Abstract
Background
During reperfusion of ischemic myocardium nitric oxide (NO) reacts with superoxide radicals to form cardiotoxic peroxynitrite, which causes lipid peroxidation. Our hypothesis was that infusion of a NO donor S-nitroso-N-acetylpenicillamine (SNAP) during ischemia–reperfusion would exacerbate the oxidative damage to the myocardium by increased formation of nitrogen radicals.
Methods and results
In 19 open-chest dogs, left anterior descending (LAD) coronary occlusion (15 min)–reperfusion (15 min) sequences were created. Using electron paramagnetic resonance (EPR), we monitored the coronary sinus concentration of ascorbate free radical (Asc•–), a measure of free radical generation (total oxidative flux). Seven control dogs (Group 1) received intravenous saline infusion during occlusion–reperfusion, while 12 dogs received SNAP infusion (Group 2: 2.5 μg/min per kg SNAP, and Group 3: 5 μg/min per kg SNAP). Left ventricular fractional area shortening was determined by echocardiography. Dogs in Group 3 receiving a high dose of SNAP (5 μg/min per kg) demonstrated a higher Asc•– concentration increase than the control group. Percent fractional area shortening in Group 1 declined from 77±4.0 (baseline) to 54±9.0% during ischemia (P <0.05), and then fully recovered to 74±3.7% with reperfusion. In the SNAP-treated dogs, the percent fractional area shortening during reperfusion was significantly lower than baseline in Group 2 (55±3.9 vs. baseline 74±4.4%, P <0.05) and in Group 3 (49±5.0 vs. baseline 71±4.5%, P <0.01). In five additional dogs, nitrotyrosine immunohistochemistry showed heavy staining of the ischemic–reperfused myocardium.
Conclusions
The NO donor SNAP increased free radical concentration and exacerbated myocardial oxidative damage after ischemia–reperfusion.
Keywords: Nitric oxide, Ischemia, Reperfusion, Free radicals, Ascorbate, Stunning, S-nitroso-N-acetylpenicillamine
1. Introduction
The acute generation of oxygen free radicals during reperfusion of ischemic myocardium contributes to ischemia–reperfusion injury [1–4]. These free radicals can react with nucleic acids, proteins and lipids, causing lipid peroxidation, cellular dysfunction and myocardial stunning [5].
Nitric oxide (NO) is an important mediator of both physiological and pathological vascular function [6]. NO can maintain coronary vasodilator tone, inhibit platelet aggregation and the adhesion of neutrophils and platelets to vascular endothelium, all of which are beneficial effects [7]. However, NO can also be cytotoxic when it is involved in inflammatory reactions [8]. Thus, the role of NO in myocardial ischemia and reperfusion is contro-versial. Several studies have demonstrated that NO has a cardioprotective effect in myocardial ischemia–reper-fusion injury [9–13]. Supplementation of NO production exogenously, such as by infusion of NO precursors (l-arginine) and NO donors during reperfusion, has been found to be cardioprotective in regional [9,10] or global ischemic heart models [11,12]. However, other studies have found that NO exacerbates ischemia–reperfusion injury via the NO–superoxide–peroxynitrite pathways [14–17]. NO synthase (NOS) inhibitors may be cardioproteciive by prevention of peroxynitrite (O=NOO–) formation from NO and superoxide during reperfusion [14–18].
The hypothesis of this study was that increasing NO availability by administering a NO donor would increase oxidative flux and worsen reperfusion injury, thus further establishing the NO–peroxynitrite pathway as an important mechanism of reperfusion injury. Our objective was to show that the NO donor S-nitroso-N-acetylpenicillamine (SNAP), administered before and during a coronary occlusion reperfusion sequence, increases Asc•– generation and worsens LV dysfunction after reperfusion.
2. Methods
2.1. Animal preparation
The study was done in mongrel dogs weighing 15–25 kg. Dogs were anesthetized with Ketamine (20 mg/kg) and Acepromazine (0.2 mg/kg). Sodium pentobarbital at a concentration of 50 mg/ml was used for supplemental intravenous anesthesia as necessary. After tracheal intubation, ventilation was begun with a volume-cycled respirator. The arterial blood gas was maintained in the physiologic range of pH (7.35–7.45) and pO2 > 100 Torr by adjusting the tidal volume, respiratory rate and fraction of inspired oxygen content as necessary.
