Significance
An ancient deep-sea mud-inhabiting 1,800-million-year-old sulfur-cycling microbial community from Western Australia is essentially identical both to a fossil community 500 million years older and to modern microbial biotas discovered off the coast of South America in 2007. The fossils are interpreted to document the impact of the mid-Precambrian increase of atmospheric oxygen, a world-changing event that altered the history of life. Although the apparent 2-billion-year-long stasis of such sulfur-cycling ecosystems is consistent with the null hypothesis required of Darwinian evolution—if there is no change in the physical-biological environment of a well-adapted ecosystem, its biotic components should similarly remain unchanged—additional evidence will be needed to establish this aspect of evolutionary theory.
Keywords: Great Oxidation Event, microbial evolution, null hypothesis, Precambrian microorganisms, sulfur bacteria
Abstract
The recent discovery of a deep-water sulfur-cycling microbial biota in the ∼2.3-Ga Western Australian Turee Creek Group opened a new window to life's early history. We now report a second such subseafloor-inhabiting community from the Western Australian ∼1.8-Ga Duck Creek Formation. Permineralized in cherts formed during and soon after the 2.4- to 2.2-Ga “Great Oxidation Event,” these two biotas may evidence an opportunistic response to the mid-Precambrian increase of environmental oxygen that resulted in increased production of metabolically useable sulfate and nitrate. The marked similarity of microbial morphology, habitat, and organization of these fossil communities to their modern counterparts documents exceptionally slow (hypobradytelic) change that, if paralleled by their molecular biology, would evidence extreme evolutionary stasis.
We here describe a Paleoproterozoic marine deep-water sediment-inhabiting sulfur-cycling microbial community permineralized in cherts of the ∼1.8-Ga Duck Creek Formation of Western Australia, the second such subseafloor biocoenose reported from the geological record. Comparable modern communities, well studied in marine mud, are characterized by the presence of large populations of bacteria that metabolically cycle sulfur and by a low content of dissolved oxygen that, in the subsurface parts of the community, is essentially zero. Physically, they typically are composed of two regions: a quiescent subsurface anoxic zone consisting of an interlaced web-like fabric of randomly oriented and commonly exceedingly long filamentous anaerobic microbes ≤10 μm in diameter and an overlying, surface-to-subsurface zone characterized by the presence of such large-diameter microaerophilic taxa as Thioploca and Beggiatoa. Metabolically, sulfur-cycling in such ecosystems is fueled by seawater sulfate that, in the anoxic zone, is bacterially reduced to hydrogen sulfide; such sulfide can then be oxidized to elemental sulfur, either by nitrate-using anaerobes or by microaerophiles, using dissolved oxygen derived from overlying waters, and the sulfur, in turn, can be microbially oxidized to sulfate.
The Duck Creek biota is ∼500 Ma younger than the first such fossil community discovered, a sulfur-cycling microbial biocoenose recently described from the Turee Creek Group Kazput Formation, also of northwestern Australia (1). Previously considered to be a cyanobacterium-dominated photic-zone assemblage (2, 3) or a deeper water community using iron-based metabolism (4), our reinterpretation of the Duck Creek biota is consistent with a proliferation of sulfur-cycling biocoenoses following the upsurge of biologically available sulfate and nitrate from the ∼2.4- to ∼2.2-Ga Great Oxidation Event, the “GOE” (5). Further, the Duck Creek fossils evidence the “hypobradytely” of early-evolved microbes, an exceptionally low rate of discernable evolutionary change and a term coined (6) to parallel the three other distinct rate distributions in evolution proposed in 1944 by G. G. Simpson (7) on the basis of morphological comparisons of fossil and living taxa. This striking degree of conservatism, documented initially for Proterozoic cyanobacteria (6, 8), is shown here for colorless sulfur bacteria by the marked similarities in habitat, web-like fabric, and organismal morphology and composition of the Duck Creek biota to the ∼2.3-Ga Turee Creek biocoenose and modern anaerobic sulfur-cycling biotas known from subseafloor sediments off the west coast of South America (9, 10).
The two mid-Precambrian communities, closely similar in all salient characteristics, differ markedly from fossil and modern photoautotroph-dominated stromatolitic/microbial mat-forming microbiotas (refs. 11 and 12, Fig. 1). The fossil filaments are also unlike modern iron-metabolizing microbes, whether Fe+2 oxidizing or Fe+3 reducing. That they are neither aerobic iron oxidizers nor anaerobic photosynthetic iron oxidizers is evidenced by their anoxic subseafloor habitat, discussed in Geological Setting and Microbial Composition and Physiology. Their possible affinity to nitrate-dependent and typically rod-shaped anaerobic iron oxidizers (13) is ruled out by their differing morphology and community composition and fabric, as well as by the absence of iron oxide minerals from the fossiliferous cherts. In addition, their lack of similarity to modern anaerobic iron-reducing bacilliform bacteria such as Geobacter and Shewanella, particularly well-studied members of this group (14–16), is shown not only by their filamentous morphology but also by the absence of magnetite and siderite from the fossil-bearing units, principal products of anaerobic iron reduction (17).
