Abstract
BACKGROUND
Hypertension is common in autosomal dominant polycystic kidney disease (ADPKD) and is associated with increased total kidney volume, activation of the renin–angiotensin–aldosterone system, and progression of kidney disease.
METHODS
In this double-blind, placebo-controlled trial, we randomly assigned 558 hypertensive participants with ADPKD (15 to 49 years of age, with an estimated glomerular filtration rate [GFR] >60 ml per minute per 1.73 m2 of body-surface area) to either a standard blood-pressure target (120/70 to 130/80 mm Hg) or a low blood-pressure target (95/60 to 110/75 mm Hg) and to either an angiotensin-converting–enzyme inhibitor (lisinopril) plus an angiotensin-receptor blocker (telmisartan) or lisinopril plus placebo. The primary outcome was the annual percentage change in the total kidney volume.
RESULTS
The annual percentage increase in total kidney volume was significantly lower in the low-blood-pressure group than in the standard-blood-pressure group (5.6% vs. 6.6%, P = 0.006), without significant differences between the lisinopril–telmisartan group and the lisinopril–placebo group. The rate of change in estimated GFR was similar in the two medication groups, with a negative slope difference in the short term in the low-blood-pressure group as compared with the standard-blood-pressure group (P<0.001) and a marginally positive slope difference in the long term (P = 0.05). The left-ventricular-mass index decreased more in the low-blood-pressure group than in the standard-blood-pressure group (−1.17 vs. −0.57 g per square meter per year, P<0.001); urinary albumin excretion was reduced by 3.77% with the low-pressure target and increased by 2.43% with the standard target (P<0.001). Dizziness and light-headedness were more common in the low-blood-pressure group than in the standard-blood-pressure group (80.7% vs. 69.4%, P = 0.002).
CONCLUSIONS
In early ADPKD, the combination of lisinopril and telmisartan did not significantly alter the rate of increase in total kidney volume. As compared with standard blood-pressure control, rigorous blood-pressure control was associated with a slower increase in total kidney volume, no overall change in the estimated GFR, a greater decline in the left-ventricular-mass index, and greater reduction in urinary albumin excretion.
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by gradual cyst enlargement over a period of decades before the loss of kidney function.1-3 Total kidney volume in ADPKD is accurately measured with the use of magnetic resonance imaging (MRI).4-6
Hypertension occurs early6,7 and is associated with progression to end-stage renal disease (ESRD) and death from cardiovascular causes in patients with ADPKD.8,9 Immunohistologic studies10,11 and clinical studies12,13 support a central role of the renin–angiotensin–aldosterone system (RAAS) in the pathogenesis of hypertension in patients with ADPKD. Activation of the RAAS may promote renal-cyst growth by means of its mitogenic effects.14,15 Although this hypothesis has been supported by studies in animals,16-18 it has not been fully evaluated in patients with ADPKD. It is unclear whether more aggressive antihypertensive therapy or an increased use of RAAS inhibitors delays progression to ESRD in patients with ADPKD.19-24
In this randomized, double-blind, placebo-controlled clinical trial, we examined the efficacy and safety of combined treatment with an angiotensin-converting–enzyme inhibitor (lisinopril) and an angiotensin-receptor blocker (telmisartan) versus treatment with lisinopril alone, as well as standard versus low blood-pressure targets, in patients 15 to 49 years of age with ADPKD who had an estimated glomerular filtration rate (GFR) of more than 60 ml per minute per 1.73 m2 of body-surface area. The primary outcome was the annual percentage change in total kidney volume.
METHODS
TRIAL DESIGN, PARTICIPANTS, AND INTERVENTIONS
Detailed information about the trial design has been published previously.25,26 The study protocol is available with the full text of this article at NEJM.org. Eligible participants were enrolled at seven clinical sites from February 2006 through June 2009. All the participants provided written informed consent. Participants were randomly assigned in a 1:1 ratio to lisinopril plus telmisartan or lisinopril plus placebo. Randomization was performed centrally with the use of permuted blocks. In addition, participants were randomly assigned in a 1:1 ratio to a standard blood-pressure target (120/70 to 130/80 mm Hg) or a low blood-pressure target (95/60 to 110/75 mm Hg), with stratification according to age, sex, race, baseline estimated GFR, and clinical site. The last study visit was in June 2014.
Participants underwent standardized imaging27 in a 1.5-T MRI scanner to determine total kidney volume, left-ventricular-mass index, and renal blood flow at baseline and at 24, 48, and 60 months. Renal vascular resistance was calculated on the basis of blood flow and mean arterial pressure.28 Image analysis was performed,27 and strict quality-control measures were maintained throughout the study.
After randomization, treatment with lisinopril and the masked study medication (telmisartan or placebo) was initiated, and the doses were adjusted in a stepwise fashion to achieve the desired blood-pressure targets (with the use of home blood-pressure measures) while the plasma levels of creatinine and potassium were monitored. Second-, third-, and fourth-line antihypertensive agents were added as needed (Table S1 in the Supplementary Appendix, available at NEJM .org). Central measurements of the serum creati-nine level and local measurements of blood urea nitrogen and electrolytes were obtained at all clinical-site visits, and 24-hour urine collections were obtained for central measurements of albumin, sodium, potassium, creatinine, and aldosterone excretion annually. Adherence to therapy was calculated as the number of drug cards (32 pills per card) given to patients minus the number returned unused during the study period, divided by the number of months of study participation.
