Abstract
A general synthetic route to the first Xantphos nickel alkyne and alkene complexes has been discovered. Various Ni complexes were prepared and characterized. NMR experiments indicate benzonitrile undergoes ligand exchange with a Xantphos ligand of (Xant)2Ni, a compound that was previously believed to be unreactive. The Ni π-complexes were also shown to be catalytically competent in cross coupling and cycloaddition reactions. (Xant)2Ni is also catalytically active for these reactions when activated by a nitrile or coordinating solvent.
Xantphos,1 a bidentate phosphine ligand with a rigid butterfly structure and a large bite angle, is a prolific ligand for a variety of nickel catalyzed reactions including hydrocyanation,2 alkylcyanation,3 cross coupling,4 conversion of ethylene into 1-butene,5 and more recently, cycloaddition.6 One limitation of these reactions and Ni(0) catalyzed reactions7 in general is that the catalyst is formed in situ either from addition of Xantphos to Ni(COD)2 (COD = 1,5-cyclooctadiene) or by reduction of a Ni(II) species in the presence of added Xantphos. Unfortunately, Ni(COD)2 must be handled in a glove box and stored at low temperatures. Furthermore, COD acts as a competitive inhibitor in some of these reactions. The alternative route, namely catalyst formation from a Ni(II) species, typically requires elevated temperatures or addition of a reductant. As such, the need for air- and thermally-stable Ni pre-catalysts that are easily activated is high. The use of LnNi (where L is the desired ligand for catalysis) as a pre-catalyst is not prevalent. In particular, (Xant)2Ni has been avoided as a precatalyst because it is thought to be unreactive owing to its full valence shell and coordination sphere.2c In fact, formation of (Xant)2Ni is generally considered detrimental to catalysis. Not surprisingly, formation of (Xant)2Ni has also thwarted efforts to prepare Xantphos-Ni π-complexes, an important intermediate in a variety of Ni/Xantphos catalyzed reactions.2–6 Herein, we report the serendipitous discovery of a synthetic route to Xantphos Ni π−complexes, an investigation into the mechanism of their formation, and an evaluation of their use as pre-catalysts. We also report the effectiveness of (Xant)2Ni to serve as a viable pre-catalyst in cross-coupling and cycloaddition reactions.
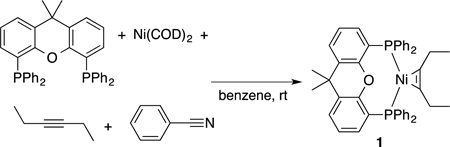 |
(1) |
We recently discovered the combination of Ni and Xantphos is one of the most effective catalysts for the cycloaddition of diynes and nitriles to afford pyridines in excellent yields.6 Unfortunately, this catalyst system does not convert untethered alkynes to the desired pyridines. In our efforts to promote the desired 3-component coupling reaction, we reacted stoichiometric amounts of Ni(COD)2 and Xantphos with an equimolar concentrations of benzonitrile and two equivalents of 3-hexyne (eq 1). Surprisingly, we serendipitously isolated (Xant)Ni-alkyne complex 1 in 88% yield rather than the expected pyridine product that would result from cycloaddition of 3-hexyne with benzonitrile. In contrast, reduction of (Xant)NiBr25 by Zn dust in the presence of 3-hexyne only afforded unidentifiable paramagnetic products.