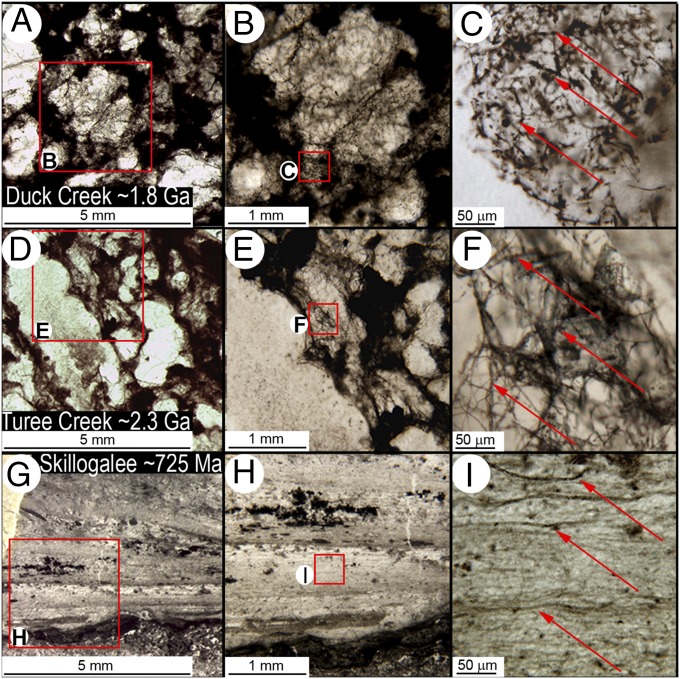
Fig. 1.
Fabrics of the Precambrian fossil sulfur-cycling biotas and a microfossiliferous stromatolite. (A−C) Wispy irregular cobweb-like fabric of the ∼1.8-Ga Duck Creek fossiliferous chert (PPRG sample 062) and (D−F) the closely similar ∼2.3-Ga Turee Creek (Kazput Formation) chert, compared with (G−I) the regularly laminated fabric of a cyanobacterium- (photototroph-) dominated biota permineralized in stromatolitic cherts of the ∼725-Ma South Australian Skillogalee Dolomite. Arrows point to fossil microbial filaments.
On these bases and supporting lines of evidence, we interpret the striking similarities in organismal morphology, community structure, habitat, and evident physiology between the ancient and modern sulfur-cycling biotas as evidencing stasis resulting from adaptation to a physically quiescent subseafloor environment that has remained essentially unchanged over billions of years.
Results
Geological Setting.
The Paleoproterozoic mid-Wyloo Group Duck Creek Formation, overlain conformably in its northern reaches by the June Hill Volcanics and capped to the southwest by strata of the Ashburton Formation, is a 1,000-m-thick sedimentary carbonate platform succession overlying siltstones of the McGrath Formation that disconformably overlie the Neoarchean−Paleoproterozoic Mount Bruce Supergroup (18). The age of the Duck Creek Formation is bracketed by a 2,209 ± 15 Ma U-Pb date on underlying volcanics of the Cheela Springs Basalt and a 1,795 ± 7 Ma U-Pb date on the overlying June Hill Volcanics (4).
The microbial biota reported here, in rocks from Duck Creek Gorge (Fig. S1), occurs in fine-grained black or mottled gray-and-black carbonaceous cherts at two localities from which fossils were first reported in 1983 (19), Precambrian Paleobiology Research Group (PPRG) samples 049−053, collected at Wyloo 1:250,000 map sheet area grid reference 436195 (latitude 22.29.208°S, longitude 116.18.886°E) and 059−062, at grid reference 459195 (lat. 22.29.213°S, long. 116.18.986°E) (20). These fossil-bearing cherts, recollected in 2012 (Fig. S1), are thin (5- to 15-cm-thick) discontinuous lenses enclosed in flat-bedded buff to light brown weathered fine-grained ferruginous dolostone (20).
Although peritidal stromatolites occur in this stratigraphically upward-deepening sedimentary succession (4, 20), the cherts and their encompassing carbonates do not contain such shallow-water indicators as stromatolites, interclasts, cross-lamination, ripple marks, mud cracks, or other desiccation features. The position of the fossiliferous strata in the well-documented sequence stratigraphy of the Duck Creek strata (4) substantiates their deposition in a relatively deep-water facies of the marine basin.
Like the fossil-containing cherts of the ∼2.3-Ga Turee Creek Group (1), we interpret those of the ∼1.8-Ga Duck Creek Formation to have formed beneath storm wave base and likely subphotic zone. The fossil-bearing chert lenses of the two units are similar: In both, they occur in deeper water regions off distally steepened carbonate ramps (1, 4); are dominantly subparallel to the bedding of encompassing carbonates but locally pinch, swell, and transgress bedding planes, attributes supporting their subseafloor formation; and contain carbonate rhombs “floating” in microcrystalline quartz and microbes preserved before cellular disintegration that indicate the lenses to be early diagenetic replacements of carbonate marls.
Microbial Composition and Physiology.