OUTCOME MEASURES
The primary outcome was the percentage change in the total kidney volume over time. The first secondary outcome was the rate of change in the estimated GFR. Other secondary outcomes included rates of change in urinary aldosterone excretion, urinary albumin excretion, left-ventricular-mass index, and renal blood flow; frequency of hospitalizations for any cause and for cardiovascular causes; quality of life; frequency of pain associated with symptoms of ADPKD; and adverse effects related to the study medication. Hospitalizations were adjudicated by investigators who were unaware of the treatment-group assignments and who determined the principal diagnosis and related procedures, whether hospitalization was related to ADPKD, and whether acute kidney injury occurred during hospitalization. Acute kidney injury was defined as an elevation in the serum creatinine level of 0.3 mg per deciliter (30 μmol per liter) or more29 from the time of hospital admission or from the most recent value obtained at the study site. A discharge diagnosis of acute kidney injury was accepted if no creatinine values were available.
STUDY OVERSIGHT
A steering committee of investigators designed the trial, and the protocol was approved by the institutional review board at each study site. Data collected by site investigative teams were entered into a central database managed by the data coordinating center and analyzed by study statisticians. An external advisory committee selected by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health reviewed the protocol and served as the data and safety monitoring board. Antihypertensive medications were donated by Merck (lisinopril) and Boehringer Ingelheim Pharmaceuticals (telmisartan and matched placebo). Neither company was involved in any aspect of study design, data collection or analysis, or manuscript preparation. The first author wrote the first draft of the manuscript, with substantial contributions from the coauthors, all of whom had access to the data and jointly decided to submit the manuscript for publication. All the authors vouch for the accuracy and completeness of the reported data, as well as for the fidelity of this report to the study protocol.
STATISTICAL ANALYSIS
All the analyses were based on the intention-to-treat principle. Owing to the absence of interaction between drug therapy and blood-pressure target (P = 0.30), the analysis of each main effect is reported separately. The primary outcome, total kidney volume, was transformed with the use of the natural logarithm and analyzed with the use of a linear mixed-effects model30 as a function of month, month by treatment group, and month by blood-pressure group, with adjustment for age, sex, race, baseline estimated GFR, and clinical site. The intercept and slope were allowed to vary randomly. We converted the per-month slope (β) into an annual percentage change according to the following formula:
where e is the base of the natural logarithms.
The estimated GFR was calculated by means of the Chronic Kidney Disease Epidemiology Collaboration equation31 with the use of central serum creatinine measurements at baseline, at the 4-month and 12-month visits, and at every subsequent 6-month visit. We used a piecewise linear mixed model32 with separate slopes for the short-term phase (baseline to 4 months) and the long-term phase (>4 months) to compare treatment groups. We tested slopes for overall changes and for changes in the short-term and long-term phases.
Remaining secondary outcomes were analyzed with the use of generalized linear mixed models with either the identity or logit-link function, depending on the measure. We conducted prespecified subgroup analyses according to age at screening, sex, baseline total kidney volume, baseline total kidney volume for participants younger than 30 years of age, and baseline estimated GFR.
The data and safety monitoring board performed yearly reviews, with planned interim analyses for the primary outcome when all imaging studies were completed at 24 months and again at 48 months. A Haybittle–Peto stopping boundary33,34 was used for each interim analysis with a nominal significance level of 0.001, and a two-tailed significance level of 0.05 was used for the final analysis.
Assuming no interaction in the 2-by-2 factorial design, we estimated that 466 participants would need to be enrolled for the study to detect a 25% reduction in the total kidney volume slope, from 5.4 to 4.1% per year, with 90% power at a 5% significance level. Accounting for a 15% withdrawal rate, this number was increased to 548 participants. With the final study design, the study had more than 95% power to detect a 25% reduction in the slope.
RESULTS
PARTICIPANTS
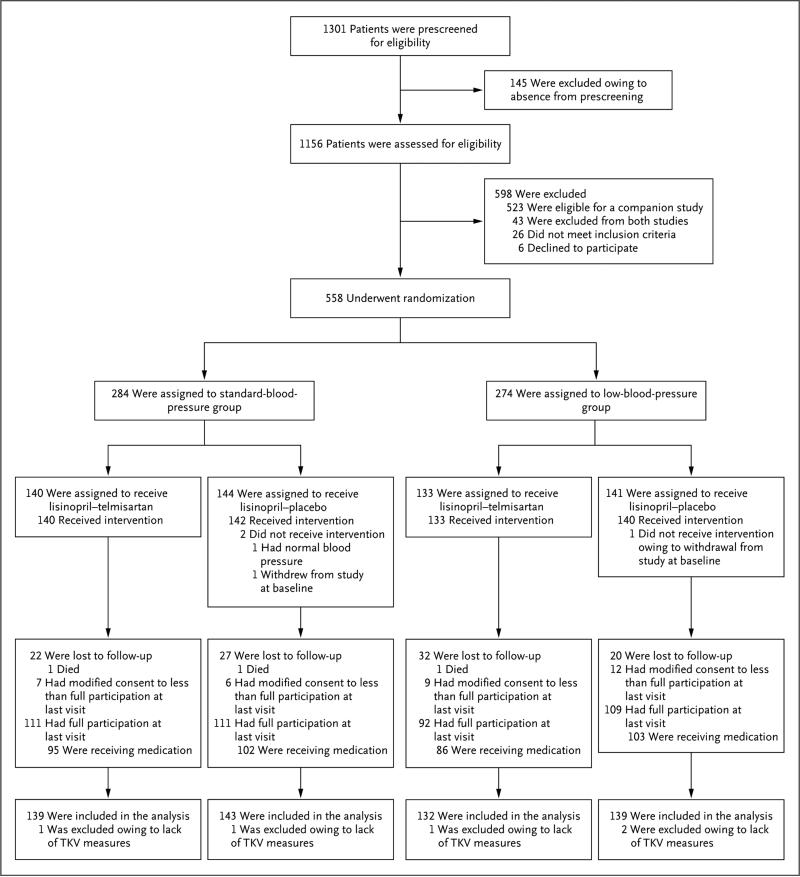
Of 1156 screened participants, 558 underwent randomization (Fig. 1). Overall, 423 participants (75.8%) completed the trial according to the protocol (i.e., full study participation), of whom 37 (8.7%) discontinued the study medication before the end of the trial; 6.1% of the participants modified their consent to less than full study participation (i.e., discontinued the medication, reduced the number of study visits or assessments, or both), and 18.1% were lost to follow-up, with similar proportions in each study group. The mean (±SD) rate of adherence to treatment was significantly lower in the lisinopril–telmi sartan group than in the lisinopril–placebo group (72.1±24.9% vs. 79.5±18.6%, P<0.001). Poor image quality resulting in rescanning requests occurred in 1.8% of the measures of total kidney volume (approximately 40% were baseline measures). There were no significant differences in baseline characteristics between the standard-blood-pressure group and the low-blood-pressure group or between the lisinopril–telmisartan group and the lisinopril–placebo group (Table 1, and Tables S2A and S2B in the Supplementary Appendix).