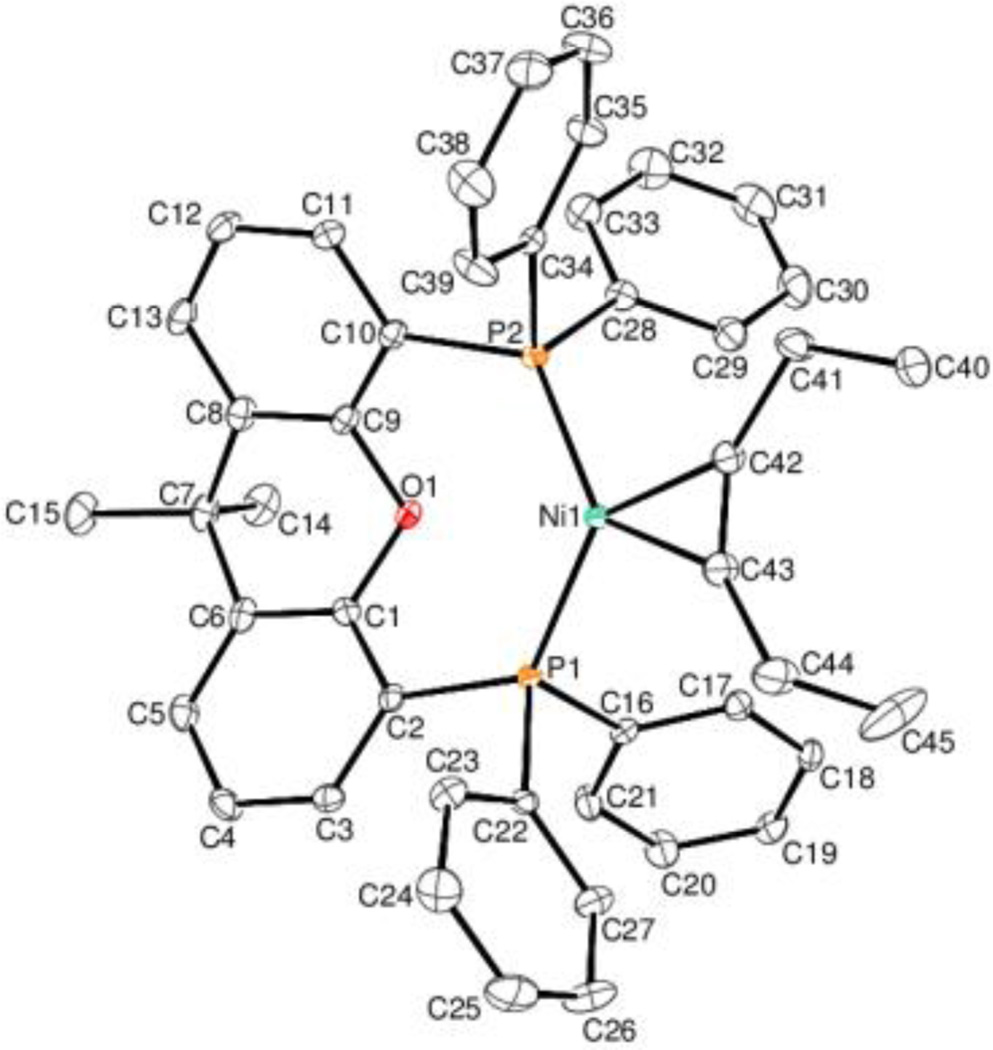
The 1H NMR of (Xant)Ni(3-hexyne) 1 displayed peaks in the aromatic region and a singlet at 1.29 ppm indicative of a Xantphos ligand. In addition, the spectrum displayed a triplet at 1.12 ppm that integrated to six protons as well as a quartet at 2.15 ppm with an integration of four protons, indicating a species with a 1:1 ratio of Xantphos: 3-hexyne. The 13C NMR spectrum included a multiplet at 135.5 ppm, consistent with an alkyne coordinated to Ni(0).8 IR spectroscopy revealed an alkyne peak at 1821 cm−1, which is about 300 cm−1 shifted down from a free alkyne. These data are in accord with a (Xant)Ni(3-hexyne) structure. Crystals of this complex suitable for x-ray crystallography were grown by diffusion of pentane into a benzene solution of the complex (Fig 1). Notably, when 3-hexyne is added to a solution of Ni(COD)2 and Xantphos, only marginal amounts of (Xant)Ni(3-hexyne) is formed in conjunction with copious (Xant)2Ni as observed by 31P NMR.
Fig. 1.
ORTEP diagram of 1. Ellipsoids are set at 35% probability level. Selected bond lengths (Å) and angles (°): Ni(1)-C(42): 1.894(2); Ni(1)-C(43): 1.905(2); C(42)-C(43): 1.265(3); C(42)-Ni(1)-C(43): 38.89(10); C(43)-C(42)-Ni(1): 71.04(15); C(42)-C(43)-Ni(1): 70.07(15).