Under fluoroscopic guidance a 6Fr USCI Gensini catheter was placed via the jugular vein into the right atrium and advanced into the coronary sinus. The electrocardiogram (lead II) and arterial pressure were monitored continuously, using a computerized system, throughout the experiment.
The heart was exposed through a midline sternotomy. A snare was placed around the left anterior descending (LAD) coronary artery for coronary occlusion and reperfusion. Heparin was administered (3000 IU IV bolus followed with intermittent flushes of heparinized saline–10 IU/ml every 30 min) to prevent thrombosis in the indwelling cannulas.
2.2. Electron paramagnetic resonance
Instead of using spin-trapping techniques to demonstrate free radical generation [19,20] we used the ascorbate free radical (Asc•–), a resonance-stabilized tricarbonyl species that is readily formed by the one-electron oxidation of ascorbate, AscH•–. Because of the low reduction potential of the Asc•–/AscH− couple, ascorbate is the terminal small molecule antioxidant [21–24]. Nearly every oxidant, including peroxynitrite, which could be present in a biological system, will bring about the one-electron oxidation of ascorbate [19]. Thus, the concentration of Asc•–, as monitored by electron paramagnetic resonance (EPR), is an excellent measure of the degree of free radical stress (total oxidative flux) in chemical, biochemical, and biologic systems [23].
For this study, we used a method we have previously described in detail [21]. A Varian E-4 spectrometer with a TM110 cavity and an aqueous flat cell was used to monitor Asc•–. Blood from the coronary sinus was directed into the lower end of the flat cell with the aid of Teflon tubing (OD 0.5 mm) and a manifold. The upper end of the flat cell was connected to the femoral vein with a variable speed infusion pump. Thus, coronary venous blood way circulated continuously through the spectrometer at 10 ml/min. In order to scan the arterial blood the manifold was switched from coronary sinus to femoral artery. The blood sample was scanned within 5 s of leaving the vein or artery.
The EPR instrument settings used to acquire the Asc•– spectra were: nominal power 40 mW; modulation amplitude 1 Gauss; time constant 1 s; scan rate = 1 Gauss per 24 s. The spectrum of Asc•– (aH = 1.8 Gauss) is typically a doublet. The peak-to-peak height (in mm) of the Asc•– signal was used to determine radical concentration as previously described using 3-carboxy-proxyl as a standard and accounting for saturation effects [24]. For our experimental conditions, 1 mm of normalized Asc•– signal height corresponds to 0.073 nM Asc•– in the blood being sampled since the volume of blood in the EPR flat cell remains constant.
Without ascorbic acid supplementation, a weak Asc•– signal (about 1 nM) can often be detected in the arterial blood, but not in the coronary sinus blood. In order to obtain an adequate EPR signal, 1 g of 1-ascorbic acid was infused intravenously as a rapid infusion (76.4 mg/min), followed by a slow continuous infusion (usually 15.3–76.4 mg/min). The ascorbate infusion does not cause changes in arterial pressure or heart rate. The Asc•– signal could then be obtained from arterial and coronary venous blood; the arterial Asc•– level was rechecked frequently. The Asc•– concentration varied from animal to animal; the arterial Asc•– signal was usually about 14 nM; the venous Asc•– signal was usually 8 nM. After we obtained a steady state level of arterial Asc•– the infusion rate of ascorbic acid was kept constant through the periods of baseline, occlusion and reperfusion. The negative arterial-venous gradient results from the repair of the ascorbate radical by the nicotinamide adenine dinucleo-tide (NAD) system as red blood cells pass through the myocardium. We maintained a steady-state level of arterial Asc•– by adjusting the rate of ascorbic acid infusion (usually 15.3–76.4 mg/min); the arterial Asc•– level was rechecked frequently. Arterial pH was maintained between 7.35 and 7.45 by respirator adjustments.
2.3. Left ventricular function by echocardiograpy
Two-dimensional transthoracic echocardiograms were obtained to determine the functional injury to the myocardium as a result of the ischemia–reperfusion sequences and to correlate this injury with the ascorbate radical generation. Two-dimensional left ventricular cavity images in the parasternal short axis view were obtained at the papillary muscle level at baseline, after 10 min of coronary occlusion and after 15 min of reperfusion. Left ventricular end-diastolic and endsystolic areas were determined by planimetry by an observer blinded as to treatment group, and the percent area shortening was determined using the formula: enddiastolic area minus end-systolic area divided by enddiastolic area.
