Abstract
Objectives
Development of a simple and accurate technique for detecting active inflammation in the joints and other tissues of patients with inflammatory disorders is an unmet need in rheumatic diseases. This study is a preliminary assessment of the safety and usage of a radiopharmaceutical, FolateScan™ (Technetium-99m EC20; 99mTc-EC20), for detecting disease activity in patients with rheumatoid arthritis.
Methods
EC20 is a folate-targeted diagnostic radiopharmaceutical which binds to the folate receptor and is preferentially taken up by activated macrophages. In this open-label, cross-sectional study, a total of 40 patients with RA (26 with one or more swollen joints, 14 with clinically quiescent joint disease; 0/66 joint count) as well as 6 patients with osteoarthritis, 12 patients with other inflammatory conditions and 5 healthy subjects received 0.1 mg of EC20 labeled with 20–25mCi of technetium-99m. Disease activity was scored in each joint and other target tissues by a radiologist blinded to the clinical assessment, and results were compared to the rheumatologist’s physical examination, which served as the test standard.
Results
The 40 patients (78% female) with RA had a mean age of 56.9 years. Assessment of uptake of 99mTc-EC20 in joints of patients with RA based on image analysis was compared to the clinical examination. FolateScan detected more actively involved joints in 27 patients (68%) than joints recorded as “swollen”, and more actively involved joints in 25 patients (63%) than joints recorded as “painful and/or swollen”. The number of swollen joints by clinical exam was correlated with ESR (r=0.43; p=0.006) and C-rp (r=0.35; p=0.03). The number of actively involved joints by FolateScan was also correlated with ESR (r=0.47; p=0.002) and C-rp (r=0.36; p=0.02). Joint uptake was also seen in patients with osteoarthritis.
Conclusions
FolateScan is a potentially useful tool for detection of disease activity in patients with RA and may be more sensitive than the physical examination.
Keywords: Folate receptor, FolateScan, rheumatoid arthritis, radiopharmaceutical
Introduction
Assessment of disease activity in rheumatoid arthritis (RA) is based on clinical and laboratory measures. Such analyses, however, often inadequately estimate the true inflammatory burden, with the consequence that patients are either under- or overtreated. Therapies for RA, while directed at reducing joint inflammation, have unwanted systemic effects that increase the risk of adverse events. There is a need for improved measures of disease activity, as well as methods to better target therapies to actively involved tissues.
Recent studies have suggested that activated macrophages are key effector cells in RA (1–4). There is a direct correlation between the level of macrophage activity and observed joint inflammation, joint symptoms, presence in the synovial lining, and bone erosion. Activated macrophages secrete mediators of inflammation and tissue destruction such as IL-I, IL-6, TNF-α, prostaglandins and metalloproteinases, among others (1). They also promote activation and proliferation of antigen-specific T-cells and are important in antigen presentation (2, 5). Activated, but not resting macrophages (or most other cells), express a functional receptor for the vitamin folic acid, termed folate receptor beta (FR-β) (6–10). FR-β is a 38 kDa glycosylphos-phatidylinositol-anchored glycoprotein that binds folic acid and folate-linked molecules with subnanomolar affinity and transports these molecules into cells by receptor-mediated endocytosis (11–15). Because other isoforms of the folate receptor (predominantly FR-α) are only accessible in the kidneys and various cancer tissues (11–15), healthy individuals display little or no uptake of folate-linked molecules in the brain, liver, blood cells, bone marrow, heart, spleen, lungs, skeletal muscle or intestines (16–18).
FolateScan (EC20), a folate-targeted 99mTc-labeled imaging agent, consists of the vitamin folic acid conjugated to a chelating agent with specificity for 99mTc (19). The chelating moiety of EC20 consists of β-L-diaminopropionic acid linked to aspartic acid, which in turn is linked to cysteine via peptide bonds (19).
The limited distribution of FR in healthy tissues has enabled use of folate-linked radioimaging agents to image FR expressing cancers in both animals and humans (17, 18, 20, 21). EC20 has been administered intravenously to cancer patients and solid tumors which have been visualized within 2h by gamma scintigraphy (22).