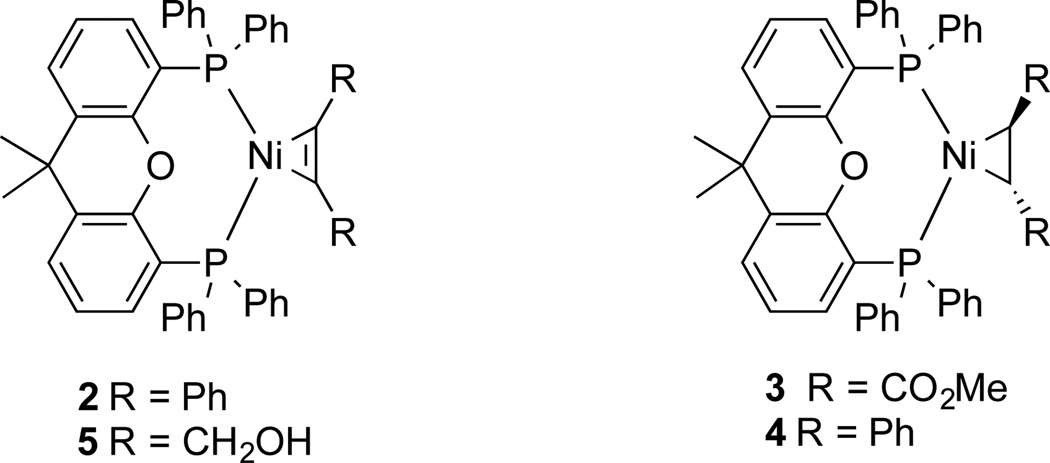
Using the reaction conditions to synthesize 1, Ni π−complexes of diphenylacetylene (2), dimethylfumarate (3), and trans-stilbene (4) were synthesized in 94%, 89%, and 76% yields, respectively (Figure 2). In addition, a Ni-π complex of 2-butyne-1,4-diol (5) was synthesized, albeit in lower yield. The formation of 5 is particularly notable in that the preferred coordination of 2-butyne-1,4-diol is by the alkyne rather than the alcohol -OH.
Fig. 2.
Xantphos Ni π−complexes synthesized from the reaction of Ni(COD)2, Xantphos, 2 equiv. of alkyne or alkene, and 1 equiv. of benzonitrile.
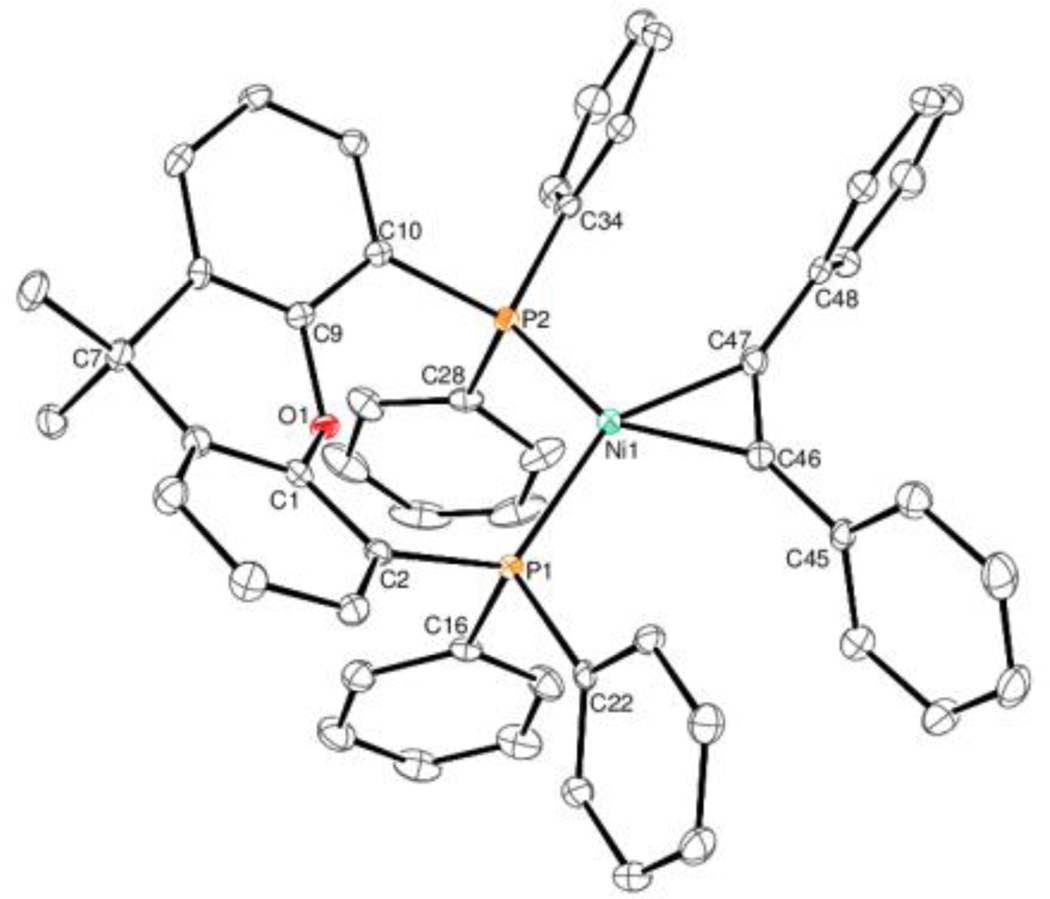
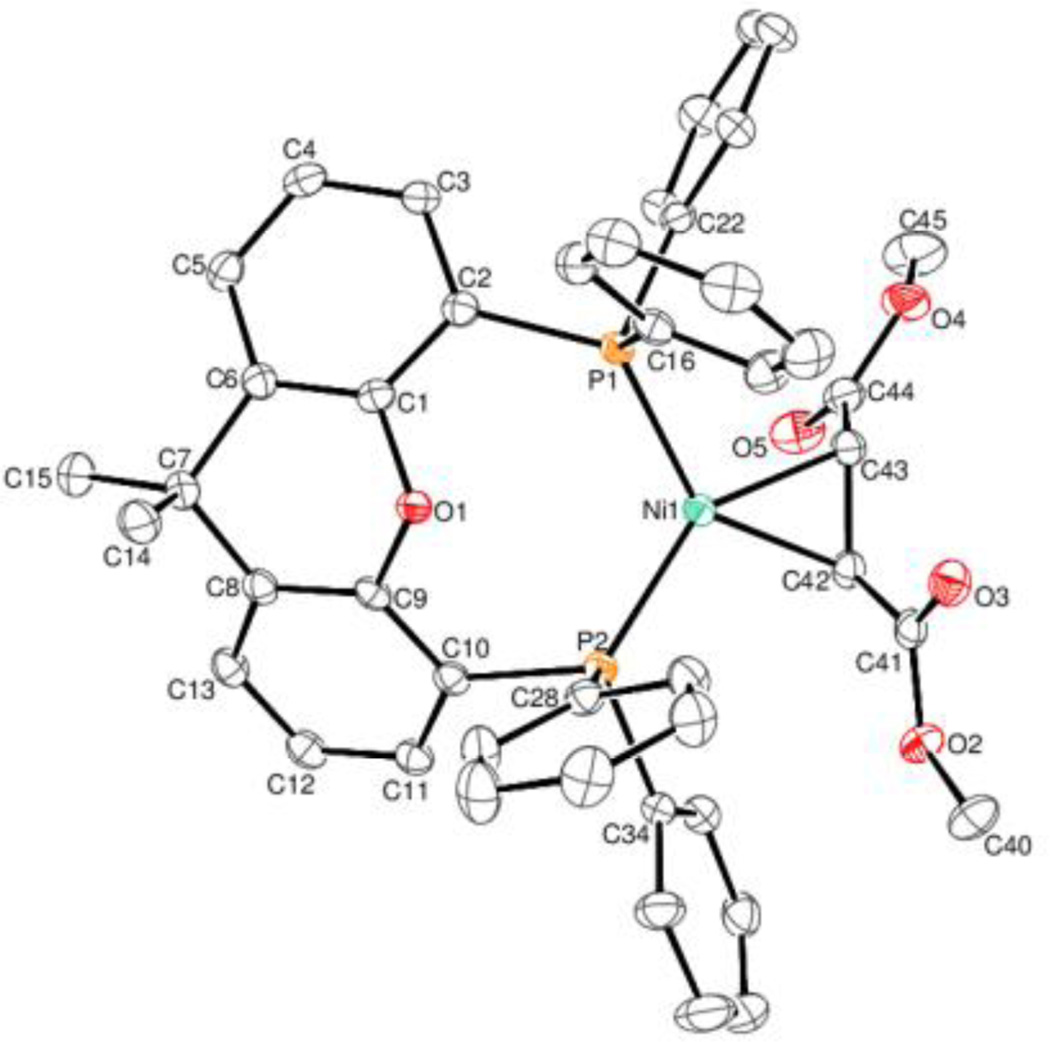
ORTEP diagrams of 2 and 3 are shown in figures 3 and 4. Alkyne complex 1 has a Ni-C(42) bond length of 1.894(2) Å and Ni-C(43) bond length of 1.905(2) Å. Complex 2 has a Ni-C(46) bond length of 1.899(3) Å and Ni-C(47) bond length of 1.895(3) Å. These bond lengths are similar to a class of (dippe)Ni(C2R2) (dippe = 1,2-bis(diisopropylphosphino)ethane) alkyne complexes prepared by Jones8a and (dtbpe)Ni(C2R2) (dtbpe = 1,2- bis(di-tert-butylphosphino)ethane) complexes prepared by Hillhouse.8b Alkene complex 3 has a Ni(1)-C(42) bond length of 1.972(2) and a Ni(1)-C(43) bond length of 1.997(2), which are surprisingly similar Ni-C bond lengths to (IMes)2Ni(dimethylfumarate) (Ni-C bond