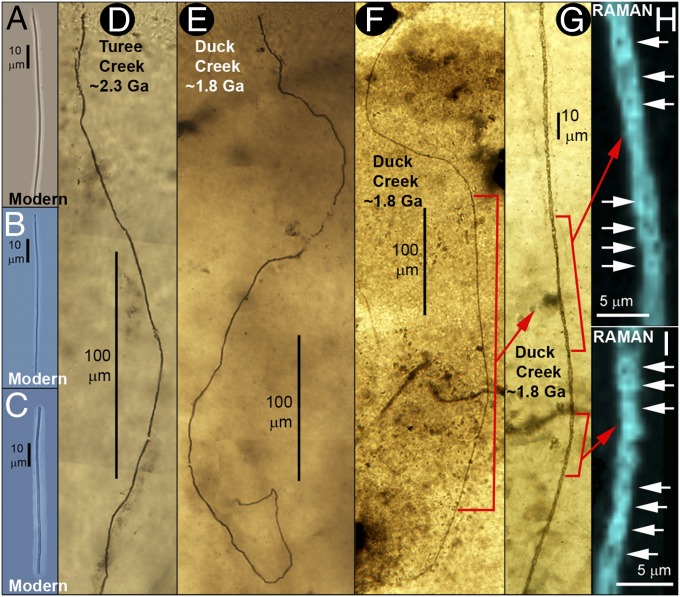
The distinctive, irregular web-like fabric of the Duck Creek biota differs markedly from the layered structure of Precambrian phototroph- (cyanobacterium-) dominated stromatolitic communities (8, 21) but is indistinguishable from that of the ∼500-Ma-older Turee Creek Group sulfur-cycling biota (Fig. 1). In both units, random meshworks of microbial filaments enclose ellipsoidal domains of clear quartz interpreted to represent replaced anhydrite (1). Similarly, the dominant microbial components of the two assemblages are essentially identical: relatively large diameter (∼7- to 9-μm-broad) elongate-celled filaments (Fig. 2 B−D and F−H), smaller diameter (∼1- to 4-μm-wide) filaments composed of bead-shaped cells (Fig. 2 L−U), and very narrow (≤1-μm-diameter) thread-like cellular filaments (Fig. 3 D−I)—morphotypes that differ from phototrophic cyanobacteria (6, 10, 11) but closely resemble principal components of the anoxic subseafloor parts of modern sulfur-cycling communities (9, 10) off the western coast of South America (Figs. 2 and 3), modern communities that exhibit an irregular web-like fabric similar to that of the fossil assemblages (compare Fig. 1 A−F with figure 10 in ref. 10) in which many of the “bead-celled” filaments contain sulfur granules (ref. 10, figures 6 and 7), products of anaerobic sulfide oxidation. Moreover, in all three communities—the Duck Creek, Turee Creek, and modern sulfur-cycling biotas—the randomly interlaced microbial filaments that comprise their web-like fabric (refs. 1, 10, and 15, Fig. 1) can be exceedingly long (Figs. 2 A−D and N−R and 3 D−F), more than a thousand microns in length, lengths not recorded in wave-agitated photic-zone stromatolitic assemblages that further evidence a physically quiescent subseafloor environment.
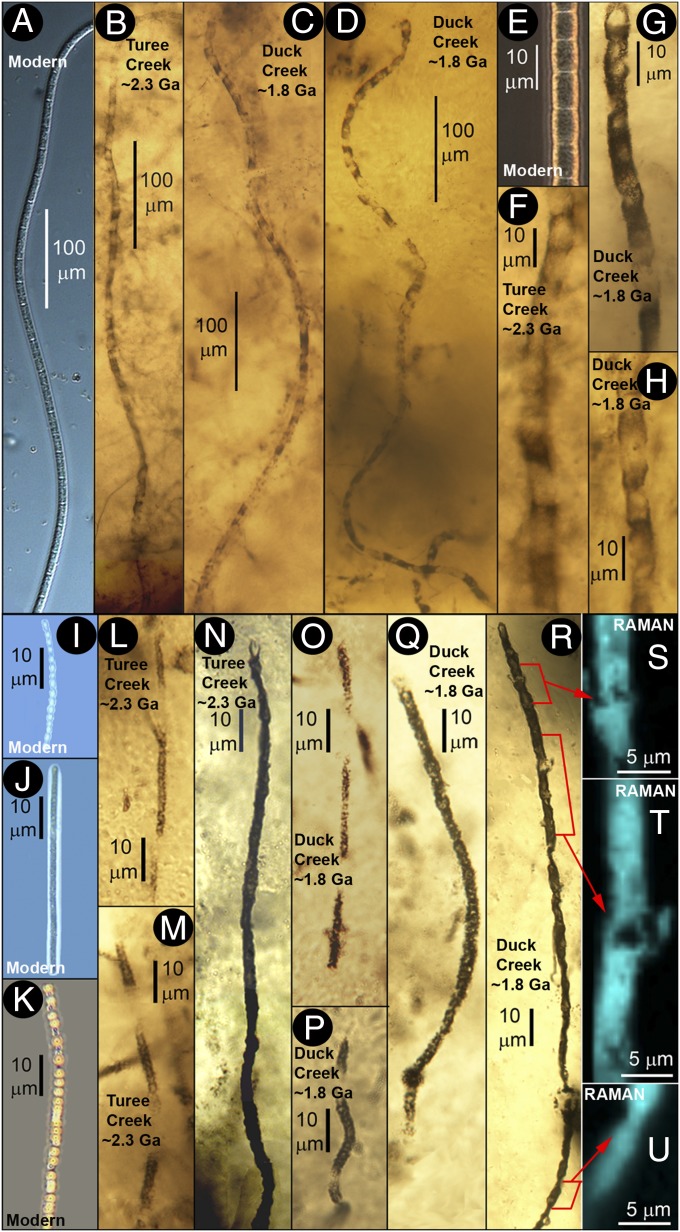
Fig. 2.
Modern and Precambrian filamentous sulfur-cycling microorganisms. (A−E) Broad, ∼7- to 9-μm-diameter modern microbes (9, 10) compared with similar fossils of the ∼2.3-Ga Turee Creek (B and F) and ∼1.8-Ga Duck Creek cherts (C, D, G, and H: PPRG sample 062). (I−K) More narrow, ∼1- to 4-μm-diameter bead-celled (I−K) modern microbes (9, 10) compared with similar fossils of the ∼2.3-Ga Turee Creek (L−N) and ∼1.8-Ga Duck Creek cherts (O−Q, PPRG sample 062; and R−U, PPRG sample 049). (S−U) Raman images of the filament in R, acquired at the ∼1,605 cm−1 band of kerogen (Fig. S5) that establish its carbonaceous (kerogenous) composition (shown in blue).