Figure 1. Enrollment, Randomization, and Follow-up of the Study Participants.
We screened 1156 participants, of whom 558 were randomly assigned to receive either lisinopril–telmisartan or lisinopril–placebo. Overall, 423 participants completed the trial according to the protocol (i.e., full study participation). Some patients modified their consent to less than full participation (i.e., discontinued the study drug, reduced the number of study assessments or visits, or both). TKV denotes total kidney volume.
Table 1.
Demographic, Clinical, and Laboratory Characteristics at Baseline, According to Study Group of the 2-by-2 Factorial Design Trial.*
| Characteristic | Lisinopril–Telmisartan (N = 273) | Lisinopril–Placebo (N = 285) | Standard Blood Pressure (N = 284) | Low Blood Pressure (N = 274) |
|---|---|---|---|---|
| Age — yr | 37.0±8.3 | 36.3±8.3 | 36.3±8.4 | 36.9±8.2 |
| Male sex — no. (%) | 141 (51.6) | 142 (49.8) | 143 (50.4) | 140 (51.1) |
| Race — no. (%)† | ||||
| White | 255 (93.4) | 262 (91.9) | 258 (90.8) | 259 (94.5) |
| Black | 6 (2.2) | 8 (2.8) | 7 (2.5) | 7 (2.6) |
| Other | 10 (3.7) | 17 (6.0) | 18 (6.3) | 9 (3.3) |
| Data missing | 2 (0.7) | 0 | 2 (0.7) | 0 |
| PKD genotype — no./total no. (%)‡ | ||||
| PKD1 | 190/252 (75.4) | 192/260 (73.8) | 204/260 (78.5) | 178/252 (70.6) |
| PKD2 | 42/252 (16.7) | 42/260 (16.2) | 34/260 (13.1) | 50/252 (19.8) |
| No mutation detected | 20/252 (7.9) | 26/260 (10.0) | 22/260 (8.5) | 24/252 (9.5) |
| Body-mass index§ | 27.4±5.2 | 27.1±5.1 | 27.3±5.4 | 27.1±4.9 |
| Estimated GFR — ml/min/1.73 m2¶ | 90.4±17.5 | 92.6±17.4 | 91.7±17.8 | 91.4±17.2 |
| Urinary aldosterone — μg/24 hr | 12.2±10.0 | 12.2±9.1 | 13.0±10.6 | 11.4±8.2 |
| Urinary albumin — mg/24 hr | ||||
| Median | 19.3 | 17.6 | 19.1 | 17.7 |
| Interquartile range | 12.7–35.2 | 11.7–30.6 | 12.8–31.8 | 11.7–33.3 |
| Total kidney volume — ml | 1264.6±786.2 | 1164.0±661.0 | 1240.6±747.1 | 1185.2±704.0 |
| Renal blood flow — ml/min/1.73 m2 | 607.7±195.3 | 609.2±216.2 | 592.4±206.1 | 624.7±205.3 |
| Left-ventricular-mass index — g/m2 | 64.1±13.2 | 63.7±12.9 | 63.8±13.8 | 63.9±12.2 |
Plus–minus values are means ±SD. No significant differences in the baseline characteristics were found between the lisinopril–telmisartan group and the lisinopril–placebo group or between the low-blood-pressure group and the standard-blood-pressure group. PKD denotes polycystic kidney disease.
Race was self-reported. Some participants selected more than one category, so percentages may sum to more than 100.
The mutated genes PKD1 and PKD2 encode polycystin-1 and polycystin-2, respectively.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The estimated glomerular filtration rate (GFR) was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration equation.31
LOW VERSUS STANDARD BLOOD-PRESSURE TARGET
Significant differences in systolic and diastolic blood-pressure levels, as measured at home, were found between the low-blood-pressure group and the standard-blood-pressure group (Fig. S1A in the Supplementary Appendix) and were maintained throughout the trial (difference in home systolic pressure at 96 months, 13.4 mm Hg; difference in home diastolic pressure at 96 months, 9.3 mm Hg). The systolic and diastolic blood pressures, as measured at home, were on target across all study visits in 40 to 66% and 58 to 75% of participants in the low-blood-pressure group, respectively, and in 32 to 48% and 33 to 52% of those in the standard-blood-pressure group, respectively. Urinary aldosterone excretion decreased significantly from baseline throughout the study in the two groups (P<0.001 for both comparisons) (Fig. S1B in the Supplementary Appendix).
