Abstract
Allogeneic stem-cell transplantation for patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) has been performed primarily with an HLA matched donor. Outcomes of haploidentical transplantation have recently improved, and a comparison between these donor sources in a uniform cohort of patients has not been performed. We evaluated outcomes of 227 patients with AML/MDS treated with melphalan-based conditioning. Donors were matched related (MRD) (N=87, 38%), matched unrelated (MUD) (N=108, 48%), or haploidentical (N=32, 14%). No significant differences were found between haploidentical and MUD transplant outcomes; however, there was a trend for improved outcomes in the MRD group with a 3-year progression-free survival for patients in remission of 57%, 45% and 41% for MRD, MUD and haploidentical, respectively (P=0.417). Recovery of T-cell subsets was similar for all groups. These results suggest that haploidentical donors can safely extend transplantation for AML/MDS patients without an HLA matched donor. Prospective studies comparing haploidentical and MUD transplants are warranted.
Keywords: myeloablative reduced-intensity conditioning regimen, fludarabine-melphalan, hematopoietic stem cell transplantation, acute myeloid leukemia, myelodysplastic syndromes, haploidentical transplantation, post-transplant cyclophosphamide
Introduction
Hematopoietic stem cell transplantation (HSCT) is an effective treatment for patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS).1,2 Reduced-intensity conditioning regimens emerged as a need to decrease transplant-related toxicity and allow transplantation of older patients or with significant comorbidities.3,4 Our group has developed a fludarabine-melphalan (FM) - based conditioning regimen.5-7 Several studies reported outcomes with FM conditioning for HLA matched related (MRD) and unrelated donor (MUD) transplants.8-13 A modified version of this regimen (including thiotepa) was used for haploidentical transplants (HaploSCT), initially with a T-cell deplete graft,14 then with a T-cell-replete graft and post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate mofetil (MMF) for graft-versus-host-disease (GVHD) prevention, as this latter strategy was associated with lower non-relapse mortality (NRM) with non-myeloablative conditioning.15,16 We previously compared these 2 strategies and showed that T-cell-replete HaploSCT was associated with better immunologic reconstitution, lower treatment-related mortality and improved outcomes, when compared with T-cell-depleted HaploSCT in adult recipients.17
Here we compared outcomes of AML/MDS patients treated with FM-based conditioning at our institution who received either a haploidentical or a fully HLA matched related and unrelated donor, and evaluated immunologic reconstitution in all 3 groups.
Materials and Methods
Patients
All 227 patients with AML/MDS who received an allograft using FM conditioning between 01/2005-09/2012 were included in this study. Eighty-seven patients (38%) received a MRD, 108 (48%) a 10/10 MUD and 32 (14%) a haploidentical transplant. All patients provided written informed consent, and the University of Texas MD Anderson Cancer Center IRB approved this retrospective analysis. Complete remission (CR) was defined as less than 5 percent bone marrow (BM) blasts with neutrophils ≥1×109/L and platelets ≥100×109/L. Cytogenetic risk was classified according to the SWOG risk category.16
Conditioning regimen
All patients received fludarabine (120-160 mg/m2 in 4 daily doses) and melphalan 140 mg/m2 (N=190, 84%) or 100 mg/m2 (N=37, 16%) as a single dose. Thiotepa 5-10 mg/kg was added to haploidentical transplant patients to enhance engraftment (N=32). Older patients and those with major comorbidities received reduced doses of melphalan or thiotepa. GVHD prophylaxis for matched transplants consisted of tacrolimus and mini-methotrexate +/- antithymocyte globulin added for MUD transplants only,9 and post-transplant cyclophosphamide 50 mg/kg on day +3 and +4, tacrolimus and MMF for haploidentical transplants.15 Tacrolimus was started on day -2 for MRD and +5 for haploidentical (to maximize allo-activation, after performing allodepletion with post-Cy, in an immune-suppressive free environment) and MUD transplants, and discontinued after 6 months if no evidence of GVHD. MMF was discontinued at day 100, unless otherwise indicated. All patients received granulocyte colony stimulating factor (GCSF) starting on day +5 or +7, according with the clinical protocol, and standard antimicrobial prophylaxis with fluconazole or voriconazole, pentamidine or trimethoprim-sulfamethoxazole and valacyclovir, for fungal, pneumocystis jiroveci and herpes simplex, respectively.