lengths for this complex are 1.984(2) and 1.988(2)) considering the electronic and steric differences between two IMes ligands and Xantphos.8c Interestingly, the P-Ni-P angle is significantly different for complexes 1, 2, and 3 (angles are 118.92(2)°, 108.83(3)°, and 112.11(2)°, respectively). As the angle approaches Xantphos’ natural bite angle of 108°,1c one phenyl ring on each P atom also comes closer together. In the case of complex 2, the rings containing C16 and C28 have a plane-plane angle of 15.71° and a centroid-centroid distance of 3.754 Å. Each pair of carbon atoms are directly overlapping, indicating a sandwich π-stacking interaction.9 Each diphenylacetylene phenyl ring is also π-stacking with one of the other Xantphos phenyl rings. The rings containing C34 and C48 have a plane-plane angle of 22.94 and a centroid-centroid distance of 3.960. The rings containing C22 and C45 have a plane-plane angle of 19.33 and a centroid-centroid distance of 3.821. The carbon atoms in each ring are not directly overlapping, consistent with a parallel-displaced π-stacking interaction.
Fig. 3.
ORTEP diagram of 2. Ellipsoids are set at 35% probability level. Selected bond lengths (Å) and angles (°): Ni(1)-C(46): 1.899(3); Ni(1)-C(47): 1.895(3); C(46)-C(47): 1.278(4); C(46)-Ni(1)-C(47): 39.36(12); C(47)-C(46)-Ni(1): 70.15(19); C(46)-C(47)-Ni(1): 70.5(2).
Fig. 4.