A median of 2.0 open-label medications were prescribed in the low-blood-pressure group and 1.0 in the standard-blood-pressure group. The proportion of patients who used various medications was significantly greater in the low-blood-pressure group than in the standard-blood-pressure group, including diuretics (44.9% vs. 26.8%, P<0.001), beta- or alpha-blockers (31.4% vs. 14.4%, P<0.001), and calcium-channel blockers (10.2% vs. 5.3%, P = 0.03) (Table S3 in the Supplementary Appendix). The mean daily lisinopril dose was more than 8 mg per day greater in the low-blood-pressure group than in the standard-blood-pressure group throughout the study (Table S4 in the Supplementary Appendix).
Primary Outcome
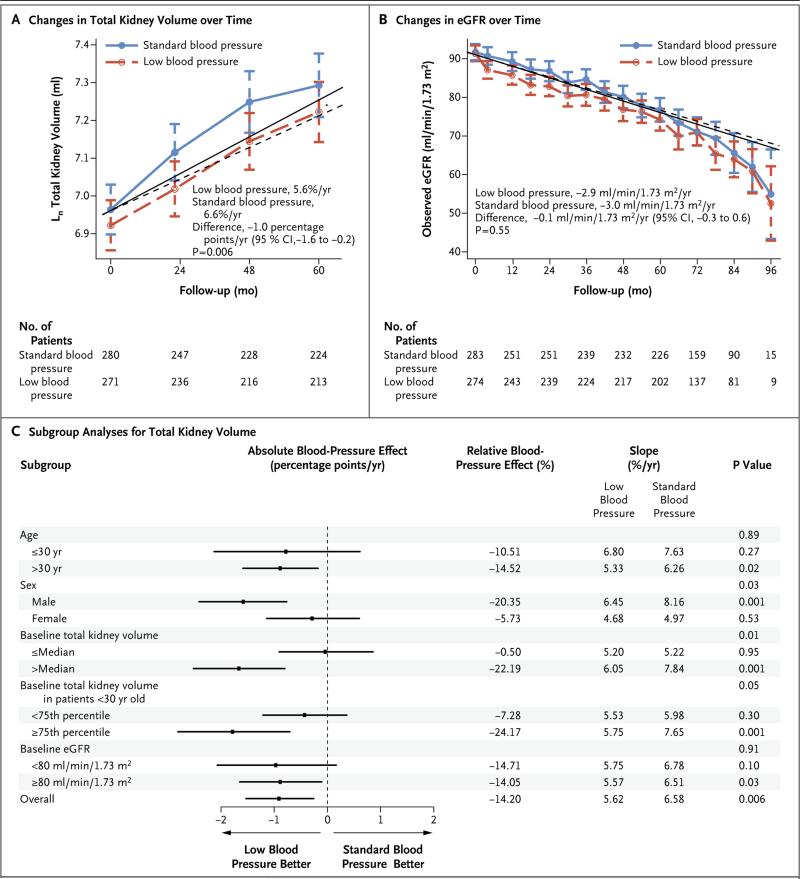
Participants in the low-blood-pressure group had a 14.2% slower annual increase in total kidney volume, as compared with those in the standard-blood-pressure group (5.6% vs. 6.6%, P = 0.006) (Fig. 2A). The total kidney volume increased by 38.0% from baseline in the low-blood-pressure group and by 44.2% from baseline in the standard-blood-pressure group (total kidney volume at 60 months, 1636 ml [95% confidence interval {CI}], 1489 to 1782] and 1788 ml [95% CI, 1639 to 1938], respectively). Forest plots showed significant subgroup interactions: the effect of low blood pressure on the rate of increase in total kidney volume was greatest for men, participants with a baseline total kidney volume greater than the median, and those with large kidneys (≥75th percentile) who were younger than 30 years of age (Fig. 2C).
Figure 2. Changes in Total Kidney Volume and Estimated Glomerular Filtration Rate (eGFR) during Follow-up and Subgroup Analyses, According to Blood-Pressure Group.
The logarithm-transformed total kidney volume (Panel A) and eGFR (Panel B) over time according to blood-pressure group and prespecified subgroup analyses (Panel C) are presented. In Panels A and B, symbols represent means, and I bars 95% confidence intervals (CI); the solid lines (standard blood pressure) and dashed lines (low blood pressure) represent model-based trajectories. In Panel C, the absolute treatment effect is the between-group difference in the annual slope. P values associated with overall subgroups correspond to interactions of subgroup by month by study group; all other P values correspond to interactions of month by study group within a particular subgroup. The slope estimates for the total kidney volume were based on linear mixed-effects models with natural log transformations on the outcome and were converted to the annual percentage change. Follow-up time ranged from 5 to 8 years.
Secondary Outcomes
The overall change in the estimated GFR was similar in the low-blood-pressure group and the standard-blood-pressure group (−2.9 and −3.0 ml per minute per 1.73 m2 per year, respectively; P = 0.55). The decline in the estimated GFR in the short-term phase was significantly greater in the low-blood-pressure group than in the standard-blood-pressure group (−3.1 vs. 0.5 ml per minute per 1.73 m2 per 4 months, P<0.001) (Fig. 2B). In the long-term phase, the slope of the estimated GFR did not differ significantly between the two groups (−2.7 and. −3.1 ml per minute per 1.73 m2 per year, respectively; difference, 0.4 ml per minute per 1.73 m2 per year [95% CI, 0.0 to 0.9]; P = 0.05).