Hematopoietic progenitor cells were obtained from the bone marrow or GCSF-mobilized peripheral blood progenitor cells collected by apheresis. All donors provided written informed consent. Hematopoietic stem cells procured from unrelated donors were obtained through the National Marrow Donor Program.
Immunologic reconstitution studies
We performed lymphocyte reconstitution on available samples from MRD, MUD and haploidentical transplant recipients between day+30 and +365 post-transplant. The median number of samples analyzed by group per each time point was 14 for haploidentical, 17 for MUD, and 5 for MRD. The antibodies used were CD19-PE, CD8-APC, CD-3PECy7, CD4-PerCP-Cy5.5, CD56-V450, and CD45-V500 (BD Biosciences). At least 10,000 live events in each sample were acquired. Data were analyzed by using FCS Express software (de novo Software, Los Angeles, CA). The percentage of donor chimerism was determined by PCR-based microsatellite polymorphism analysis.
Statistical analysis
Descriptive statistics are presented for all patients as well as by donor type. Categorical measures were assessed using Fisher's exact test while continuous measures were assessed by Kruskal-Wallis test.18 NRM, relapse, and GVHD were assessed by the cumulative incidence (CI) function using the competing risks method. The competing risk included for NRM was relapse while the competing risk included for relapse was death. For GVHD, the competing risks included were relapse and death. The Kaplan-Meier (KM) method was used to estimate overall survival (OS) and progression-free survival (PFS).19 Differences between groups for the CI measures were determined using Gray's test20, whereas the log-rank test was used to assess group differences for OS and PFS. Cox proportional hazards regression models were fit to evaluate prognostic effects of demographic and clinical measures of interest on PFS. 21 All statistical analyses were performed using SAS 9.3 for Windows (Copyright © 2011 by SAS Institute Inc., Cary, NC).
Results
Transplant outcomes based on different donor types
Demographics and characteristics of all treated patients (N=227) are presented in Table 1, and for patients in remission (N=70) before transplant in Table 2. A statistically significant difference between donor types was observed for age, source of stem cells and hematopoietic cell transplantation specific comorbidity index.22 Considering that a significantly higher proportion of patients were in remission in the HaploSCT group compared with the matched groups, we analyzed outcomes for patients in remission in addition to outcomes of all treated patients.
Table 1. Demographics for all patients.
| All patients | By donor type | ||||
|---|---|---|---|---|---|
| Measure | N=227 | MRD (N=87) | MUD (N=108) | Haplo (N=32) | p-value |
|
| |||||
| Age at HSCT (years) | |||||
|
| |||||
| Median (range) | 60 (20-76) | 60 (24-76) | 62 (21-76) | 52 (20-67) | <0.001a |
|
| |||||
| Gender, n (%) | |||||
|
| |||||
| Male | 128 (56) | 52 (60) | 60 (56) | 16 (50) | 0.610b |
|
| |||||
| Female | 99 (44) | 35 (40) | 48 (44) | 16 (50) | |
|
| |||||
| HCT-CI total scores | |||||
|
| |||||
| Median (range) | 3 (0-12) | 3 (0-12) | 3 (0-9) | 1.5 (0-5) | <0.001a |
|
| |||||
| HPC, n (%) | |||||
| Peripheral blood | 143 (63) | 84 (97) | 58 (54) | 1 (3) | <0.001b |
|
| |||||
| Bone marrow | 84 (37) | 3 (3) | 50 (46) | 31 (97) | |
|
| |||||
| Diagnosis, n (%) | |||||
|
| |||||
| AML, MDS/AML | 151 (67) | 58 (67) | 71 (66) | 22 (69) | 0.994b |
|
| |||||
| MDS | 33 (15) | 12 (14) | 17 (16) | 4 (13) | |
|
| |||||
| Secondary | 43 (19) | 17 (20) | 20 (19) | 6 (19) | |
|
| |||||
| Disease status, n (%) | |||||
|
| |||||
| CR | 70 (31) | 25 (29) | 26 (24) | 19 (59) | <0.001b |
|
| |||||
| No CR | 157 (69) | 62 (71) | 82 (76) | 13 (41) | |
| Cytogenetic risk, n (%) | |||||
| n | 220 | 83 | 106 | 31 | |
|
| |||||
| Good | 22 (10) | 7 (8) | 11 (10) | 4 (13) | 0.663b |
|
| |||||
| Intermediate | 110 (50) | 38 (46) | 55 (52) | 17 (55) | |
|
| |||||
| Poor | 88 (40) | 38 (46) | 40 (38) | 10 (32) | |
CR: complete remission; haplo: haploidentical donor; HCT-CI: hematopoietic cell transplantation comorbidity index22; HSCT: (hematopoietic) stem cell transplantation; MEL: melphalan; MRD: matched related donor; MUD: matched unrelated donor; NA: not applicable; n: number.