Fig. 3.
Modern and Precambrian filamentous sulfur-cycling microorganisms. Very narrow, ≤1-μm-diameter modern microbes (A−C) (9, 10) compared with similar fossils of the ∼2.3-Ga Turee Creek (D) and ∼1.8-Ga Duck Creek cherts (E−I; PPRG sample 062). (G) Higher-magnification image of a part of the filament in F, parts of which are shown in Raman images (H and I) acquired at the ∼1,605 cm−1 band of kerogen (Fig. S5) that document its carbonaceous composition (shown in blue) and cellularity (white arrows).
Morphometric comparison of the principal components of the fossil and modern sulfur-cycling communities with oscillatoriacean cyanobacteria, the dominant microorganisms of photic-zone stromatolitic/mat-forming assemblages, documents their dissimilarity (11, 12). The broadest of the fossil sulfur bacteria are ∼8 μm in diameter, composed of 12- to 15-μm-long cells. Such dimensions are highly unusual for cyanobacteria. Of 436 taxa of modern oscillatoriaceans analyzed, only two (<1%) have similar dimensions (11), and of 94 taxa of septate unbranched filaments reported from 69 Proterozoic geological units, only one (Partitiofilum tungusum), preserved in a photic-zone intertidal setting unlike that of the fossil sulfur bacteria, is morphometrically comparable (11, 12). Similarly, the ∼1- to 4-μm-wide bead-celled fossil filaments are of appreciably smaller diameter than Nostoc or other morphologically comparable cyanobacteria and, unlike many such taxa, are enclosed by a prominent cylindrical sheath and lack heterocysts and akinetes (Fig. 2 L−U); and the ≤1-μm-diameter and commonly exceedingly long thread-like fossil bacteria are morphometrically decidedly unlike cyanobacteria, both fossil and modern (11, 12).
For extant prokaryotes, phylogenetic relations and physiological characteristics can be determined by molecular biology backed by growth experiments. For fossil communities, however, the biomolecules required (DNA, rRNA, enzymes, etc.) have been lost during preservation. Thus, analyses of fossil communities such as those described here must rely on evidence provided by proxies—the morphometrics of the preserved fossils backed by studies of their regional and local geological setting and their paleoecology and community fabric.
On the basis of such proxies and the notable similarities in habitat, fabric, and microbial composition of the fossilized biocoenoses of the ∼1.8-Ga Duck Creek and ∼2.3-Ga Turee Creek to the anoxic zone of modern sulfur-cycling communities, we interpret the Duck Creek fossils to represent an anoxic sediment-inhabiting sulfur bacterial biocoenose like that of their modern counterparts in which marine sulfate is microbially reduced to H2S that, by use of nitrate, is then anaerobically oxidized to elemental sulfur and subsequently to sulfate (9, 10, 22).
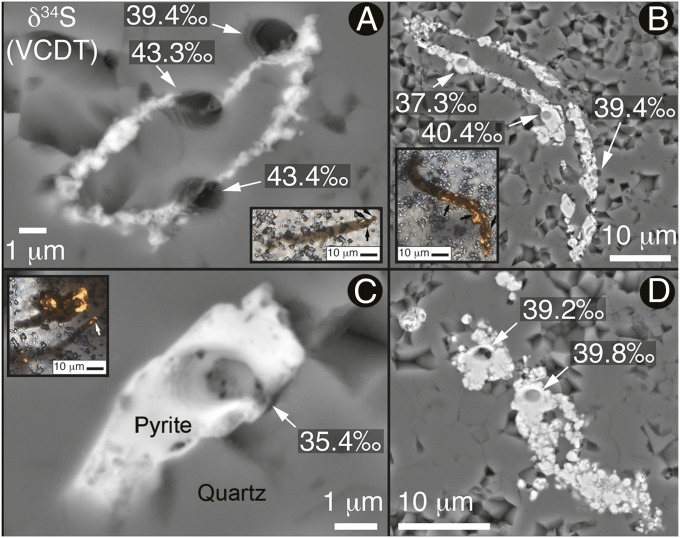
This interpretation is supported by δ34S(Vienna Canyon Diablo Troilite, VCDT) values determined by secondary ion mass spectroscopy (SIMS) analyses of Duck Creek pyritized filaments and associated pyrite, 66 measurements that span a range of >50‰, from −9.4 to +43.4‰ (Fig. S2 and Dataset S1). Of these, values measured on individual Duck Creek pyritized fossils are typically ∼40‰ (Fig. 4). The most plausible explanation for the broad range and virtually uniformly positive δ34S values of the Duck Creek pyrite (Dataset S1) is the precipitation of pyrite by sulfate-reducing bacteria in an environment exhibiting very low seawater sulfate concentrations, like that indicated for other Proterozoic units of comparable age (23), in a relatively closed subseafloor system where microbial sulfur cycling would have been prevalent and/or at a rate similar to that of the diffusion of marine sulfate into the zone of its reduction. Under such conditions, the microbially precipitated pyrite would have the same or similar δ34S as the metabolized seawater sulfate, indicating that the maximum value measured (43.4‰; Fig. 4 and Dataset S1) approximates the δ34S of mid-Precambrian oceanic sulfate (see SI Text, Fig. S2, and Dataset S1).