ORTEP diagram of 3. Ellipsoids are set at 35% probability level. Selected bond lengths (Å) and angles (°): Ni(1)-C(42): 1.972(2); Ni(1)-C(43): 1.997(2); C(42)-C(43): 1.427(3); C(42)-Ni(1)-C(43): 42.14(9); C(43)-C(42)-Ni(1): 69.86(13); C(42)-C(43)-Ni(1): 68.00(12).
We embarked on a series of NMR experiments to evaluate the ability of nitrile to disrupt the formation of the typically unreactive (Xant)2Ni. Not surprisingly, when diphenylacetylene was added to a saturated solution of (Xant)2Ni, no reaction occurred (eq 2). However, when 5 equiv benzonitrile was also added, alkyne complex (as well as free Xantphos) was slowly generated and the reaction ultimately reached equilibrium at 24% of alkyne complex 2 (eq 3). Similarly, when diphenylacetylene was added to a solution of 1 equiv Ni(COD)2 and 1 equiv Xantphos, which has been allowed to pre-coordinate for 2 minutes, only 5% yield of alkyne complex 2 formed while 95% of (Xant)2Ni was produced. Conversely, when diphenylacetylene and 5 equiv of benzonitrile were both added to a solution of 1 equiv Ni(COD)2 and 1 equiv Xantphos, a significant increase in the formation of alkyne complex 2 was observed, and any (Xant)2Ni that formed was completely converted to 2 within 6 hours. Taken together, these data suggests that nitrile undergoes initial ligand displacement of one Xantphos on (Xant)2Ni and subsequently is replaced with alkyne (Scheme 1, vide infra).