Because activated macrophages also express FR, and since activated macrophages can be prominent in RA, arthritic joints in animal models of RA were recently examined for uptake of 99mTc-EC20 and other folate-targeted imaging agents (6, 8, 23). These imaging studies revealed that folate conjugates concentrate in the extremities, liver and spleen of animal models, and that their uptake is mediated by FR+ macrophages. Thus, tissue accumulation was shown to be greatly reduced by addition of excess folic acid, supporting the role of FR in mediating uptake (6), and depletion of macrophages was found to abolish tissue accumulation of the imaging agents. Because these model studies suggest that EC20 may be useful in assessing activated macrophage involvement in the inflammatory processes of RA, we hypothesized that synovium and other extraarticular tissues of human RA patients might also take up folate conjugates in a manner related to their degree of active inflammation.
The primary objectives of this study were to assess the safety and articular uptake of EC20 in active joint inflammation in patients with RA. As secondary objectives, we sought to evaluate the sensitivity, specificity, and predictive value of FolateScan compared to measures of clinical disease activity, including swollen joints and acute-phase reactants. We also preliminarily assessed the sensitivity of FolateScan for extraarticular disease involvement, including lung, nodules and serositis.
Methods
Study design
This was an open-labeled, single-center cross-sectional interventional study of patients with RA (40 patients) with varying degrees of disease activity, including two patients with active RA related intersitial lung disease. We also evaluated 5 healthy controls and 6 with osteoarthritis (OA). All patients met accepted criteria for disease classification and activity assessment (24). All subjects gave informed consent for the study, which was approved by the Mayo Clinic Institutional Review Board.
Study drug
The 99mTc labeled imaging agent was prepared by 99mTc-pertechnetate (1850MBq, or 50 mCi, in a 1–2 mL volume) to the lyophilized, non-radio-active reagent in the vial and heating for 18 minutes at 100°C. The activity of 99mTc-EC20 was calibrated for a 740–925 MBq (or 20–25 mCi) dose at the time of injection.
After reconstitution, the radiochemical purity of technetium Tc 99m EC20 was measured by TLC. The radiochemical purity must be 90% to pass acceptance criteria. To optimize the specificity of the scan, the first 8 patients with RA received increasing doses of folic acid of 0 mg (2 patients), 0.5 mg (2 patients), 1.0 mg (2 patients), 2.0 mg (2 patients), followed 1–3 minutes later by 1–2 mL injection of 0.1 mg of EC20 labeled with 20–25 mCi of technetium-99m. Images were obtained 2hrs after injection.
Imaging procedure
Head-to-toe anterior and posterior planar scintigrams (whole body imaging) and spot views of selected joints (feet and hands) in anterior, posterior and/or lateral views were acquired with a dual-detector, large-field-of-view gamma camera equipped with low-energy high-resolution (LEHR) parallel-hole collimators. A 20% symmetric energy window was approximately centered over the 140-keV photopeak ofTc-99m.
Assessment
All patients were evaluated to identify swollen and tender joints using 66 joints. Both the complete 66 joint count and the modified 28 joint count used in routine clinical practice and for calcuation of the Disease Activity Score (DAS28) were used in the data analysis (25, 26). Extraarticular features were evaluated in patients with RA, and disease-specific features were evaluated for the other conditions by the specialist investigators.
The nuclear physician evaluated the FolateScan for uptake in appendicular skeletal joints (66) and other organs blinded to the results of the clinical examination. Scans were scored on a patient report form as no uptake, mild uptake, or marked uptake for each particular joint and organ. Mild uptake was scored if the region had about 2-fold greater count density (dark grey on inverted greyscale) than baseline joint activity (light grey) while marked uptake was scored if the region had had about 4-fold greater count density (black). Visual interpretation was used to best mimic general clinical practice methods of nuclear medicine scan interpretation.
C-reactive protein (C-rp) and Westergren erythrocyte sedimentation rate (ESR) were measured in all patients.
Statistical methods
Descriptive statistics (means, proportions, etc.) were used to summarize the data. Correlations were performed using Spearman methods since the ESR and C-rp values were not normally distributed. Jackknife techniques were used to estimate 95% confidence intervals for sensitivity, specificity, positive predictive value, negative predictive value and accuracy in order to account for multiple joints per patient (27).
Results
A total of 40 patients with RA (26 with one or more swollen joints, 14 with clinically quiescent joint disease; 66 joint count) as well as 6 patients with OA and 5 healthy control subjects received 0.1 mg of EC20 labeled with 20–25mCi of technetium-99m. Table I contains the demographic information and acute-phase reactant measurements of these patients.