Urinary albumin excretion decreased in the low-blood-pressure group, as compared with an increase in the standard-blood-pressure group (−3.77% per year [95% CI, −5.71 to −1.78] vs. 2.43% per year [95% CI, 0.48 to 4.41], P<0.001) (Fig. S2A in the Supplementary Appendix). The low-blood-pressure group had a greater reduction in the left-ventricular-mass index than the standard-blood-pressure group did (−1.17 vs. −0.57 g per square meter per year, P<0.001) (Fig. S2B in the Supplementary Appendix). Renal blood flow declined similarly in the two groups (Fig. S2C in the Supplementary Appendix), but renal vascular resistance increased more in the standard-blood-pressure group than in the low-blood-pressure group (P<0.001) (Fig. S2D in the Supplementary Appendix).
The physical component scores on the 36-Item Short Form General Health Survey (SF-36) did not differ significantly between the two groups, and the mental component scores improved significantly in the standard-blood-pressure group, as compared with the low-blood-pressure group (Fig. S2E and S2F in the Supplementary Appendix). The proportion of patients with one or more episodes of dizziness and light-headedness at the end of the study was greater in the low-blood-pressure group than in the standard-blood-pressure group (80.7% vs. 69.4%, P = 0.002) (Table S5 in the Supplementary Appendix). The frequencies of death, serious cardiovascular or renal events, hyperkalemia, acute kidney injury, and cancer did not differ significantly between the two groups (Table 2, and Table S6 in the Supplementary Appendix).
Table 2.
Adverse Events in the 2-by-2 Factorial-Design Trial.*
| Event | Lisinopril–Telmisartan (N = 273) | Lisinopril–Placebo (N = 285) | Standard Blood Pressure (N = 284) | Low Blood Pressure (N = 274) |
|---|---|---|---|---|
| Mean follow-up — yr | 5.6 | 5.7 | 5.7 | 5.6 |
| Acute kidney injury | ||||
| No. of events | 15 | 19 | 17 | 17 |
| No. of participants — % | 13 (4.8) | 16 (5.6) | 13 (4.6) | 16 (5.8) |
| Hyperkalemia | ||||
| No. of events | 13 | 6 | 11 | 8 |
| No. of participants — % | 11 (4.0) | 5 (1.8) | 9 (3.2) | 7 (2.6) |
| Hospitalization | ||||
| No. of events | 85 | 128 | 120 | 93 |
| Incidence — no. of events/100 person-yr | 5.55 | 7.92 | 7.43 | 6.07 |
| Cardiac-related hospitalization | ||||
| No. of events | 13 | 9 | 13 | 9 |
| Incidence — no. of events/100 person-yr | 0.85 | 0.56 | 0.80 | 0.59 |
| Cancer | ||||
| No. of events | 4 | 4 | 2 | 6 |
| No. of participants — % | 4 (1.5) | 4 (1.4) | 2 (0.7) | 6 (2.2) |
| Serious adverse event | ||||
| Death — no. of participants (%)† | 1 (0.4) | 1 (0.4) | 2 (0.7) | 0 |
| Cardiac disorder | ||||
| No. of events | 9 | 6 | 12 | 3 |
| No. of participants — % | 6 (2.2) | 5 (1.8) | 8 (2.8) | 3 (1.1) |
| Gastrointestinal disorder | ||||
| No. of events | 11 | 17 | 21 | 7 |
| No. of participants — % | 8 (2.9) | 12 (4.2) | 16 (5.6)‡ | 4 (1.5) |
| Abdominal pain | ||||
| No. of events | 3 | 9 | 7 | 5 |
| No. of participants — % | 3 (1.1) | 6 (2.1) | 6 (2.1) | 3 (1.1) |
| Nervous system disorder | ||||
| No. of events | 10 | 12 | 14 | 8 |
| No. of participants — % | 8 (2.9) | 10 (3.5) | 11 (3.9) | 7 (2.6) |
| Renal or urinary system disorder | ||||
| No. of events | 14 | 15 | 16 | 13 |
| No. of participants — % | 12 (4.4) | 14 (4.9) | 14 (4.9) | 12 (4.4) |
| Nephrolithiasis or renal colic | ||||
| No. of events | 3 | 4 | 7 | 0 |
| No. of participants — % | 3 (1.1) | 4 (1.4) | 7 (2.5)‡ | 0 |
All serious adverse events were classified with the use of the Common Terminology Criteria for Adverse Events, version 4.0.
The causes of death were cardiac arrest (in one patient in the lisinopril–placebo group) and a neurologic event (in one patient in the lisinopril–telmisartan group).
P<0.05 for the comparison with the low-blood-pressure group.
LISINOPRIL–TELMISARTAN VERSUS LISINOPRIL–PLACEBO
Although the trajectory of the systolic and diastolic blood-pressure levels, as assessed at home, during the long-term phase differed between the lisinopril–telmisartan group and the lisinopril–placebo group (P<0.001), the lisinopril–telmisartan group had significantly lower blood pressures than the lisinopril–placebo group at the 4-month and 12-month visits only (Fig. S3A in the Supplementary Appendix). The reduction in urinary aldosterone excretion was similar in the two groups (Fig. S3B in the Supplementary Appendix). Diuretic use was significantly greater in the lisinopril–placebo group than in the lisinopril–telmisartan group (41.4% vs. 29.7%, P = 0.004) (Table S3 in the Supplementary Appendix). The mean lisinopril dose was more than 5 mg per day lower in the lisinopril–telmisartan group than in the lisinopril–placebo group at all visits (Table S7 in the Supplementary Appendix).