Scheme 1.
Ligand exchange leading to formation of (Xant)Ni π−complexes
In some cases, the formation of (Xant)2Ni can be circumvented by changing the order of reactant addition. When diphenylacetylene and Ni(COD)2 were premixed for 5 minutes prior to the addition of Xantphos, only alkyne complex was observed, regardless of whether benzonitrile was added. Similarly, when a solution of Ni(COD)2 was added to a solution of Xantphos and diphenylacetylene, only alkyne complex was observed. In contrast, when trans-stilbene and Ni(COD)2 were premixed for 5 minutes prior to the addition of Xantphos, (Xant)2Ni was initially the major product instead of the desired alkene complex 3 (2:1). This is consistent with a report by Tolman indicating trans-stilbene is less than an order of magnitude better at binding Ni(0) than COD.10 In this case, addition of benzonitrile does facilitate the quantitative formation of Ni π−complex 4. These reactions were repeated with 3-hexyne instead of diphenylacetylene. The same trends were observed, albeit less pronounced than those observed with diphenylacetylene.
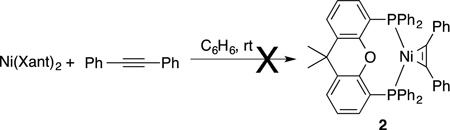 |
(2) |
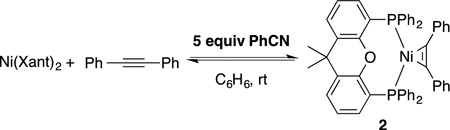 |
(3) |
These NMR experiments suggest the following series of reactions is possible (Scheme 1). When Xantphos is added to Ni(COD)2, Xantphos displaces one COD ligand to form a (Xant)Ni(COD) species (pathway A). The transient (Xant)Ni(COD) species can undergo a second ligand substitution by either Xantphos to form (Xant)2Ni (pathway B) or by an alkyne to form (Xant)Ni(alkyne) (pathway C). Alternatively, Ni(COD)2 itself can also undergo ligand substitution by an alkyne (pathway D) followed by displacement of COD by Xantphos (pathway E) as evidenced by the effects of order of addition of reagents. The (Xant)Ni(alkyne) complex can react with Xantphos to form (Xant)2Ni, (pathway F). If this happens in the absence of nitrile, this reaction is irreversible. However, in the presence of nitrile, (Xant)2Ni is converted to an intermediate nitrile complex which allows the formation of the desired alkyne complex (pathways G1 and G2).
The catalytic activity of complexes 1–5 as well as (Xant)2Ni with and without added benzonitrile in cross coupling were evaluated (Table 1). The title complexes, except 2-butyne-1,4-diol complex 5, successfully catalyzed the cross coupling of 1-bromonaphthalene and vinyl zinc bromide in THF at 50 °C to afford 1-vinylnapthalene in good yields (entries 1–4). Surprisingly, the use of (Xant)2Ni as a catalyst also provided 1-vinylnapthalene in both the presence and absence of added nitrile (entries 6–7). Importantly, product yields are comparable to yields when Xantphos and Ni(acac)2 are employed as catalyst.4 Surprised by the success of (Xant)2Ni in this reaction, we investigated the ability of N-methyl-2-pyrrolidone (NMP), an additive used to keep the vinyl zinc reagent homogenous, to cause Xantphos dissociation from (Xant)2Ni. When diphenylacetylene and NMP were added to a saturated solution of (Xant)2Ni, formation of complex 2 was observed albeit in low yield (i.e., 8%). However, NMP is less effective than benzonitrile as ten times the equivalents of NMP, relative to benzonitrile, was required to achieve even moderate yields of complex 2.
Table 1.
