Abstract
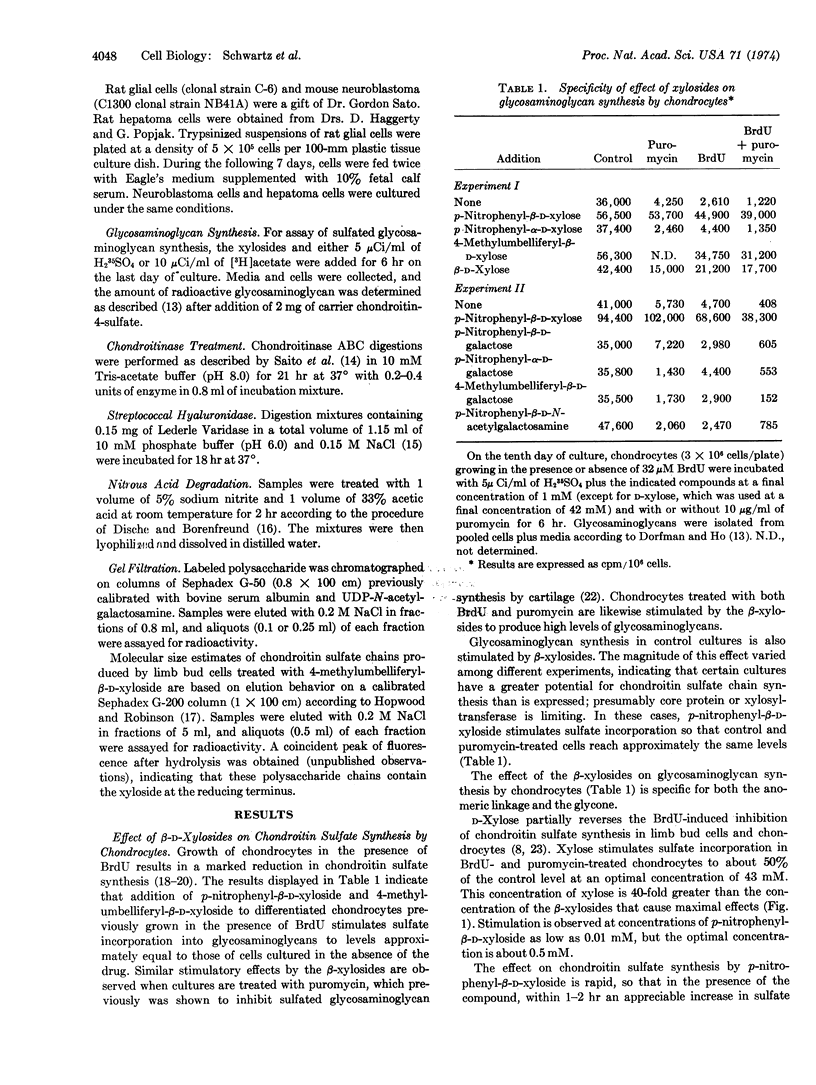
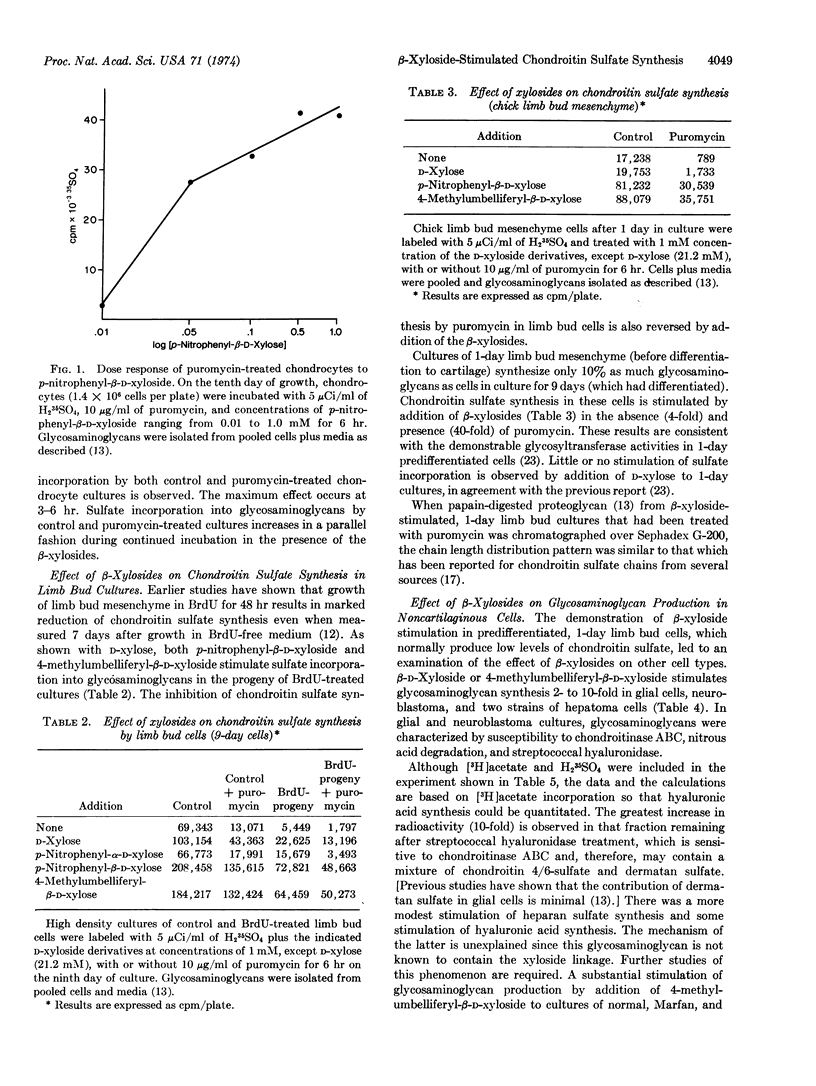
Previous studies have shown that D-xylose partially overcomes the puromycin inhibition of chondroitin sulfate synthesis in cultured chick embryo chondrocytes. Likewise, D-xylose stimulates chondroitin sulfate synthesis by limb bud mesenchyme cells previously treated with BrdU or limb bud cartilage cells treated with puromycin. The studies reported here show that p-nitrophenyl-β-D-xylopyranoside and 4-methyl-umbelliferyl-β-D-xylopyranoside cause a much greater stimulation than does D-xylose and are active at much lower concentrations. In contrast to D-xylose, the xylosides strikingly stimulate chondroitin sulfate synthesis in predifferentiated mesenchyme cells. The xylosides stimulate synthesis of chondroitin sulfate by rat glial cell tumor cells (RC-6), a mouse neuroblastoma (C1300, NB41A), and two strains of cultured rat hepatoma cells (HTC, H4). These results indicate that certain types of nonconnective tissue cells contain the enzymic machinery for synthesis of chondroitin sulfate which is normally not utilized because of limited synthesis of core protein and/or xylosyltransferase. The β-xylosides may be used as a probe of the capacity of various cell types to synthesize sulfated glycosaminoglycans.
Keywords: glycosaminoglycans, cartilage cells, mesenchyme cells, glial cells, hepatoma
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells, V. The effect of 5-bromodeoxyuridine on cloned chondrocytes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1144–1151. doi: 10.1073/pnas.59.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. R., Rodén L., Stoolmiller A. C. Biosynthesis of chondroitin sulfate proteoglycan. Xylosyl transfer to Smith-degraded cartilage proteoglycan and other exogenous acceptors. J Biol Chem. 1972 Jun 25;247(12):3838–3847. [PubMed] [Google Scholar]
- Coleman A. W., Coleman J. R., Kankel D., Werner I. The reversible control of animal cell differentiation by the thymidine analog, 5-bromodeoxyuridine. Exp Cell Res. 1970 Feb;59(2):319–328. doi: 10.1016/0014-4827(70)90606-3. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- Dorfman A., Ho P. L. Synthesis of acid mucopolysaccharides by glial tumor cells in tissue culture. Proc Natl Acad Sci U S A. 1970 Jun;66(2):495–499. doi: 10.1073/pnas.66.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helting T., Rodén L. Biosynthesis of chondroitin sulfate. I. Galactosyl transfer in the formation of the carbohydrate-protein linkage region. J Biol Chem. 1969 May 25;244(10):2790–2798. [PubMed] [Google Scholar]