Table I.
Demographics of study population receiving FolateScan.
| Study subjects | n. | Age, years (mean ± SD) |
Female n. (%) |
ESR median (range) |
C-rp median (range) |
|---|---|---|---|---|---|
| Rheumatoid arthritis | 40 | 56.9 ± 13.5 | 31 (78%) | 14.5 (0,82) | 6 (<3, 105) |
| Active | 26 | 59.2 ± 14.5 | 20 (77%) | 16.5 (2, 82) | 7 (<3, 105) |
| Inactive | 14 | 52.7 ± 10.8 | 11 (79%) | 10.5 (0, 26) | <3 (<3, 13) |
| Control | 5 | 51.0 ± 21.1 | 0 (0%) | 1 (1, 9) | <3 (<3, 3.2) |
| Osteoarthritis | 6 | 64.6 ± 11.2 | 5 (83%) | 9 (5, 21) | <3 (<3, 6.9) |
n: number; SD: standard deviation; ESR: erythrocyte sedimentation rate (Westergren); C-rp: C-reactive protein.
Pretreatment with 1 or 2 mg of folate resulted in attenuation of uptake in the parotid glands and joints, and therefore, was not used after the first 8 patients, all of whom had RA. There was no apparent influence of pretreatment with or without folate on the results of the uptake in other organs, and the remaining patients were examined without pretreatment with folate. Results of all examinations are considered in the analysis.
In patients with RA, FolateScan detected more actively involved joints (swelling and/or painful; 66 joint count) in 25 patients (63%) than joints recorded as swollen and/or painful by the examiner; fewer joints were identified by FolateScan compared to the examiner in 13 patients (22%); and in 2 patients (5%) the number of swollen or tender joints was recorded as being the same (Table II). Figure 1 demonstrates sample findings in patients with RA. Based on the 66 joint count, the sensitivity of FolateScan compared to the rheumatologist examination for detection of swelling and/or pain was 40% (95% confidence interval [CI]: 26–55%), specificity 86% (95% CI: 82–90%), positive predictive value 32% (95% CI: 21–43%), negative predictive value 90% (95% CI: 85–95%), accuracy 80% (95% CI: 75–84%). Results were similar if only swelling, or pain, or the 28 joint count were considered.
Table II.
Diagnostic testing measures for FolateScan.
| Disease | Sensitivity % |
Specificity % |
PPV % |
NPV % |
Accuracy* % |
Number (%) of patients with more joints assessed as active by clinical examination |
Number (%) of patients with same number of joints assessed as active on clinical and FolateScan examination |
Number (%) of patients with more joints assessed as active by FolateScan than by clinical examination |
|---|---|---|---|---|---|---|---|---|
| Rheumatoid arthritis only (n=40) | ||||||||
| Swelling (66 joints) | 47 | 85 | 21 | 95 | 82 | 9 (22%) | 4 (10%) | 27 (68%) |
| 19 | 96 | 31 | 93 | 90 | 19 (48%) | 6 (15%) | 15 (38%) | |
| Pain (66 joints) | 36 | 85 | 22 | 92 | 80 | 9 (22%) | 2 (5%) | 29 (72%) |
| 13 | 96 | 30 | 90 | 87 | 22 (55%) | 6 (15%) | 12 (30%) | |
| Swelling/pain (66 joints) | 40 | 86 | 32 | 90 | 80 | 13 (22%) | 2 (5%) | 25 (63%) |
| 14 | 97 | 41 | 88 | 85 | 22 (62%) | 4 (10%) | 11 (28%) | |
| Swelling (28 joints) | 46 | 79 | 26 | 90 | 74 | 9 (23%) | 5 (12%) | 26 (65%) |
| 17 | 95 | 33 | 88 | 84 | 18 (45%) | 10 (25%) | 12 (30%) | |
| Pain (28 joints) | 38 | 78 | 22 | 88 | 72 | 11 (28%) | 3 (7%) | 26 (65%) |
| 14 | 94 | 28 | 87 | 82 | 19 (48%) | 9 (22%0 | 12 (30%) | |
| Swelling/pain (28 joints) | 42 | 80 | 35 | 84 | 72 | 13 (33%) | 5 (12%) | 22 (55%) |
| 15 | 95 | 42 | 81 | 79 | 22 (55%) | 9 (22%) | 9 (22%) | |
| Osteoarthritis only (n=6) | ||||||||
| Swelling (66 joints) | 50 | 89 | 5 | 99 | 89 | 0 (0%) | 1 (17%) | 5 (83%) |
| 25 | 99 | 25 | 99 | 98 | 1 (17%) | 3 (50%) | 2 (33%) | |
| Pain (66 joints) | 37 | 92 | 39 | 92 | 86 | 3 (50%) | 0 (0%) | 3 (50%) |
| 7 | 100 | 75 | 89 | 89 | 6 (100%) | 0 (0%) | 0 (0%) | |
| Swelling/pain (66 joints) | 37 | 92 | 39 | 92 | 86 | 3 (50%) | 0 (0%) | 3 (50%) |
| 7 | 100 | 75 | 89 | 89 | 6 (100%) | 0 (0%) | 0 (0%) |
PPV: positive predictive value; NPV: negative predictive value.