2.4. Protocol
One hour before occlusion of the LAD coronary artery, an intravenous infusion of ascorbate was given (1 g bolus followed by 15.3–76.4 mg/min infusion) to amplify the endogenous Asc•– signal throughout the experiment.
Consecutive Asc•– EPR scans were obtained from the femoral artery until the signal amplitude of the spectrum reached a reasonable range. The Asc•– concentration was then monitored from the coronary sinus. We maintained a steady-state Asc•– venous signal throughout the experiment by making adjustments in the ascorbate infusion rate as necessary.
A control group (Group 1, n = 7) received continuous saline infusion from 30 min before coronary occlusion to the end of the ischemia–reperfusion sequence (total 60 mm). Group 2 (n = 6) received a low dose infusion of SNAP (2.5 μg/kg per min) and Group 3 (n = 6) received a high dose infusion of SNAP (5 μg/kg per min), beginning 30 min before coronary occlusion and continuing during the 30 min occlusion–reperfusion sequence.
Once stable Asc•– signals were obtained, a baseline echocardiogram was done. The LAD coronary artery was occluded for 15 min and then released. Reperfusion was documented by resolution of electrocardiographic ST-segment elevation and of myocardial tissue cyanosis. The Asc•– concentration was measured with consecutive EPR scans. The coronary sinus Asc•– signal was monitored from the beginning of the infusion of SNAP at baseline through 30 min of ischemia–reperfusion sequence. The femoral artery Asc•– signal was re-checked to verify Asc•– stability at baseline, prior to occlusion and at 15 min of reperfusion. An echocardio-gram was performed at baseline, during occlusion, and again after 30 min of reperfusion for each animal. If the arterial Asc•– signal varied by more than 15% from baseline values, the data were discarded. Arterial blood gases were obtained at the time of coronary artery occlusion, 10 min after occlusion, and at 5 min of reperfusion. Euthanasia was then achieved by massive intravenous barbiturate overdose.
2.5. Nitrotyrosine immunohistochemistry
In five additional dogs receiving SNAP infusion (5 μg/ kg per min), the myocardium subjected to ischemia–reperfusion sequences were analyzed by immunohisto-chemistry for the presence of nitrotyrosine, a peroxynitration product. After the experiment was completed, the hearts were removed and perfused with a 4% formaldehyde buffer (500 ml). Left ventricular myocar-dial biopsies were obtained from the ischemic area and non-ischemic areas (distal and proximal to the LAD coronary occlusion, respectively), cut into 2 mm sections, and post-fixed for 2 h in formaldehyde buffer. Tissue sections were processed through graded alcohols to paraffin blocks. Sections (4 μm) were cut from each block, mounted on Superfrost histology slides (Fisher Scientific, Pittsburgh, PA). Sections were deparaffinized, rehydrated, and then treated with Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA). After washing, sections were blocked with horse serum for 1 h.
Immunohistochemical staining for nitrotyrosine was performed using a Vectastain ABC-AP kit (Vector Laboratories). Anti-nitrotyrosine (Upstate Biotechnology Inc., Lake Placid, NY) was used at a dilution of 1:1000 and sections were incubated overnight at 4 °C. After washing, the slides were incubated with biotinylated anti-mouse antibody for 30 min, then washed and incubated for 30 min with Vectastain ABC-AP reagent. The next day, tissue sections were rinsed with phosphate-buffered saline and processed for the demonstration of immunoreactive protein by treating slides with alkaline phosphatase substrate solution and counter-staining with nuclear fast red. The presence of immunoreactive protein was assessed microscopically by an independent reviewer.
2.6. Statistical analysis
A repeated measures analysis was used to compare the percent area shortening by echocardiography among the three treatment groups (control, SNAP 2.5 μg/kg per min, and SNAP 5 μg/kg per min). The factors included in the repeated measures analysis were treatment, the between animal factor, and phase (baseline, occlusion, and reperfusion), the repeated within animal factor. The statistical tests that were performed in this analysis involved (1) the pairwise comparison between the treatment groups at each phase and (2) the pairwise comparison between the phases within each treatment group. For these two sets of comparisons, t-test statistics were computed using least squares mean and standard error estimates from the repeated measures analysis. Bonferroni's method was applied to the P-values to adjust for the number of tests performed within each set. A Bonferroni adjusted P-value < 0.05 was considered to be statistically significant. The same analysis was used to compare percent change in Asc•– among the treatment groups. The repeated measures factor for this variable was time (1.5–15 min at 1.5 min increments). The statistical analyses for this study were performed using the sas/stat procedure MIXED [25]. A one-way ANOVA analysis was used to compare Asc•– units (area under curve) among the three groups. Post-hoc test using Tukey's method was then applied to compare the difference in the Asc•– units between any two groups.