Fig. 4.
Sulfur isotopic SIMS δ34S(VCDT) analyses of Duck Creek pyrite. Backscattered electron images of pyritic filaments (A−C) and framboidal pyrite (D) (M.R.W. collection 12.06.18.2, compare PPRG samples 049–053). The Insets in A−C, acquired by combined transmitted and reflected light, show filaments plunging beneath the thin section surface; arrows point to SIMS analytical pits.
Paleoecology.
Although data summarized above indicate that both the ∼1.8-Ga Duck Creek and ∼2.3-Ga Turee Creek fossiliferous cherts are benthic and subseafloor, evidently formed beneath storm wave base and likely subphotic zone, there is no reliable geologic index to establish quantitatively their bathymetric setting. Nevertheless, their formation in such an environment is consistent with and supported by both positive and negative evidence.
Positive evidence indicating the fossil assemblages to be subseafloor include the following: Regional geology, sequence stratigraphy, local geology, disposition of the fossil-bearing beds, paleoecologic setting, fabric of the fossiliferous cherts, morphology of the fossils, sulfur isotope data showing the prevalence of strictly anaerobic sulfate reducers in a restricted environment, and the community composition and one-to-one comparison of the morphology of the fossil microbes to principal components of extant anaerobic subseafloor sulfur-cycling communities.
Negative evidence indicating the fossil assemblages not to be shallow-water phototrophic include the following: Regional and local geology (namely, sequence stratigraphy, lack of shallow-water indicators, and the fine-grained petrology of associated carbonates and their lack of clastics), morphometrics of the fossils documenting their dissimilarity from coeval shallow-water photic-zone phototrophs, and sulfur isotope data establishing the prevalence of anaerobic sulfate reducers.
Other lines of evidence further support interpretation of the Duck Creek biota as a sulfur-cycling mud-inhabiting community of anaerobic microbes: (i) Global models suggest that subsurface water masses of the latest Paleoproterozoic were essentially anoxic and in some regions sulfidic, evidenced in the rock record by pyritic shales (23–25). (ii) The fossiliferous Duck Creek chert locally contains copious authigenic pyrite, some encrusting microbial filaments, and a firm indicator of local anoxic conditions (Fig. S3). (iii) The Duck Creek biota, like others of similar age (1, 26–29), contains the asteriform microbe Eoastrion (Figs. S4 and S5) that closely resembles modern Metallogenium, a planktonic microaerophile that inhabits the hypolimnion, where it precipitates MnO2, Fe2O3, and TiO2 in a dysoxic environment (30), encrustations like those of Duck Creek specimens (Fig. S4). (iv) Present also in the Duck Creek (Fig. S4) and other Paleoproterozoic biotas (1, 26–29, 31, 32) are Huroniospora-like unicells morphologically comparable (33) to the free-swimming (34) modern microaerophilic sulfur bacterium Thiovulum that, like Metallogenium, the modern counterpart of Eoastrion, inhabits the oxic−anoxic interface and, as previously interpreted (26), a planktonic microbe evidently deposited from overlying waters. (v) The Duck Creek assemblage, like that of the older Turee Creek biocoenose (1), lacks large-diameter filamentous microbes morphologically comparable to microaerophilic “megabacteria” such as Beggiatoa, present in modern sulfur-cycling biotas at the sediment−water interface, and Thioploca, an extant sulfur cycler that commonly penetrates into underlying anoxic mud (22, 35, 36). The single large-celled partial filament reported from the Duck Creek chert is regarded as possibly cyanobacterial and allochthonous (3), an interpretation suggesting that sulfur-cycling megabacteria may have been of later origin.
Discussion
In the aftermath of the ∼2.4- to ∼2.2-Ga GOE, it is likely that surface waters of the Duck Creek basin were at least weakly oxic (37–40). The increase of atmospheric oxygen at the GOE would have increased the abundance not only of seawater sulfate (5, 25)—precursor of the bacterially generated H2S that, despite competition for reaction with dissolved ferrous iron, would have produced local sulfidic regions (38, 39) that may have promoted the spread of sulfur-cycling communities—but also of nitrate, “oxygenation of the upper parts of the oceans almost certainly [being] accompanied by a shift in the marine chemistry of nitrogen from NH4+-dominated to NO3−-dominated” (ref. 5, pages 3820–3821) with “a complete nitrogen biogeochemical cycle [being] established by about 2.0 Ga” (41, page 349). Such nitrate, diffusing from overlying waters into subseafloor sediments, would have served to fuel anaerobic oxidative phosphorylation in the anoxic zone of sulfur-cycling communities. The GOE-spurred increase of H2S may also have affected photic-zone communities where it would have been consumed by associated sulfur cyclers, by anoxygenic photosynthetic bacteria, and by cyanobacteria capable of facultative (anoxygenic or oxygenic) photosynthesis (42).
Thus, we interpret the microbial biotas of the Turee Creek and Duck Creek cherts to reflect an opportunistic response to the GOE, an interpretation consistent with the metabolic and biosynthetic pathways of modern microbes as well as fossil evidence suggesting the immediately post-GOE appearance of oxygen-protective mechanisms in nostocalean cyanobacteria and the advent of obligately aerobic eukaryotes (43).
How could the seemingly identical sulfur-cycling anoxic sediment-inhabiting biotas of the ∼1.8-Ga Duck Creek and ∼2.3-Ga Turee Creek cherts, like those of Proterozoic stromatolitic cyanobacteria (6, 8), have evidently remained fundamentally unchanged over billions of years?