Primary Outcome
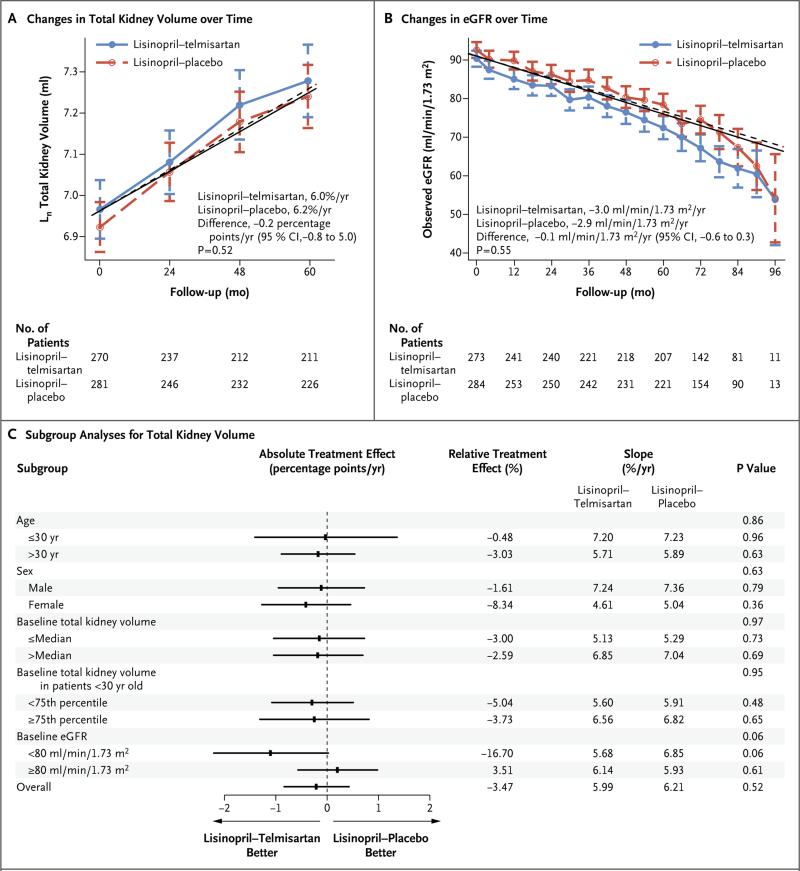
The total kidney volume increased at similar rates in the lisinopril–telmisartan group and the lisinopril–placebo group (6.0% per year and 6.2% per year, respectively; P = 0.52) (Fig. 3A). The total kidney volume increased by 40.5% in the lisinopril–telmisartan group and by 42.2% in the lisinopril–placebo group (total kidney volume at 60 months, 1777 ml [95% CI, 1617 to 1937] and 1655 ml [95% CI, 1518 to 1792], respectively). Forest plots showed no significant subgroup interactions, with the exception of a marginal benefit for patients with a baseline estimated GFR of less than 80 ml per minute in the lisinopril– telmisartan group (5.7% per year, vs. 6.9% per year in the lisinopril–placebo group, P = 0.06) (Fig. 3C).
Figure 3. Changes in Total Kidney Volume and eGFR during Follow-up, and Subgroup Analyses, According to Treatment Group.
The logarithm-transformed total kidney volume (Panel A) and eGFR (Panel B) over time according to treatment group and prespecified subgroup analyses (Panel C) are presented. In Panels A and B, symbols represent means, and I bars 95% confidence intervals; the solid lines (lisinopril–telmisartan) and dashed lines (lisinopril–placebo) represent model-based trajectories. In Panel C, the absolute treatment effect is the between-group difference in the annual slope. P values associated with overall subgroups correspond to interactions of subgroup by month by study group; all other P values correspond to interactions of month by study group within a particular subgroup. The slope estimates for total kidney volume were based on linear mixed-effects models with natural log transformations on the outcome and were converted to annual percentage change. Follow-up time ranged from 5 to 8 years.
Secondary Outcomes
The estimated GFR declined similarly in the lisinopril–telmisartan group and in the lisinopril–placebo group (−3.00 ml per minute per 1.73 m2 per year and −2.86 ml per minute per 1.73 m2 per year, respectively; P = 0.55) (Fig. 3B). Urinary albumin excretion remained unchanged in the two treatment groups (Fig. S4A in the Supplementary Appendix). A significant and similar decline from baseline in the left-ventricular-mass index occurred in the two groups (Fig. S4B in the Supplementary Appendix). Renal blood flow decreased and renal vascular resistance increased similarly in the two groups (Fig. S4C and S4D in the Supplementary Appendix).