3. Results
The hemodynamic parameters are shown in Table 1. There were no differences with respect to baseline systolic and diastolic arterial pressure. Compared with the control group (saline), dogs in the two treated groups demonstrated a significant decline in systolic and diastolic arterial pressure after 30 min infusion of SNAP, as expected.
Table 1.
Arterial pressure response to SNAP or saline (control group) infusion
| Dogs | Systolic arterial pressure (mmHg) |
Diastolic arterial pressure (mmHg) |
||
|---|---|---|---|---|
| Before infusion | After 30-min infusion | Before infusion | After 30-min infusion | |
| Control group 1 | 110.0±8.3 | 106.4±9.5 | 78.6±8.4 | 72.9±8.5 |
| SNAP group 2 (2.5 μg/kg per min) | 113.6±7.6 | 81.0±10.5* | 78.0±8.6 | 54.0±8.4* |
| SNAP group 3 (5 μg/kg per min) | 110.7±4.1 | 75.7±2.8* | 72.9±2.6 | 52.9±2.6* |
Data presented are mean value±S.E.M.
P </0.05 vs. control group.
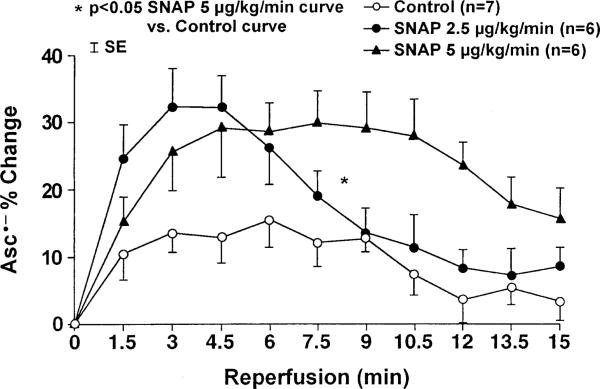
The changes in coronary venous Asc•– concentration during ischemia–reperfusion are summarized in Fig. 1. In the control dogs, the Asc•– concentration increased 15% at 6 min of reperfusion, and then gradually decreased. Dogs receiving the low dose SNAP (2.5 μg/min per kg, Group 2) showed a non-significant increase of Asc•– concentration (32% peak increase) at 3 and 4.5 min of reperfusion. The Group 3 dogs, which received a higher dose of SNAP (5 mg/min per kg), demonstrated a higher (P < 0.05) Asc•– concentration increase during reperfusion for the entire curve, but not for any individual time points compared with the control group. No significant difference in the Asc•– concentration increase between low dose and high dose SNAP groups was seen. The area under the curve of Asc•– units was 95±22 (arbitrary units) in the control dogs versus 171±30 (arbitrary units, P = NS) in Group 2 dogs receiving the low dose of SNAP and 231±40 (arbitrary units, P = 0.02) in Group 3 dogs receiving high dose of SNAP, an overall increase in total myocardial free radical flux of 80% in Group 2 and 143% in Group 3, respectively.
Fig. 1.
Coronary sinus concentration of Asc•– after ischemia reperfusion sequences. The rise in Asc•– concentration was significantly higher (P < 0.05) for the entire curve, but not for any individual time point, in the animals receiving a high dose of the NO donor SNAP (5 μg/min per kg) compared with the control group (P < 0.05).
