Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology. 2004;145(11):4997–5005. doi: 10.1210/en.2004-0569. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Orson FM, Kinsey B, Kosten T, Brimijoin S. The concept of pharmacologic cocaine interception as a treatment for drug abuse. Chem Biol Interact. 2010;187(1-3):421–424. doi: 10.1016/j.cbi.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy V, et al. Preclinical studies on neurobehavioral and neuromuscular effects of cocaine hydrolase gene therapy in mice. J Mol Neurosci. 2014;53(3):409–416. doi: 10.1007/s12031-013-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brimijoin S, et al. Anti-cocaine antibody and butyrylcholinesterase-derived cocaine hydrolase exert cooperative effects on cocaine pharmacokinetics and cocaine-induced locomotor activity in mice. Chem Biol Interact. 2013;203(1):212–216. doi: 10.1016/j.cbi.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng L, et al. Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS ONE. 2013;8(6):e67446. doi: 10.1371/journal.pone.0067446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker JJ, et al. Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psychiatry. 2012;71(8):700–705. doi: 10.1016/j.biopsych.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Loo PL, Van Zutphen LF, Baumans V. Male management: Coping with aggression problems in male laboratory mice. Lab Anim. 2003;37(4):300–313. doi: 10.1258/002367703322389870. [DOI] [PubMed] [Google Scholar]
- 11.Spencer SJ, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72(6):457–465. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Lutter M, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, et al. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70(11):1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Duysen EG, et al. Production of ES1 plasma carboxylesterase knockout mice for toxicity studies. Chem Res Toxicol. 2011;24(11):1891–1898. doi: 10.1021/tx200237a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. 2014;19(12):1284–1294. doi: 10.1038/mp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang Y-P. Low-mass molecular dynamics simulation: a simple and generic technique to enhance configurational sampling. Biochem Biophys Res Commun. 2014;452(3):588–592. doi: 10.1016/j.bbrc.2014.08.119. [DOI] [PubMed] [Google Scholar]
- 17.Sun H, et al. Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase. Engineering effective butyrylcholinesterase mutants for cocaine detoxication. J Biol Chem. 2001;276(12):9330–9336. doi: 10.1074/jbc.M006676200. [DOI] [PubMed] [Google Scholar]
- 18.Ekström F, et al. Structure of HI-6*sarin-acetylcholinesterase determined by Xray crystallography and molecular dynamics simulation: Reactivator mechanism and design. PLoS ONE. 2009;4(6):e5957. doi: 10.1371/journal.pone.0005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl) 1978;57(1):47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- 20.Bartke A. Increased sensitivity of seminal vesicles to testosterone in a mouse strain with low plasma testosterone levels. J Endocrinol. 1974;60(1):145–148. doi: 10.1677/joe.0.0600145. [DOI] [PubMed] [Google Scholar]
- 21.De Vriese C, Hacquebard M, Gregoire F, Carpentier Y, Delporte C. Ghrelin interacts with human plasma lipoproteins. Endocrinology. 2007;148(5):2355–2362. doi: 10.1210/en.2006-1281. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Duysen EG, Lockridge O. The butyrylcholinesterase knockout mouse is obese on a high-fat diet. Chem Biol Interact. 2008;175(1-3):88–91. doi: 10.1016/j.cbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 24.Giovambattista A, et al. Direct effect of ghrelin on leptin production by cultured rat white adipocytes. Obesity (Silver Spring) 2006;14(1):19–27. doi: 10.1038/oby.2006.4. [DOI] [PubMed] [Google Scholar]
- 25.Kalra SP, Ueno N, Kalra PS. Stimulation of appetite by ghrelin is regulated by leptin restraint: Peripheral and central sites of action. J Nutr. 2005;135(5):1331–1335. doi: 10.1093/jn/135.5.1331. [DOI] [PubMed] [Google Scholar]
- 26.Kamegai J, et al. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119(1-2):77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Kang K, Zmuda E, Sleeman MW. Physiological role of ghrelin as revealed by the ghrelin and GOAT knockout mice. Peptides. 2011;32(11):2236–2241. doi: 10.1016/j.peptides.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Carlini VP, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299(5):739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8(7):536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 30.Toth M, et al. The neural background of hyper-emotional aggression induced by post-weaning social isolation. Behav Brain Res. 2012;233(1):120–129. doi: 10.1016/j.bbr.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Goliasch G, et al. Butyrylcholinesterase activity predicts long-term survival in patients with coronary artery disease. Clin Chem. 2012;58(6):1055–1058. doi: 10.1373/clinchem.2011.175984. [DOI] [PubMed] [Google Scholar]
- 32.Ben Assayag E, et al. Serum cholinesterase activities distinguish between stroke patients and controls and predict 12-month mortality. Mol Med. 2010;16(7-8):278–286. doi: 10.2119/molmed.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbel Y, et al. Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events. Mol Med. 2014;20(1):38–45. doi: 10.2119/molmed.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderon-Margalit R, Adler B, Abramson JH, Gofin J, Kark JD. Butyrylcholinesterase activity, cardiovascular risk factors, and mortality in middle-aged and elderly men and women in Jerusalem. Clin Chem. 2006;52(5):845–852. doi: 10.1373/clinchem.2005.059857. [DOI] [PubMed] [Google Scholar]
- 35.National Research Council . Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 36.Nishi Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146(5):2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 37.Cornell WD, et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1995;117(19):5179–5197. [Google Scholar]
- 38.Cieplak P, Cornell WD, Bayly C, Kollman PA. Application of the multimolecule and multiconformational RESP methodology to biopolymers: Charge derivation for DNA, RNA, and proteins. J Comput Chem. 1995;16(11):1357–1377. [Google Scholar]
- 39.Jorgensen WL, Chandreskhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79(2):926–935. [Google Scholar]
- 40.Chun S, et al. Apolipoprotein E polymorphism and serum lipoprotein(a) concentrations in a Korean male population. Ann Clin Biochem. 2001;38(Pt 2):129–134. doi: 10.1258/0004563011900434. [DOI] [PubMed] [Google Scholar]
- 41.Parks RJ, et al. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93(24):13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.