: Kruskal-Wallis test.
: Fisher's exact test.
Table 2. Demographics for patients in remission prior to transplant.
| All patients | By donor type | ||||
|---|---|---|---|---|---|
| Measure | N=70 | MRD (N=25) | MUD (N=26) | Haplo (N=19) | p-value |
|
| |||||
| Age at HSCT (years) | |||||
|
| |||||
| Median (range) | 57 (22-71) | 59 (25-71) | 62 (24-70) | 55 (22-67) | 0.048a |
|
| |||||
| Gender, n (%) | |||||
|
| |||||
| Male | 36 (51) | 15 (60) | 11 (42) | 10 (53) | 0.452b |
|
| |||||
| Female | 34 (49) | 10 (40) | 15 (58) | 9 (47) | |
|
| |||||
| HCT-CI total scores | |||||
|
| |||||
| Median (range) | 2 (0-9) | 3 (0-6) | 3 (0-9) | 0 (0-4) | 0.002a |
|
| |||||
| HPC, n (%) | |||||
| Peripheral blood | 40 (57) | 23 (92) | 16 (62) | 1 (5) | <0.001b |
|
| |||||
| Bone marrow | 30 (43) | 2 (8) | 10 (38) | 18 (95) | |
|
| |||||
| Diagnosis, n (%) | |||||
|
| |||||
| AML, MDS/AML | 58 (83) | 22 (88) | 21 (81) | 15 (79) | 0.879b |
|
| |||||
| MDS | 6 (9) | 1 (4) | 3 (12) | 2 (11) | |
|
| |||||
| Secondary | 6 (9) | 2 (8) | 2 (8) | 2 (11) | |
|
| |||||
| Disease status, n (%) | |||||
|
| |||||
| CR | NA | NA | NA | NA | NA |
|
| |||||
| No CR | NA | NA | NA | NA | |
| Cytogenetic risk, n (%) | |||||
| n | 67 | 23 | 25 | 19 | |
|
| |||||
| Good | 6 (9) | 1 (4) | 4 (16) | 1 (5) | 0.216b |
|
| |||||
| Intermediate | 40 (60) | 11 (48) | 16 (64) | 13 (68) | |
|
| |||||
| Poor | 21 (31) | 11 (48) | 5 (20) | 5 (26) | |
CR: complete remission; haplo: haploidentical donor; HCT-CI: hematopoietic cell transplantation comorbidity index22; HSCT: (hematopoietic) stem cell transplantation; MEL: melphalan; MRD: matched related donor; MUD: matched unrelated donor; NA: not applicable; n: number.
: Kruskal-Wallis test.
: Fisher's exact test.
Neutrophil engraftment occurred in 99%, 96% and 97% of MRD, MUD and haploidentical patients in the entire cohort, while all patients in remission engrafted with 100% donor cells. The great majority of patients in all groups experienced full donor chimerism at day 30 post-transplant (99%, 97% and 100%, respectively), and this was sustained at day 100 post-transplant. The median time to neutrophil recovery for HaploSCT recipients was 18 days (range 8-21), longer than in MRD - median 13 days (range 10-22) and MUD - median 12 days (range 10-27) (P<0.001), while platelet engraftment was 25 days (range 18-46) for HaploSCT and 14 days (range 10-45) for MUD and 16 days (range 9-42) for MRD transplants (P<0.001). These differences were related to the use of bone marrow stem cells in the HaploSCT group (Table 1).