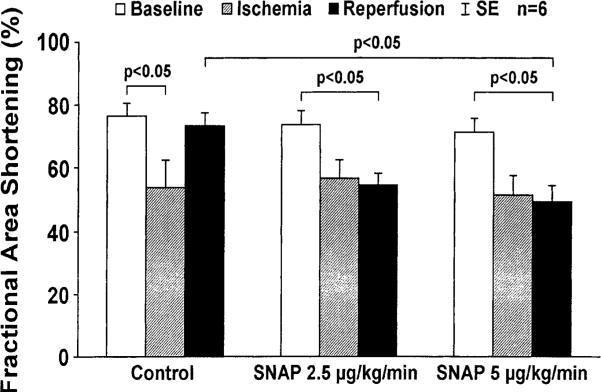
Echocardiographic measured left ventricular fractional area shortening is shown in Fig. 2. The control dogs showed a fall in fractional area shortening from 77±4.0% at baseline to 54±9.0% (P < 0.05 vs. baseline) during ischemia, and then fully recovered to 74±3.7% after reperfusion (P = NS vs. baseline). In the dogs receiving low dose SNAP (Group 2), the fractional area shortening post reperfusion (55±3.9%) was significantly lower than baseline (P < 0.05). In the dogs receiving high dose SNAP (Group 3), a trend toward lower fractional area shortening during occlusion (51±6.3%) was observed compared with baseline (71±4.5%, P < 0.08), and the fractional area shortening then showed a further fall to 49±5.0% after reperfusion (P < 0.01 vs. baseline). Percent fractional area shortening during reperfusion was significantly lower in Group 3 (49±5.0%) compared with the control group (74±3.7%, P < 0.05) and there was a trend toward lower percent fractional area shortening in Group 2 (55±3.9%, P = 0.06 vs. the control group).
Fig. 2.
Fractional area shortening by echocardiography after ischemia and reperfusion. A significant fall in fractional area shortening by echocardiography occurred during ischemia in all animal groups. In animals receiving saline (control group), fractional area shortening fully recovered to baseline after reperfusion; in high dose SNAP-treated group (Group 3, 5 μg/min per kg) post-reperfusion fractional area shortening remained low compared with baseline (P < 0.05).
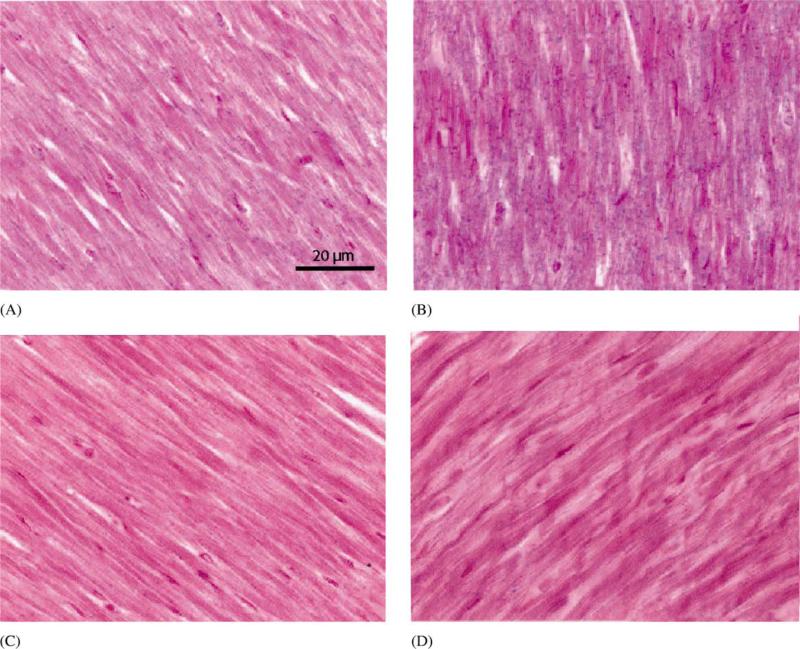
Nitrotyrosine immunohistochemistry was performed as a measure of reactive nitrogen species-mediated vascular injury. In hearts subjected to ischemia–reper-fusion and SNAP infusion, nitrotyrosine staining was markedly greater in the ischemic-reperfused myocardium as compared with non-ischemic myocardium (Fig. 3).
Fig. 3.
Detection of nitrotyrosine in myocardium subjected to an ischemia–reperfusion sequence after SNAP infusion. Nitrotyrosine is indicated by blue stain. (A) non-risk area; (B) risk area; (C) risk area, no primary antibody; (D) control animal (no SNAP given). Sections are counterstained with nuclear fast red. Sections are representative of four of the five animals studied. Nitrotyrosine staining was markedly greater in the risk area (ischemicre-perfused myocardium).
4. Discussion
The major findings of this study are: (1) the NO donor SNAP increases coronary sinus free radical concentration in an ischemia–reperfusion canine model, (2) nitrotyrosine staining confirms that reactive nitrogen species are generated in myocardium subjected to the ischemia–reperfusion sequences, and (3) the increase in free radical concentration is accompanied by exacerbated post-reperfusion left ventricular dysfunction (myocardial stunning) as measured by fractional area shortening.