Activity of Complexes in Cross-Couplinga
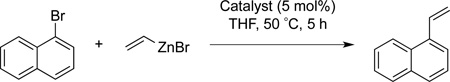 | |||
|---|---|---|---|
| Entry | Catalyst | Conversion (%)b | Yield (%)b |
| 1 | complex 1 | 89 | 86 |
| 2 | complex 2 | 97 | 96 |
| 3 | complex 3 | 97 | 96 |
| 4 | complex 4 | 99 | 99 |
| 5 | complex 5 | 15 | 14 |
| 6 | (Xant)2Ni | 97 | 97 |
| 7 | (Xant)2Ni + PhCNc | 98 | 98 |
Reaction Conditions: 1-bromonapthalene (1 eq, 0.5 M), vinyl zinc bromide (1.75 eq)
Determined by GC compared to naphthalene internal standard
10 mol% PhCN was used
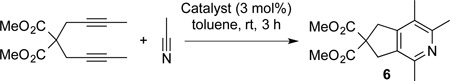
The activity of the complexes in cycloaddition was also assessed. Complexes 1, 2, and 4 (3 mol %) were added to diyne and acetonitrile at room temperature. In each case, high yields of pyridine 6 were obtained (entries 1–2 and entry 4, Table 2). Importantly, these yields are comparable to an isolated yield of 94% when Xantphos and Ni(COD)2 are employed.6 In contrast, complex 3 was not competent in this reaction presumably due to the high affinity of dimethylfumarate for Ni(0) (entry 3). Complex 5, which did not catalyse the cross coupling reaction (vide supra), was also not an effective catalyst for this reaction (entry 5). In addition, (Xant)2Ni did catalyse the cycloaddition with an without added benzonitrile (entries 6 and 7), A higher conversion was observed with added benzonitrile due to activation of the complex, although a slightly lower yield was observed due to incorporation of benzonitrile. It is likely that acetonitrile serves to activate the (Xant)2Ni in the same manner as benzonitrile.
Table 2.
Activity of Complexes in Cycloadditona
 | |||
|---|---|---|---|
| Entry | Catalyst | Conversion (%)b | Yield (%)b |
| 1 | complex 1 | 97 | 97 |
| 2 | complex 2 | >99 | 91 |
| 3 | complex 3 | 6 | 5 |
| 4 | complex 4 | 99 | 91 |
| 5 | complex 5 | 1 | 1 |
| 6 | (Xant)2Ni | 65 | 60 |
| 7 | (Xant)2Ni + PhCNd | 81 | 57 |
Reaction Conditions: Diyne (1 eq, 0.1 M), acetonitrile (1.5 eq)
Determined by GC relative to naphthalene internal standard
10 mol% of benzonitrile was used.
To assess the possibility for using (Xant)Ni-π complexes as air stable pre-catalysts, the stability of complexes 1 were evaluated. To our dismay, when a solution of 1 was exposed to air, the solution turned from yellow to brown with a marked decrease in the intensity of the singlet in 31P NMR compared to internal standard. After 10 minutes only 13% of the complex remained; within 20 minutes complex 1 had completely decomposed. The sensitivity of 1 in the solid state to air was also investigated. Within 10 minutes of exposing 1 in the solid state to air, only a trace amounts of 1 could be observed by 31P and 1H NMR spectroscopy; complete decomposition of 1 was observed after exposing a sample to air for 30 minutes. In contrast, (Xant)2Ni displayed surprising stability to air. This complex was slightly more stable towards air in solution. After 25 minutes in solution, only trace (Xant)2Ni was detected by 31P NMR; no complex was detected after 35 minutes wherein the solution turned from orange to colourless. However, samples of (Xant)2Ni in the solid state that were exposed to atmosphere for up to 8 h remained intact (as examined by 31P NMR spectroscopy). After 12 h, trace Xantphos and Xantphos oxides were detected, and over time the intensity of these peaks slowly increased. Importantly, after exposure to air for 3 days, ~90% of the solid sample was identified as (Xant)2Ni by 31P NMR spectroscopy, indicating this complex is reasonably air-stable as a solid.