Clinical outcomes were similar between the 3 donor groups for all patients and for those in remission. PFS for all patients with a MRD, MUD, and haploidentical donor, at 1 and 3 years post-transplant were 52%, 42%, 43% and 36%, 27%, 30% (P=0.120), lower for patients not in remission, respectively 42%, 30%, 10% and 27%, 21%, 10% (P=0.105). For patients in remission, PFS for the MRD, MUD, and haploidentical groups, at 1 and 3 years post-transplant, were 80%, 76%, 64% and 57%, 45%, and 41%, respectively (P=0.417) (Table 3, Figure 1A-B). We have also compared outcomes for patients in remission between haploidentical and the matched transplant group combined (Figure S1). The 1 and 3 year PFS were 78% and 51% in the matched group and 64% and 41% in the haploidentical group (p=0.316), whereas OS at 1 and 3 years was 82% and 56% in the matched group and 77% and 66% in the haploidentical group (p=0.646). A multivariable analysis was also performed for PFS for patients in remission at transplant to determine prognostic effects of specific measures of interest, including age at transplant, disease risk at diagnosis, melphalan dose, and donor type. None of these factors were significantly associated with PFS (Figure 1C).
Table 3. Transplant outcomes for all patients.
| All patients | By donor type | ||||
|---|---|---|---|---|---|
| Measure | N=227 | MRD (N=87) | MUD (N=108) | Haplo (N=32) | |
|
| |||||
| Day 30 chimerism | |||||
|
| |||||
| Donor engraftment | |||||
|
| |||||
| n | 216, | 87 | 98 | 31, | |
|
| |||||
| n (%) | 210 (97) | 86 (99) | 94 (96) | 30 (97) | 0.441a |
|
| |||||
| 100% donor | |||||
|
| |||||
| n | 208, | 85, | 93, | 30, | |
|
| |||||
| n (%) | 204 (98) | 84 (99) | 90 (97) | 30 (100) | 0.798a |
|
| |||||
| CMV reactivation | |||||
|
| |||||
| n | 224 | 87 | 106 | 31 | |
|
| |||||
| n (%) | 121 (54) | 42 (48) | 57 (54) | 22 (71) | 0.098a |
|
| |||||
| EBV reactivation | 0 | 0 | 0 | 0 | - |
|
| |||||
| CI aGVHD 2-4 (%) Day 100 | 30 | 31 | 29 | 29 | 0.709b |
|
| |||||
| CI aGVHD 3-4 (%) Day 100 | 7 | 11 | 6 | 0 | 0.044b |
|
| |||||
| CI cGVHD (Ext+Lim) (%) Year 3 | 34 | 43 | 30 | 19 | 0.094b |
|
| |||||
| CI cGVHD (Ext) (%) Year 3 | 24 | 31 | 21 | 11 | 0.125b |
|
| |||||
| Follow-up survivors Median (range), mo | 31 (0.8–85.4) | 45 (6.4-78.8) | 40 (0.8-85.4) | 13 (1.6-31.2) | <0.001c |
|
| |||||
| CI Relapse (%) | 0.750b | ||||
| Day 100 | 8 | 7 | 8 | 10 | |
| 4 | 0 | 0 | |||
| Year 1 | 26 | 28 | 23 | 33 | |
|
| |||||
| CI Non-relapse mortality (%) | 0.099b | ||||
| Day 100 | 18 | 10 | 26 | 13 | |
| Year 1 | 27 | 20 | 35 | 24 | |
|
| |||||
| Progression free survival (%) | 0.120d | ||||
| Day 100 | 74 | 83 | 65 | 78 | |
| Year 1 | 46 | 52 | 42 | 43 | |
| Year 3 | 31 | 36 | 27 | 30 | |
| Median (95% confidence interval) days | 284 (215, 404) | 411 (237, 757) | 238 (160, 386) | 276 (173, NE) | |
|
| |||||
| 10/10 | 10/10 | 5/10 | |||
a: acute; c: chronic; CI: cumulative incidence; CMV: cytomegalovirus; EBV: Epstein-Barr virus; CR: complete remission; ext: extensive; haplo: haploidentical donor; lim.: limited; Mel: melphalan; mo: month; MRD: matched related donor; MUD: matched unrelated donor;, n: number; NE = not estimable;
: Fisher's exact test.
: Gray's test.
: Kruskal-Wallis test.
: Log-rank test.
Figure 1. Transplant outcomes for recipients of haploidentical, matched related and 10/10 HLA matched unrelated donor transplants.