We used EPR to allow immediate quantitative determination of the Asc•– concentration [21]. This technique provides real-time analysis of total oxidative flux. Previous studies in our laboratory have shown that this technique can be used to continuously quantitate the changes of free radical concentration after ischemia–reperfusion sequences [21,26,27] and after defibrillating shocks [28]. Ascorbate itself is an antioxidant, but it has been shown that exogenous ascorbate, as we administered, does not alter or attenuate myocardial stunning [21]. Furthermore, there is no known direct interaction between NO and ascorbate.
Our present study demonstrated heavy nitrotyrosine staining of the myocardium subjected to an ischemia–reperfusion sequence with SNAP infusion, indicating the generation of peroxynitrite. There was only minimal nitrotyrosine staining of the non-ischemic myocardium. This finding is in agreement with our previous study[18], which showed the NOS inhibitor NG-nitro-l-arginine (l-NNA) decreased the nitrotyrosine staining in ischemic-reperfused myocardium, supporting the role of the NO–peroxynitrite pathway in reperfusion injury.
4.1. Comparison with previous studies of the NO pathway in ischemia–reperfusion injury
The focus of the present study was to investigate the effect of the NO pathway on myocardial stunning after brief ischemia, not after prolonged ischemia producing infarction [29–32]. The conclusions of previous studies of NO and NOS inhibition in models of brief ischemia have varied. Some studies have shown a cardioprotective effect of NO on myocardial stunning. Nitroglycerin (NTG), a precursor of NO, has been reported to attenuate myocardial stunning in open-chest rabbits [33,34]. In conscious dogs subjected to 10 min of ischemia followed by 30 min of reperfusion, Hasebe et al. [35] demonstrated that infusion of the NOS inhibitor l-NNA aggravated myocardial stunning independent of regional perfusion. However, a detrimental role of NO in myocardial stunning has been reported by other studies. Mori et al. [36] have shown that the intracoronary administration of the NO precursor, l-arginine, before reperfusion exacerbates myocardial stunning because of the formation of peroxynitrite. Wang et al. [37] reported that NOS inhibitor l-NAME inhibited NO generation by over 80% and tripled the recovery of contractile function in the isolated perfused rat heart subjected to 30 min of global ischemia. The results of the present study, showing that infusion of the NO donor SNAP worsens the recovery of the LV fractional area shortening, are consistent with a detrimental effect of NO in myocardial stunning.
A cardioprotective effect of NO may result from preservation of endothelial function, prevention of leukocyte adhesion, and promotion of vasodilation. In contrast, a cardiotoxic effect of NO may result from its combination with superoxide after ischemia–reperfusion sequences to generate toxic peroxynitrite. In an isolated perfused rat heart model subjected to 20 min of ischemia and 30 min of reperfusion. Yasmin et al. [16] demonstrated the acute production of peroxynitrite during reperfusion in the ischemic heart; the NOS inhibitor l-NAME caused improvement in post-ischemic function. Liu et al. [38] measured levels of NO, superoxide and peroxynitrite in rats subjected to myocardial ischemia (20 min) and reperfusion (5 h) and concluded that elevated NO, superoxide and peroxynitrite played a critical role in the pathogenesis of reperfusion injury.
4.2. Limitations
We performed only brief coronary artery occlusions (15 min) in this study to avoid causing myocardial infarction. Shorter or longer coronary durations of coronary occlusion might yield different results. Similarly, periods of reperfusion lasting several hours or more might produce more recovery of contractile function than the 15 min recovery period we studied.
SNAP was administered prior to and during coronary occlusion–reperfusion. We do not know what effect SNAP would have if given only upon reperfusion. Only two doses of SNAP were selected for this study. The SNAP dose was based on previously published studies [10,39] and it was arbitrary. Our conclusions are only applicable to these two doses of SNAP.
In the present study, SNAP lowered the arterial pressure to the lower autoregulatory limits of the heart (~ 76/53 mmHg, calculated mean arterial pressure of 61). Therefore, part of the LV dysfunction seen with SNAP administration could simply be due to incomplete reperfusion, secondary to hypotension and failure of autoregulation.
The Asc•– technique described in the present study cannot measure the concentration of individual radicals such as the superoxide and hydroxyl radical. Instead, the ascorbate technique yields information on total oxidative flux, an indicator of oxidative stress.