- Helting T., Rodén L. Biosynthesis of chondroitin sulfate. II. Glucuronosyl transfer in the formation of the carbohydrate-protein linkage region. J Biol Chem. 1969 May 25;244(10):2799–2805. [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasher R., Cahn R. D. The effects of 5-Bromodeoxyuridine on the differentiation of chondrocytes in vitro. Dev Biol. 1969 May;19(5):415–435. doi: 10.1016/0012-1606(69)90080-3. [DOI] [PubMed] [Google Scholar]
- Levitt D., Dorfman A. Control of chondrogenesis in limb-bud cell cultures by bromodeoxyuridine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2201–2205. doi: 10.1073/pnas.70.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Dorfman A. The irreversible inhibition of differentiation of limb-bud mesenchyme by bromodeoxyuridine. Proc Natl Acad Sci U S A. 1972 May;69(5):1253–1257. doi: 10.1073/pnas.69.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Hurler's syndrome: biosynthesis of acid mucopolysaccharides in tissue culture. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1310–1316. doi: 10.1073/pnas.56.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Robinson H. C., Telser A., Dorfman A. Studies on biosynthesis of the linkage region of chondroitin sulfate-protein complex. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1859–1866. doi: 10.1073/pnas.56.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBERT J. E. INCORPORATION OF 14C AND 3H FROM LABELED NUCLEOTIDE SUGARS INTO A POLYSACCHARIDE IN THE PRESENCE OF A CELL-FREE PREPARATION FROM CARTILAGE. J Biol Chem. 1964 May;239:1310–1315. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Telser A., Robinson H. C., Dorfman A. The biosynthesis of chondroitin sulfate. Arch Biochem Biophys. 1966 Sep 26;116(1):458–465. doi: 10.1016/0003-9861(66)90053-1. [DOI] [PubMed] [Google Scholar]
- Telser A., Robinson H. C., Dorfman A. The biosynthesis of chondroitin-sulfate protein complex. Proc Natl Acad Sci U S A. 1965 Sep;54(3):912–919. doi: 10.1073/pnas.54.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]