We suggest differing answers for these two early-evolved hypobradytelic lifestyles:
-
i)
For cyanobacteria, the answer evidently lies in a genetically encoded ecological flexibility derived from their early adaptation to geologically exceedingly slow changes of the photic-zone environment (e.g., of solar luminosity, UV flux, day length, and CO2, O2, and usable sulfur and nitrogen). Because of their large population sizes, global dispersal by ocean currents and hurricanes, and capability to generate oxygen toxic to anaerobic competitors for photosynthetic space, these ecologic generalists adapted to and survived in a wide range of habitats (6).
-
ii)
Once subseafloor sulfur-cycling microbial communities had become established, however, there appears to have been little or no stimulus for them to adapt to changing conditions. In their morphology and community structure, such colorless sulfur bacteria—inhabitants of relatively cold physically quiescent anoxic sediments devoid of light-derived diel signals and a setting that has persisted since early in Earth history—have exhibited an exceedingly long-term lack of discernable change consistent with their asexual reproduction (6).
Given these observations, it might be tempting to interpret such sulfur-cycling communities as evidencing the “negative” null hypothesis of Darwinian evolution—if there is no change in the physical-biological environment of a well-adapted ecosystem, there should be no speciation, no evolution of the form, function, or metabolic requirements of its biotic components—a confirmation of Darwin's theory that seems likely to be provided only by ecosystems fossilized in an environment that has remained essentially unaltered over many hundreds of millions of years.
Although logically required, this aspect of evolutionary theory has yet to be established. Unlike stromatolitic cyanobacteria-dominated biocoenoses, for which evolutionary conservatism has been documented by comparison of more than 120 fossil species and some 24 modern genera in scores of deposits investigated by many workers (6), comparison of fossil sulfur-cycling communities with the anaerobic components of their modern analogs is based only on the two fossil assemblages described here and the subseafloor microbes of modern communities off the west coast of South America (9, 10), none of which has received detailed morphometric, taxonomic, and systemic evaluation.
Moreover, and although, like Simpson's classic rate distributions of evolution (7), the morphology-based “concept of hypobradytely does not necessarily imply genomic, biochemical, or physiological identity between modern and fossil taxa” (6, page 6736), a claim of extreme evolutionary stasis—a lack of speciation over billions of years—would be strengthened not only by discovery of additional fossil communities but by firm evidence of their molecular biology. Although speciation-based evolution occurs at the phenotypic rather than genotypic level of biologic−environmental interaction, the biomolecules underlying such change are not preserved in the rock record in which such assessment can be based only on indirect proxies and inferences of physiology based on isotopic analyses.
For cyanobacterial communities, such problems have been overcome by nearly 50 y of studies that have documented their evolutionary conservatism in many deposits and scores of taxa belonging to diverse cyanobacterial families. In addition, their hypobradytelic evolution is evidenced by numerous proxies—not only the habitat, community fabric, cellular morphology, and isotopic composition of preserved microbes but also their taphonomy, patterns of cell division, modes of colony and filament formation, inferred behavior, and the structure and form of the distinctively layered millimetric to decimetric photic-zone stromatolites and bioherms they produce (e.g., refs. 6, 19–21, 26–31, and 44). A comparable array of data are not available for sulfur-cycling biotas.
Finally, unlike cyanobacteria for which “essentially all of the salient morphological features used in the taxonomic classification of living cyanobacteria can be observed in well-preserved fossils” (45, page 453), sulfur-cycling bacteria are classified primarily on the basis of their (geologically unpreservable) genomic composition. Moreover, large-diameter (“giant”) sulfur bacteria of differing phylogenetic lineages can exhibit similar morphologies and patterns of behavior suggesting convergent evolution of morphologic “look-alikes” adapted to a same or similar function (46, 47). Although it remains to be established whether such morphological “mimicry” is exhibited also by the more narrow ≤10-μm-diameter sulfur bacteria described here—the two modern sulfur bacterial taxa of similar dimensions being aerobes (ref. 46, table 2) rather than anaerobes like the Duck Creek and Turee Creek fossils—it remains conceivable that the marked similarities between the two mid-Precambrian communities and their modern counterparts could be an example of the so-called Volkswagen Syndrome, a lack of change in organismal form that masks the evolution of internal biochemical machinery (6).
We regard it likely that ancient subseafloor microbial biocoenoses will be discovered to fill the gap between the mid-Precambrian and the present and that these communities will be fundamentally similar in their form, function, and metabolic requirements to those of the Duck Creek and Turee Creek cherts. Such findings may eventually be regarded as having confirmed the null hypothesis required of Darwinian evolution, but such an assessment would be, at present, premature.
Methods
Optical Microscopy and Raman Spectroscopy.
Optical studies of all specimens, embedded in petrographic thin sections, were performed using standard techniques. Raman analyses of these specimens were acquired by use of a triple-stage laser-Raman system that has macro-Raman and confocal micro-Raman capabilities (for details, see SI Methods and ref. 48).
Secondary Ion Mass Spectroscopy.
Pyritic specimens were analyzed in petrographic thin sections using newly developed sample holders that provide high precision and accuracy for targets within 8 mm (in comparison with 5 mm for standard sample holders) of the center of the specimen-containing discs (see SI Methods for details).