5. Conclusions
In conclusion, infusion of the NO donor SNAP increased total oxidant flux during early reperfusion; the increase in total oxidant flux was accompanied by impaired recovery of LV function. The NO–peroxynitrite pathway plays an important role in ischemia–reperfusion injury.
Acknowledgements
Supported by AHA grant #0020656Z (Y.Z.), NHLBI grants HL53284 (R.E.K.), HL62984 (F.J.M.), HL03669 (F.J.M.) and CA84462 (G.R.B., S.M.M.).
Abbreviations
- Asc•–
ascorbate free radical
- EPR
electron paramagnetic resonance
- LAD
left anterior descending
- NO
nitric oxide
- NOS
NO synthase
- O=NOO–
peroxynitrite
- SNAP
S-nitroso-N-acetylpenicillamine
References
- 1.Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992;26:101–8. doi: 10.1093/cvr/26.2.101. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991;5(Suppl. 2):249–68. doi: 10.1007/BF00054747. [DOI] [PubMed] [Google Scholar]
- 3.Kramer JH, Misik V, Weglicki MB. Lipid peroxidation-derived free radical production and postischemic myocardial reperfusion injury. Ann New York Acad Sci. 1994;723:180–96. [PubMed] [Google Scholar]
- 4.Kukreja RC, Hess ML. The oxygen free radical system: from equations through membrane-protein interaction to cardiovascular injury and protection. Cardiovasc Res. 1992;26:641–55. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R. Basic and clinical aspects of myocardial stunning. Prog Cardiovasc Dis. 1998;40:477–516. doi: 10.1016/s0033-0620(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 6.Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit Care Med. 2000;28(Suppl.):N37–52. doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. New Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 8.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–56. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 9.Weyrich AS, Ma XL, Lefer AM. The role of L-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation. 1992;86:279–88. doi: 10.1161/01.cir.86.1.279. [DOI] [PubMed] [Google Scholar]
- 10.Shinmura K, Tang XL, Takamo H, et al. Nitric oxide donors attenuate myocardial stunning in conscious rabbits. Am J Physiol. 1999;277:112495–503. doi: 10.1152/ajpheart.1999.277.6.H2495. [DOI] [PubMed] [Google Scholar]
- 11.Sato H, Zhao ZQ, McGee D, et al. Supplemental L-arginine during cardioplegic arrest and reperfusion avoids regional post-ischemic injury. J Thorac Cardiovasc Surg. 1995;110:302–14. doi: 10.1016/S0022-5223(95)70226-1. [DOI] [PubMed] [Google Scholar]
- 12.Amrani M, Chester AH, Jayakumar J, et al. L-argininc reverses low coronary reflow and enhances postischemic recovery of cardiac mechanical function. Cardiovasc Res. 1995;30:200–4. [PubMed] [Google Scholar]
- 13.Lefer AM. Attenuation of myocardial ischemia-reperfusion injury with nitric oxide replacement therapy. Ann Thorac Surg. 1995;60:847–51. doi: 10.1016/0003-4975(95)00423-I. [DOI] [PubMed] [Google Scholar]
- 14.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad and the ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 15.Muijsers RBR, Folkerts G, Henricks PAJ, et al. Peroxynitrite: a two-faced metabolite of nitric oxide. Life Sci. 1997;60:1833–45. doi: 10.1016/s0024-3205(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 16.Yasmin W, Strynadka K, Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion in isolated rat hearts. Cardiovasc Res. 1997;33:422–32. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- 17.Naseem SA, Kontos MC, Rao PS, et al. Sustained inhibition of nitric oxide by NG-nitro-L arginine improves myocardial function following ischemia/reperfusion in isolated perfused rate heart. J Mol Cell Cardiol. 1995;27:419–26. doi: 10.1016/s0022-2828(08)80038-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Bissing JW, Xu L, et al. Nitric oxide synthase inhibitors decrease coronary sinus-free radical concentration and ameliorate myocardial stunning in an ischemia-reperfusion model. J Am Coll Cardiol. 2001;38:546–54. doi: 10.1016/s0735-1097(01)01400-0. [DOI] [PubMed] [Google Scholar]