In conclusion, we have discovered a method to synthesize (Xant)Ni-alkene and -alkyne complexes, characterized them, and evaluated their catalytic activity. The mechanism of their formation was also investigated. Interestingly, we discovered nitrile is capable of facilitating the dissociation of a Xantphos ligand from (Xant)2Ni for productive alkene or alkyne coordination. However, nitrile is not necessary to form Xantphos Ni π-complexes if a strongly coordinating alkene or alkyne is allowed to pre-coordinate to Ni before Xantphos is added. These complexes showed similar catalytic activity to Xantphos and Ni(COD)2 in cycloaddition of diynes and nitriles as well as to Xantphos and Ni(acac)2 in Negishi coupling. Although (Xant)Ni π–complexes 1–5 are not bench stable, they do not need to be stored at low temperatures like Ni(COD)2, and do not require activation like many Ni(II) pre-catalysts. Of particular interest, despite being Ni(0), (Xant)2Ni was shown to be reasonably air stable as a solid and was found to be a competent pre-catalyst when activated by nitrile or a coordinating solvent. This discovery should aid in the experimental simplicity of future Ni Xantphos catalyzed reactions. We believe that further work with these complexes could lend further insight into how nickel Xantphos catalyzed reactions operate and why Xantphos is an excellent ligand for a variety of transition metal catalyzed reactions.
Supplementary Material
Acknowledgments
We would like to acknowledge the NIH (GM076125), the NSF (0911017), and the DOE for financial support and Dr. Atta Arif at the University of Utah for crystal structure determination.
Footnotes
Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/c000000x/
Notes and references
- 1.For reviews on Xantphos and related ligands in transition metal catalysis see: Kamer PCJ, van Leeuwen PWNM, Reek JNH. Acc. Chem. Res. 2001;34:895. doi: 10.1021/ar000060+. Birkholz M, van Leeuwen PWNM. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2008 Birkholz M, Freixa Z, van Leeuwen PWNM. Chem. Soc. Rev. 2009;38:1099. doi: 10.1039/b806211k.
- 2.a) Kranenburg M, Kamer PCJ, van Leeuwen PWNM, Vogt D, Keim W. J. Chem. Soc. Chem. Commun. 1995:2177. [Google Scholar]; b) Goertz W, Kamer PCJ, van Leeuwen PWNM, Vogt D. Chem. Commun. 1997:1521. [Google Scholar]; c) Goertz W, Keim W, Vogt D, Englert U, Boele Maarten DK, van der Veen LA, Kamer Paul CJ, van Leeuwen PWNM. J. Chem.Soc. Dalton Trans. 1998:2981. [Google Scholar]; d) Goertz W, Kamer PCJ, van Leeuwen PWNM, Vogt D. Chem. Eur. J. 2001:1614. doi: 10.1002/1521-3765(20010417)7:8<1614::aid-chem16140>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Hirata Y, Tanaka M, Yada A, Nakao Y, Hiyama T. Tetrahedron. 2009;65:5037. [Google Scholar]
- 4.Yamamoto T, Yamakawa R. J. Org. Chem. 2009;74:3603. doi: 10.1021/jo900290t. [DOI] [PubMed] [Google Scholar]
- 5.Mora G, Van Zutphen S, Klemps C, Louis JY, Le Floch P. Inorg. Chem. 2007;46:10365. doi: 10.1021/ic701529a. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P, Zhang K, Louie J. Angew. Chemie. Int. Ed. 2011;50:10694. doi: 10.1002/anie.201104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For a reviews on Ni catalysis see Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299. doi: 10.1038/nature13274. Tamaru Y, editor. Modern Organonickel Chemistry. Wiley-VCH; 2005. Montgomery J. Angew. Chemie. Int. Ed. 2004;43:3890. doi: 10.1002/anie.200300634.
- 8.a) Edelbach BL, Lachicotte RJ, Jones WD. Organometallics. 1999;18:4040. [Google Scholar]; b) Waterman R, Hillhouse GL. Organometallics. 2003;22:5182. [Google Scholar]; c) Clement ND, Cavell KJ, Ooi L. Organometallics. 2006;25:4155. [Google Scholar]
- 9.a) Sinnikrot MO, Valeev EF, Sherill CD. J. Am. Chem. Soc. 2002;124:10887. doi: 10.1021/ja025896h. [DOI] [PubMed] [Google Scholar]; b) Sinnokrot MO, Sherrill CD. J. Phys. Chem. A. 2004;108:10200. [Google Scholar]; c) Hunter CA, Sanders JKM. J. Am. Chem. Soc. 1990;112:5525. [Google Scholar]
- 10.a) Tolman CA, Seidel WC. J. Am. Chem. Soc. 1974;96:2774. [Google Scholar]; b) Tolman CA. J. Am. Chem. Soc. 1974;96:2780. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.