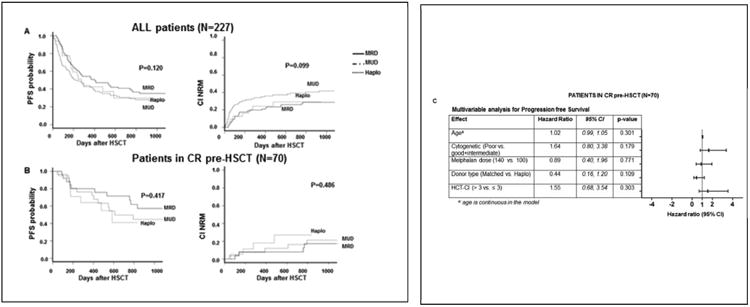
(A) All treated patients and (B) patients in remission at transplant. Progression free survival (PFS), overall survival (OS) and cumulative incidence of non-relapse mortality are shown. C) Multivariable analysis for progression free survival for patients in remission prior to transplant, in relation to age, cytogenetic risk (SWOG), melphalan dose, donor type (matched vs. haploidentical), and HCT-CI.
The incidence of GVHD was also similar between these groups. For patient in remission, the 100-day CI of grade 2-4 aGVHD was 24%, 19% and 26%, while the CI of grade 3-4 aGVHD were 4%, 4%, and 0% for the MRD, MUD, and haploidentical groups, respectively (P=0.685). The CI rates of cGVHD at 3 years post-transplant, limited and extensive, were 46%, 42%, and 24% (P=0.518), and extensive only, were 29%, 23% and 17% (P=0.919) for MRD, MUD haploidentical transplant patients, respectively. NRM for patients in remission at day 100 were 0%, 4%, and 5% and at 1 year, 8%, 8%, and 18% for MRD, MUD, and haploidentical transplant patients, respectively (P=0.486) (Table 3). The incidence of viral reactivation for all 3 groups is presented in Table 3. There was a trend towards higher incidence of CMV reactivation in the haplo group (71% versus 48% for MRD and 54% for MUD groups) while no EBV reactivation or PTLD was observed in any of the 3 groups.
Immunologic reconstitution
We compared recovery of lymphocyte subsets for patients who received a MRD and MUD and haploidentical recipients between day+30 and +365 post-transplant. A detailed representation of immune reconstitution of T-cell subsets for the three donor groups is reported in Figure 2. Regardless of donor type, all patients approached normal CD3+ counts at approximately day 180 post-transplant, with a subsequent steep increase from day 270 to day 365. Median absolute CD3+ count/μL at day 30 were 448 for MRD, 111 for MUD, and 71 for haploidentical (P=0.047), while at day 90 were 252, 365 and 273, at day 180 were 606, 497 and 601, and at day 365 were 1221, 853 and 1576, respectively (P=NS). CD3+CD8+ cell counts predominated at all-time points. No other significant differences in reconstitution of CD3+/CD4+ were found between samples of MRD, MUD and haploidentical transplant patients. NK-cell (CD3-CD56+) reconstitution was similar between the matched and haploidentical groups at days 90, 180, and 270. Matched transplants had a significantly higher median absolute NK-cell numbers very early post-transplant as compared with haploidentical patients [day 30 - 197, 221 and 47/μL, respectively; P=0.019] while haploidentical recipients had the highest median number of NK- cells at day 365 [48, 201, and 387/μL, respectively (P=0.002)]. The median absolute number of CD20+ cells (B-cells)/μL at day 30 were low in all patients and had similar reconstitution in all 3 groups thereafter (Figure 2).
Figure 2. Recovery of T cell subsets for recipients of haploidentical, matched siblings and 10/10 HLA matched unrelated donor transplants.
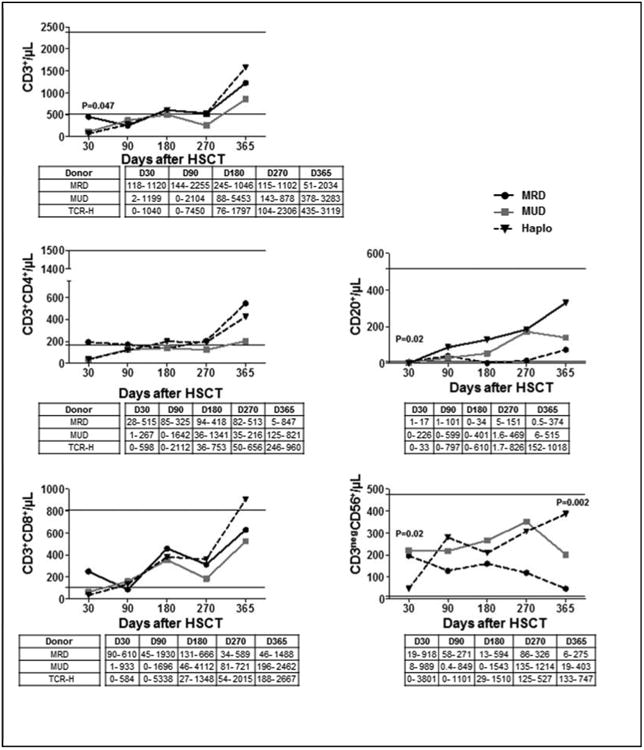
Median number of T-lymphocyte subssets, B cells (CD20+) and Natural Killer cells (CD3negCD56+) are shown for each donor type (horizontal lines indicate reference values), and table displays range value.