- 19.Bolli R, Patel BS, Jeroudi MO, et al. Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin a-phenyl-N-tertbutyl nitrone. J Clin Invest. 1988;82:476–85. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JE, Felix CC, Olinger GN, et al. Myocardial ischemia and reperfusion: direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci USA. 1988;85:2786–9. doi: 10.1073/pnas.85.8.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma MK, Buettner GR, Spencer KT, et al. Aseorbyl free radical as a real-time marker of free radical generation in briefly ischemia and reperfusion hearts. An electron paramagnetic resonance study. Circ Res. 1994;74:650–8. doi: 10.1161/01.res.74.4.650. [DOI] [PubMed] [Google Scholar]
- 22.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, a-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 23.Buettner GR, Jurkiewiez BA. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic Biol Med. 1993;14:49–55. doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- 24.Buettner GR, Kiminyo KP. Optimal EPR detection of weak nitroxide spin adduct and ascorbate free radical signals. J Biochem Biophys Methods. 1992;24:147–51. doi: 10.1016/0165-022x(92)90054-e. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute . SAS/STAT Software: Changes and Enhancements through Release 6.11. SAS Institute; Cary, NC: 1996. pp. 531–56. Chap. 18. [Google Scholar]
- 26.Lindower PD, Spencer KT, Caterine MR, et al. Prolonged coronary artery occlusion-reperfusion sequences reduce myocar-dial free radical production: an electron paramagnetic resonance study. Am Heart J. 1996;132:1147–55. doi: 10.1016/s0002-8703(96)90457-3. [DOI] [PubMed] [Google Scholar]
- 27.Garcia LA, DeJong SC, Martin SM, et al. Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J Am Coll Cardiol. 1998;32:536–9. doi: 10.1016/s0735-1097(98)00231-9. [DOI] [PubMed] [Google Scholar]
- 28.Caterine MR, Spencer KT, Pagan-Carlo LA, et al. Direct current shocks to the heart generate free radicals: an electron paramagnetic resonance study. J Am Coll Cardiol. 1996;28:1598–609. doi: 10.1016/s0735-1097(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi K, Vinten-Johansen J, Lefer DJ, et al. Intracoronary L-arginine during reperfusion improves endothelial function and reduces infarct size. Am J Physiol. 1992;263:H1650–8. doi: 10.1152/ajpheart.1992.263.6.H1650. [DOI] [PubMed] [Google Scholar]
- 30.Lefer DJ, Nakanishi K, Johnson WE, et al. Antineutrophil and myocardial protection actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion in dogs. Circulation. 1993;88:2337–50. doi: 10.1161/01.cir.88.5.2337. [DOI] [PubMed] [Google Scholar]
- 31.Patel VC, Yellon DM, Singh KJ, et al. Inhibition of nitric oxide limits infarct size in the in situ rabbit heart. Biochem Biophys Res. 1993;194:234–8. doi: 10.1006/bbrc.1993.1809. [DOI] [PubMed] [Google Scholar]
- 32.Woolfson RG, Patel VC, Neild GH, et al. Inhibition of nitric oxide synthesis reduces infarct size by an adenosine-dependent mechanism. Circulation. 1995;91:1545–51. doi: 10.1161/01.cir.91.5.1545. [DOI] [PubMed] [Google Scholar]
- 33.Iwamoto T, Miura T, Urabe K, et al. Effect of nicorandil on post-ischemic contractile dysfunction in the heart: roles of its ATP-sensitive K+ channel opening property and nitrate property. Clin Exp Pharmacol Physiol. 1993;20:595–602. doi: 10.1111/j.1440-1681.1993.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 34.Gross GJ, Pieper GM, Warltier DC. Comparative effects of nicorandil, nitroglycerin, nicotinic acid: and SG-86 on the metabolic status and functional recovery of the ischemic-reper-fused myocardium. J Cardiovasc Pharmacol. 1987;10:S76–84. [PubMed] [Google Scholar]
- 35.Hasebe N, Shen YT, Vatner SF. Inhibition of endothelium-derived relaxing factor enhances myocardial stunning in conscious dogs. Circulation. 1993;88:2862–71. doi: 10.1161/01.cir.88.6.2862. [DOI] [PubMed] [Google Scholar]
- 36.Mori E, Haramaki N, Ikeda H, et al. Intracoronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res. 1998;40:113–23. doi: 10.1016/s0008-6363(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemie heart: evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–30. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 38.Liu P, Hock CE, Nagele R, et al. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol. 1997;272:H2327–36. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- 39.Takano H, Tang XL, Qiu Y, et al. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]