Supplementary Material
Acknowledgments
We thank J. Shen-Miller, A. K. Garcia, and S. Loyd for reviews of a draft of this manuscript; Thomas N. Taylor for serving as editor of this contribution; and Bo Barker Jørgensen, Timothy W. Lyons, and two anonymous referees for helpful comments on the manuscript submitted. J.W.S. and M.R.W. thank their colleagues in the Precambrian Paleobiology Research Group for collection of the samples studied and acknowledge particularly the late H. J. Hofmann, who, with J.W.S., was first to investigate fossil-bearing cherts from the studied localities. V.A.G. and C.E. thank A. Fonseca and N. Ruiz-Tagle for contributing to data analysis and Fondo Nacional de Desarrollo Científico y Tecnológico (Projects 1070552 and 1110786) and the project International Census of Marine Microbes. A.B.K. was supported by the UCLA Center for the Study of Evolution and the Origin of Life and the Penn State Astrobiology Research Center. M.R.W. acknowledges support by Australian Research Council Discovery Grant DP1093106. M.J.V.K. acknowledges support by the International Commission of Stratigraphy and the University of New South Wales. K.H.W., R.K., and J.W.V. were supported by the University of Wisconsin Astrobiology Research Consortium, funded by the NASA Astrobiology Institute. For his current work at Jet Propulsion Laboratory, K.H.W. acknowledges a grant from NASA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419241112/-/DCSupplemental.
References
- 1.Grice K, et al. 2012 A 2.3 Ga sulfuretum at the GOE: Microfossils and organic geochemistry evidence from the Turee Creek Group, Western Australia. Astrobiology Science Conference 2012 (NASA Astrobiol Program, Atlanta, GA), Abstract 2084. Available at abscicon2012.arc.nasa.gov/abstracts.
- 2.Knoll AH, Barghoorn ES. A gunflint-type microbiota from the Duck Creek dolomite, western Australia. Orig Life. 1976;7(4):417–423. doi: 10.1007/BF00927937. [DOI] [PubMed] [Google Scholar]
- 3.Knoll AH, Strother PK, Rossi S. Distribution and diagenesis of microfossils from the lower Proterozoic Duck Creek Dolomite, Western Australia. Precambrian Res. 1988;38:257–279. doi: 10.1016/0301-9268(88)90005-8. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JP, et al. Geobiology of the late Paleoproterozic Duck Creek Formation, Western Australia. Precambrian Res. 2010;179:135–149. [Google Scholar]
- 5.Holland HD. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim Cosmochim Acta. 2002;66:3811–3826. [Google Scholar]
- 6.Schopf JW. Disparate rates, differing fates: Tempo and mode of evolution changed from the Precambrian to the Phanerozoic. Proc Natl Acad Sci USA. 1994;91(15):6735–6742. doi: 10.1073/pnas.91.15.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson GG. Tempo and Mode in Evolution. Columbia Univ Press; New York: 1944. [Google Scholar]
- 8.Schopf JW. Microflora of the Bitter Springs Formation, Late Precambrian, Central Australia. J Paleontol. 1968;42:651–688. [Google Scholar]
- 9.Gallardo VA, Espinoza C. New communities of large filamentous sulfur bacteria in the eastern South Pacific. Int Microbiol. 2007;10(2):97–102. [PubMed] [Google Scholar]
- 10.Gallardo VA, Espinoza C. Large multicellular filamentous bacteria under the oxygen minimum zone of the eastern South Pacific: A forgotten biosphere. Proc SPIE. 2007;6694:H1–H11. [Google Scholar]
- 11.Schopf JW. In: The Proterozoic Biosphere, A Multidisciplinary Study. Schopf JW, Klein C, editors. Cambridge Univ Press; New York: 1992. pp. 195–218. [Google Scholar]
- 12.Schopf JW. In: The Proterozoic Biosphere, A Multidisciplinary Study. Schopf JW, Klein C, editors. Cambridge Univ Press; New York: 1992. pp. 1123–1166. [Google Scholar]
- 13.Hedrich S, Schlömann M, Johnson DB. The iron-oxidizing proteobacteria. Microbiology. 2011;157(Pt 6):1551–1564. doi: 10.1099/mic.0.045344-0. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan R, Palsson BØ, Lovley DR. In situ to in silico and back: Elucidating the physiology and ecology of Geobacter spp. using genome-scale modelling. Nat Rev Microbiol. 2011;9(1):39–50. doi: 10.1038/nrmicro2456. [DOI] [PubMed] [Google Scholar]
- 15.Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4(10):752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- 16.Fredrickson JK, et al. Towards environmental systems biology of Shewanella. Nat Rev Microbiol. 2008;6(8):592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 17.Roh Y, Moon H-S. Iron reduction by a psychrotolerant Fe(III)-reducing bacterium isolated from ocean sediment. Geosci J. 2001;5:183–190. [Google Scholar]
- 18.Thorne AM, Seymour DB. 1991. Geology of the Ashburton Basin Geol Surv West Aust Bull 139:1–141.