Discussion
In the present study we analyzed outcomes of a uniform cohort of AML/MDS patients treated during the same period of time with the same conditioning regimen and compared outcomes for different donor types including matched related, unrelated and haploidentical donors. We found that outcomes of patients transplanted with a haploidentical donor performed with a T-cell-replete graft and post-transplant cyclophosphamide, tacrolimus and MMF for GVHD prophylaxis were comparable to matched transplants treated with conventional GVHD prophylaxis. These findings were noted not only for all patients but also for patients in remission, as more patients in the matched transplant group were not in remission at transplant.
Overall, there was a non-significant trend for improved outcomes with MRD transplants while the survival curves were superimposable for haploidentical and MUD transplants. As previously reported by others and us, cGVHD was lower in the HaploSCT group likely owing to the use of PTCy, consistent with results from another recent study.23 As compared with the recent study from Bashey et al., here we report on results on a homogenous cohort of patients with the same diagnosis and conditioning regimen. In addition, our analysis showed that haploidentical transplantation was not associated with a higher relapse rate, as previously suggested,24 when we compared transplant outcomes with matched transplants for patients with AML/MDS treated with the same conditioning regimen. Burroughs retrospectively compared outcomes of Hodgkin's disease patients treated with non-myeloablative conditioning with haploidentical, matched sibling and unrelated donor transplants.25 In their study, haploidentical recipients experienced lower treatment-related mortality as well as a significant decrease risk in relapse compared to the HLA-matched related and unrelated recipients, with outcomes at least as good as matched transplants.25 Taken together these 2 retrospective studies (including ours), suggest that haploidentical transplants may now offer similar outcomes to matched transplants.
Regarding the role of type/conditioning intensity and outcomes, results from retrospective studies suggest that survival after administration of reduced-intensity conditioning for AML/MDS is comparable to myeloablative regimens, and whether the drug used for conditioning chemotherapy (for example busulfan vs. melphalan), might affect outcome is currently unknown. Other studies reported comparable results for haploidentical transplants using busulfan-based conditioning, suggesting that both melphalan-based or busulfan-based conditioning may be adequate if a more intense conditioning regimen (rather than non-myeloablative) is considered.26
Immune-suppression prophylaxis may diminish the GvL effect, although it can significantly reduce the mortality risk from GvHD. In our study, ATG was added to tacrolimus and mini-methotrexate as GVHD prophylaxis for MUD transplants only. Here, we used a lower dose of rabbit ATG that is unlikely to influence significantly the outcomes as a large CIBMTR study showed that only higher ATG doses (>7 mg/kg) were associated with high incidence of infections and relapse.27
Immunologic reconstitution of lymphocyte subsets was also similar between the 3 groups for all post-transplant time points analyzed, except on day 30, where we have found that MRD transplants reconstituted earlier CD4+ and CD8+ cells compared with the other 2 groups. Interestingly, the use of post-transplant cyclophosphamide in this setting did not appear to delay immune recovery of T cell subsets in the haploidentical transplant group, which has been another concern with this form GVHD prevention. Future studies will need to investigate in depth the immune recovery between transplants with different donors and different forms of GVDH prevention.
Limitations of this study are primarily related to the relatively small number of patients and the retrospective nature of this study. Patients in the haploidentical transplant group were younger, tended to have lower HCT-CI scores then matched transplants and had overall shorter median follow-up. Patients in the MRD group appeared to experience better PFS compared with the haploidentical and MUD groups. Due to a smaller sample size and the potential lack of power, a statistically significant difference was not detected. In addition, while enough samples were available for the MUD and haploidentical groups, the relatively small sample size available to evaluated immune reconstitution in the matched related donor group could have impaired our ability to detect other differences between this group and the other two. However, despite these limitations, our study is the first analysis which compares transplant outcomes and immune reconstitution for patients with AML/MDS in a uniform cohort, treated with the same conditioning regimen during the same period of time. These results suggest that patients with a transplant indication may proceed safely with transplantation using a haploidentical donor as alternative to a matched donor.