No significant differences in the physical component scores of the SF-36 were found between the lisinopril–telmisartan group and the lisinopril–placebo group, although the mental component scores were significantly better in the lisinopril–telmisartan group (P = 0.02) (Fig. S4E and S4F in the Supplementary Appendix). Hyperkalemia, acute kidney injury, and cancer occurred at similar, low rates in the two groups (Table 2, and Table S6 in the Supplementary Appendix). The risk of hospitalization was lower in the lisinopril–telmisartan group than in the lisinopril–placebo group (hazard ratio, 0.71; 95% CI, 0.53 to 0.95). The proportion of patients who had one or more serious adverse events and symptoms was similar in the two groups (Table 2, and Tables S5 and S6 in the Supplementary Appendix). The risk of having kidney stones was decreased each month in the lisinopril–telmisartan group (odds ratio, 0.99; 95% CI, 0.98 to 1.00), as compared with virtually no change each month in the lisinopril–placebo group (odds ratio, 1.00; 95% CI, 0.99 to 1.01) (P = 0.048).
DISCUSSION
This study involved young hypertensive patients (15 to 49 years of age) with ADPKD, who had relatively preserved kidney function and were at risk for progression to ESRD.9 The safety of both dual blockade of the RAAS and rigorous blood-pressure control was excellent, although dizziness and lightheadedness occurred more often in the low-blood-pressure group than in the standard-blood-pressure group.
Lisinopril–telmisartan treatment did not show a benefit, as compared with lisinopril alone, with regard to the change in total kidney volume or estimated GFR. Urinary aldosterone levels declined similarly in the two medication groups, and diuretics and vasodilators were used more often in the lisinopril–placebo group than in the lisinopril–telmisartan group, differentially affecting the level of RAAS suppression in the two treatment groups.
Rigorous blood-pressure control attenuated the annual rate of increase in total kidney volume by 14.2% and was associated with reduced urinary albumin excretion. The rates of urinary albumin excretion are typically low, and elevations occur in less than 30% of patients with ADPKD. However, urinary albumin excretion is associated with increased total kidney volume35 and is an independent predictor of faster progression to ESRD.9 Patients younger than 30 years of age with the largest kidneys were more likely to benefit from rigorous blood-pressure control than were patients of similar age with smaller kidneys. Men, but not women, also had evidence of a benefit from low blood pressure.
The overall rate of change in the estimated GFR was similar in the two blood-pressure groups, with a negative slope difference in the short term in the low-blood-pressure group, as compared with the standard-blood-pressure group (P<0.001), and a marginally positive slope difference in the long term (P = 0.05). The evaluation of change in the estimated GFR was hampered by the decline in the short-term phase and the lack of creatinine measure at the end of the study, after the study drug had been discontinued. A beneficial effect on cyst burden (rate of total kidney-volume growth) was not associated with an improvement in the slope of the estimated GFR. Whether a time lag between the therapeutic effect on total kidney volume and stabilization of the estimated GFR occurs in patients with ADPKD is not yet known. Given the combination of the decline in the estimated GFR in the short-term phase and a potential temporal delay between the change in total kidney volume and the change in the estimated GFR, this study was not of sufficient size or duration to show a potential benefit of blood-pressure control on kidney function.
In conclusion, the rate of total kidney-volume growth and the slope of the estimated GFR were not affected by dual blockade of the RAAS. Aggressive blood-pressure control was safe in young, hypertensive patients with ADPKD and preserved kidney function. This level of blood-pressure control, as compared with standard blood-pressure control, was associated with a modest reduction in total kidney volume over time, without differences in the slope of the estimated GFR. Improvement in markers of the secondary outcomes, including the left-ventricular-mass index and urinary albumin excretion, also suggests a benefit of aggressive blood-pressure control.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK62402 to Dr. Schrier, DK62411 to Dr. Perrone, DK62410 to Dr. Torres, DK082230 to Dr. Moore, DK62408 to Dr. Chapman, and DK62401 to Washington University in St. Louis) and the National Center for Research Resources General Clinical Research Centers (RR000039 to Emory University, RR000585 to the Mayo Clinic, RR000054 to Tufts Medical Center, RR000051 to the University of Colorado, RR023940 to the University of Kansas Medical Center, and RR001032 to Beth Israel Deaconess Medical Center), National Center for Advancing Translational Sciences Clinical and Translational Science Awards (RR025008 and TR000454 to Emory University, RR024150 and TR00135 to the Mayo Clinic, RR025752 and TR001064 to Tufts University, RR025780 and TR001082 to the University of Colorado, RR025758 and TR001102 to Beth Israel Deaconess Medical Center, RR033179 and TR000001 to the University of Kansas Medical Center, and RR024989 and TR000439 to Cleveland Clinic), by funding from the Zell Family Foundation (to the University of Colorado), and by a grant from the PKD Foundation.
Dr. Schrier reports receiving fees for serving on advisory boards from Otsuka Pharmaceuticals, Janssen Pharmaceuticals, and Ikaria; Dr. Perrone, consulting fees from Sanofi–Genzyme and Vertex Pharmaceuticals and consulting fees and grant support through his institution from Otsuka Pharmaceuticals; Drs. Torres and Harris, grant support from Otsuka Pharmaceuticals; Dr. Braun, grant support from Wyeth–Pfizer; Dr. Steinman, grant support from Otsuka Pharmaceuticals and Kadmon; Dr. Hogan, grant support from Novartis and Otsuka Pharmaceuticals;. Dr. Rahbari-Oskoui, fees for serving on advisory boards from Otsuka Pharmaceuticals; Dr. Bae, consulting fees from Otsuka Pharmaceuticals; and Dr. Chapman, consulting fees from Kadmon, Otsuka Pharmaceuticals, and Pfizer and grant support from Otsuka Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
We thank the participants for taking part in the study, the research program coordinators and program managers at Washington University (Gigi Flynn and Robin Woltman) and the University of Pittsburgh (Susan Spillane and Patty Smith), the staff at the Image Analysis Center (Johana Schafer and Cheng Tao), the study coordinators at the clinical centers (Sabira Bacchus, Rita Brienza, Sheri Copeland, Elizabeth Courtney, Maria Fish-man, Michelle Garcia, Diana George, Amy Haun, Carol Horner, Cathy Jackman, Andee Jolley, Pamela Lanza, Barbara Maxwell, Pamela Morgan, Sarah Nicholls, Troy Ofstie, Kristine Otto, Heather Ondler, Sue Saunders, Gertrude Simon, Rita Spirko, Lydia Sweeney, Veronika Testa, and Diane Watkins), and all the other members of the Halt Progression of Polycystic Kidney Disease teams at the study sites, including those who left before the end of the study.