- 19.Hofmann HJ, Schopf JW. In: Earth's Earliest Biosphere: Its Origin and Evolution. Schopf JW, editor. Princeton Univ Press; Princeton, NJ: 1983. pp. 321–360. [Google Scholar]
- 20.Walter MR, Hofmann HJ, Schopf JW. In: Earth's Earliest Biosphere: Its Origin and Evolution. Schopf JW, editor. Princeton Univ Press; Princeton, NJ: 1983. pp. 385–413. [Google Scholar]
- 21.Walter MR, Grotzinger JP. Schopf JW. In: Schopf JW, Klein C, editors. The Proterozoic Biosphere, A Multidisciplinary Study. Cambridge Univ Press; New York: 1992. pp. 253–260. [Google Scholar]
- 22.Jørgensen BB, Gallardo VA. Thioploca spp.: Filamentous sulfur bacteria with nitrate vacuoles. FEMS Microbiol Ecol. 1999;28:301–313. [Google Scholar]
- 23.Shen Y, Knoll AH, Walter MR. Evidence for low sulphate and anoxia in a mid-Proterozoic marine basin. Nature. 2003;423(6940):632–635. doi: 10.1038/nature01651. [DOI] [PubMed] [Google Scholar]
- 24.Canfield DE, Habicht KS, Thamdrup B. The Archean sulfur cycle and the early history of atmospheric oxygen. Science. 2000;288(5466):658–661. doi: 10.1126/science.288.5466.658. [DOI] [PubMed] [Google Scholar]
- 25.Canfield DE. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–453. [Google Scholar]
- 26.Barghoorn ES, Tyler SA. Microorganisms from the Gunflint Chert: These structurally preserved Precambrian fossils from Ontario are the most ancient organisms known. Science. 1965;147(3658):563–575. doi: 10.1126/science.147.3658.563. [DOI] [PubMed] [Google Scholar]
- 27.Walter MR, Goode ADT, Hall WDM. Microfossils from a newly discovered Precambrian stromatolitic iron formation in Western Australia. Nature. 1976;261:221–223. [Google Scholar]
- 28.Knoll AH, Simonson B. Early proterozoic microfossils and penecontemporaneous quartz cementation in the sokoman iron formation, Canada. Science. 1981;211(4481):478–480. doi: 10.1126/science.211.4481.478. [DOI] [PubMed] [Google Scholar]
- 29.Tobin KJ. The paleoecology and significance of the Gunflint-type microbial assemblages of the Frere Formation (Early Proterozoic), Nabberu Basin, Western Australia. Precambrian Res. 1990;47:71–81. [Google Scholar]
- 30.Gregory E, Perry RS, Staley JT. Characterization, distribution, and significance of Metallogenium in Lake Washington. Microb Ecol. 1980;6(2):125–140. doi: 10.1007/BF02010551. [DOI] [PubMed] [Google Scholar]
- 31.Amard B, Bertrand-Sarfati J. Microfossils in 2000 Ma old cherty stromatolites of the Franceville Group, Gabon. Precambrian Res. 1997;81:197–221. [Google Scholar]
- 32.Muir MD. Microfossils from the Middle Precambrian McArthur Group, Northern Territory, Australia. Orig Life. 1974;5(1):105–118. [PubMed] [Google Scholar]
- 33.de BOER W, La Riviere JWM, Houwink AL. Observations on the morphology of Thiovulum majus Hinze. Antonie van Leeuwenhoek. 1961;27:447–456. doi: 10.1007/BF02538470. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Pichel F. Rapid bacterial swimming measured in swarming cells of Thiovulum majus. J Bacteriol. 1989;171(6):3560–3563. doi: 10.1128/jb.171.6.3560-3563.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jørgensen BB, Revsbech NP. Colorless sulfur bacteria, Beggiatoa spp. and Thiovulum spp., in O2 and H2S microgradients. Appl Environ Microbiol. 1983;45(4):1261–1270. doi: 10.1128/aem.45.4.1261-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz HN, Jørgensen BB. Big bacteria. Annu Rev Microbiol. 2001;55:105–137. doi: 10.1146/annurev.micro.55.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Partin CA, et al. Large-scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales. Earth Planet Sci Lett. 2013;369-370:284–293. [Google Scholar]
- 38.Reinhard CT, et al. Proterozoic ocean redox and biogeochemical stasis. Proc Natl Acad Sci USA. 2013;110(14):5357–5362. doi: 10.1073/pnas.1208622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506(7488):307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 40.Planavsky NJ, et al. Earth history. Low mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science. 2014;346(6209):635–638. doi: 10.1126/science.1258410. [DOI] [PubMed] [Google Scholar]
- 41.Thomazo C, Papineau D. Biogeochemical cycling of nitrogen on the early Earth. Elements. 2013;9:345–351. [Google Scholar]
- 42.Cohen Y, Jørgensen BB, Shilo M. Sulphide-dependent anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. Nature. 1975;257:489–492. doi: 10.1128/jb.123.3.855-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schopf JW. Geological evidence of oxygenic photosynthesis and the biotic response to the 2400-2200 ma “great oxidation event.”. Biochemistry (Moscow) 2014;79(3):165–177. doi: 10.1134/S0006297914030018. [DOI] [PubMed] [Google Scholar]
- 44.Schopf JW, Kudryavtsev AB. A renaissance in studies of ancient life. Geol Today. 2010;26:141–146. [Google Scholar]
- 45.Knoll AH, Golubić S. In: Early Organic Evolution. Schidlowski M, Golubić S, Kimberly MM, McKirdy DM, Trudinger PA, editors. Springer; New York: 1992. pp. 450–462. [Google Scholar]
- 46.Salman V, et al. A single-cell sequencing approach to the classification of large, vacuolated sulfur bacteria. Syst Appl Microbiol. 2011;34(4):243–259. doi: 10.1016/j.syapm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Salman V, Bailey JV, Teske A. Phylogenetic and morphologic complexity of giant sulphur bacteria. Antonie van Leeuwenhoek. 2013;104(2):169–186. doi: 10.1007/s10482-013-9952-y. [DOI] [PubMed] [Google Scholar]
- 48.Schopf JW, et al. Gypsum-permineralized microfossils and their relevance to the search for life on Mars. Astrobiology. 2012;12(7):619–633. doi: 10.1089/ast.2012.0827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.