In conclusion, our analysis found that outcomes of patients with AML/MDS treated with HLA-haploidentical donors are comparable at least with outcomes of matched unrelated donor transplants. Prospective randomized trials are needed to compare directly these two donor sources, as similar outcomes may allow patients with more aggressive or advanced disease to proceed faster to transplantation.
Supplementary Material
Similar progression free survival, (left panels) overall survival (center panels) and cumulative incidence of non-relapse mortality (right panels) between haploidentical, matched related and 10/10 matched unrelated donors transplants treated with melphalan-based conditioning, for all patients (A) or for patients in complete remission before transplant (B).
Highlights.
Comparable progression-free survival for patients with AML/MDS treated with a haploidentical versus an HLA matched donor
Similar recovery of the immune function post-transplant for haploidentical versus matched related or unrelated donor transplants
Acknowledgments
The authors thank the patients and their families for their support. We acknowledge the excellent contribution to patient care of nurses, physician assistants, pharmacists, research and data coordinators of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center.
Footnotes
Authorship Contributions: AD: contributed with data collection and interpretation, wrote the manuscript; DM performed the statistical analysis, contributed to data analysis and writing of manuscript; AH, LMP, SRP collaborated in data collection and figures preparation; PK, GR and JC contributed with data collection and verification; KR and DAL collaborated in immune-reconstitution data analysis; AA, MHQ, SA,QB,GA, CMH, IFK, BO, PK, UP, EJS, MDL: provided patient care and data analysis; REC and SOC provided patient care, formulated the hypothesis, contributed to data analysis and writing of the manuscript. All the authors, read, reviewed and approved the final version of the manuscript.
Disclosure of Conflicts of Interest: The authors have no conflict of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49(4):511–533. [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. New Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19(12):2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 4.Litzow M, Tarima S, Perez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115(9):1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97(3):631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 6.Champlin R, Khouri I, Shimoni A, et al. Harnessing graft-versus-malignancy: non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111(1):18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 7.Bayraktar UD, Bashir Q, Qazilbash M, et al. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(3):344–356. doi: 10.1016/j.bbmt.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):865–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 9.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13(4):454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Besien K, Kunavakkam R, Rondon G, et al. Fludarabine-melphalan conditioning for AML and MDS:alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15(5):610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popat U, de Lima MJ, Saliba RM, et al. Long-term outcome of reduced-intensity allogeneic hematopoietic SCT in patients with AML in CR. Bone Marrow Transplant. 2012;47(2):212–216. doi: 10.1038/bmt.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Besien K, Stock W, Rich E, et al. Phase I-II study of clofarabine-melphalan-alemtuzumab conditioning for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(6):913–921. doi: 10.1016/j.bbmt.2011.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lima M, Champlin RE, Thall PF, et al. Phase I/II study of gemtuzumab ozogamicin added to fludarabine, melphalan and allogeneic hematopoietic stem cell transplantation for high-risk CD33 positive myeloid leukemias and myelodysplastic syndrome. Leukemia. 2008 Feb;22(2):258–264. doi: 10.1038/sj.leu.2405014. [DOI] [PubMed] [Google Scholar]
- 14.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45(3):429–436. doi: 10.1038/bmt.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luznik L, Jalla S, Engstrom LW, et al. Durable engraftment of major histocompatibility complex-incompatible cells after non-myeloablative conditioning with fludarabine, low-dose total body irradiation, and post-transplantation cyclophosphamide. Blood. 2001;98:3456–64. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell PV, Luznik L, Jones RJ, et al. Non-myeloablative bone marrow transplantation from partially HLA-mismatched related donors using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 17.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T-cell replete graft compared with T-cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman GH, Halton JH. Note on exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(1-2):141–149. [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–202. with discussion. [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 24.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burroughs LM, O'Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Similar progression free survival, (left panels) overall survival (center panels) and cumulative incidence of non-relapse mortality (right panels) between haploidentical, matched related and 10/10 matched unrelated donors transplants treated with melphalan-based conditioning, for all patients (A) or for patients in complete remission before transplant (B).