Footnotes
(Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; HALT-PKD [Study A] ClinicalTrials.gov number, NCT00283686.)
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grantham JJ. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–85. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW, Brosnahan G, Cadnapaphornchai MA, et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014 Jun 12; doi: 10.1681/ASN.2013111184. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabow PA, Chapman AB, Johnson AM, et al. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990;38:1177–80. doi: 10.1038/ki.1990.330. [DOI] [PubMed] [Google Scholar]
- 7.Schrier RW. Hypertension and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2011;57:811–3. doi: 10.1053/j.ajkd.2011.02.379. [DOI] [PubMed] [Google Scholar]
- 8.Orskov B, Sørensen VR, Feldt-Rasmussen B, Strandgaard S. Changes in causes of death and risk of cancer in Danish patients with autosomal dominant polycystic kidney disease and end-stage renal disease. Nephrol Dial Transplant. 2012;27:1607–13. doi: 10.1093/ndt/gfr467. [DOI] [PubMed] [Google Scholar]
- 9.Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–9. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 10.Graham PC, Lindop GB. The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 1988;33:1084–90. doi: 10.1038/ki.1988.115. [DOI] [PubMed] [Google Scholar]
- 11.Torres VE, Donovan KA, Scicli G, et al. Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;42:364–73. doi: 10.1038/ki.1992.297. [DOI] [PubMed] [Google Scholar]
- 12.Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin–angiotensin–aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–6. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 13.Torres VE, Wilson DM, Burnett JC, Jr, Johnson CM, Offord KP. Effect of inhibition of converting enzyme on renal hemodynamics and sodium management in polycystic kidney disease. Mayo Clin Proc. 1991;66:1010–7. doi: 10.1016/s0025-6196(12)61724-8. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe G, Lee RJ, Albanese C, Rainey WE, Batlle D, Pestell RG. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–7. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- 15.Thomas W, Dooley R, Harvey BJ. Aldosterone as a renal growth factor. Steroids. 2010;75:550–4. doi: 10.1016/j.steroids.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Jia G, Kwon M, Liang HL, et al. Chronic treatment with lisinopril decreases proliferative and apoptotic pathways in autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2010;25:1139–46. doi: 10.1007/s00467-010-1477-2. [DOI] [PubMed] [Google Scholar]
- 17.Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am J Kidney Dis. 1994;24:491–8. doi: 10.1016/s0272-6386(12)80907-3. [DOI] [PubMed] [Google Scholar]
- 18.Zafar I, Tao Y, Falk S, McFann K, Schrier RW, Edelstein CL. Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol Renal Physiol. 2007;293:F854–E859. doi: 10.1152/ajprenal.00059.2007. [DOI] [PubMed] [Google Scholar]
- 19.Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–9. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 20.Spithoven EM, Kramer A, Meijer E, et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014 May 14; doi: 10.1038/ki.2014.120. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. J Am Soc Nephrol. 1995;5:2037–47. doi: 10.1681/ASN.V5122037. [Erratum, J Am Soc Nephrol 1995;6:1318.]
- 22.Orskov B, Rømming Sørensen V, Feldt-Rasmussen B, Strandgaard S. Improved prognosis in patients with autosomal dominant polycystic kidney disease in Denmark. Clin J Am Soc Nephrol. 2010;5:2034–9. doi: 10.2215/CJN.01460210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrier RW, McFann KK, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int. 2003;63:678–85. doi: 10.1046/j.1523-1755.2003.00776.x. [DOI] [PubMed] [Google Scholar]
- 24.Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with auto-somal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57:856–62. doi: 10.1053/j.ajkd.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5:102–9. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres VE, Chapman AB, Perrone RD, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–85. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae KT, Tao C, Zhu F, et al. MRI-based kidney volume measurements in ADPKD: reliability and effect of gadolinium enhancement. Clin J Am Soc Nephrol. 2009;4:719–25. doi: 10.2215/CJN.03750708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith HW. Lectures on the kidney. University of Kansas Press; Lawrence: 1943. Evolution of the kidney. pp. 3–23. [Google Scholar]
- 29.Reddy NP, Ravi KP, Dhanalakshmi P, Annigeri R, Ramakrishnan N, Venkataraman R. Epidemiology, outcomes and validation of RIFLE and AKIN criteria in acute kidney injury (AKI) in critically ill patients: Indian perspective. Ren Fail. 2014;36:831–7. doi: 10.3109/0886022X.2014.899432. [DOI] [PubMed] [Google Scholar]
- 30.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 31.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzmaurice G, Laird NM, Ware JH. Applied longitudinal analysis. Wiley; New York: 2004. [Google Scholar]
- 33.Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373–9. [Google Scholar]
- 34.Cook J, DeMets D. Introduction to statistical methods for clinical trials. Chapman & Hall; New York: 2008. [Google Scholar]
- 35.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–57. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.