Percentage of all joints in which the results of the clinical examination were concordant with the FolateScan findings.
Figures in bottom half of each cell in bold include only joints with “marked” uptake on the FolateScan as active by scan.
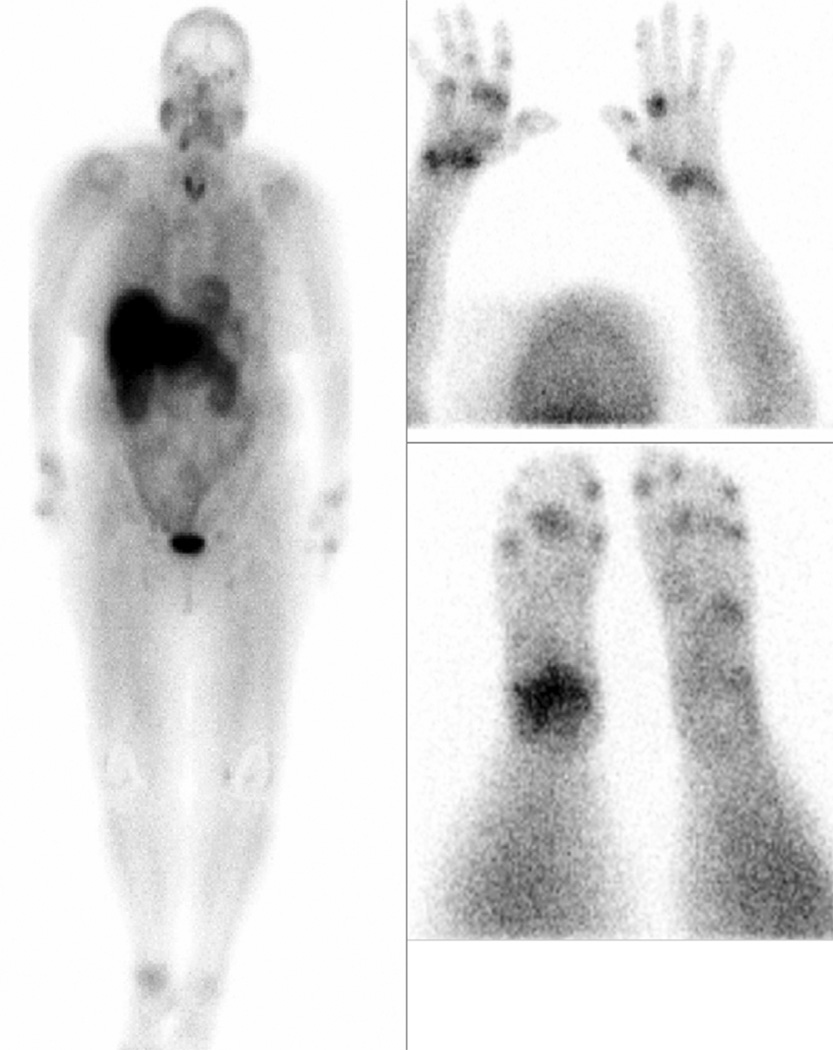
Fig. 1.
Whole body FolateScan of a patient with active rheumatoid arthritis demonstrating increased activity in multiple joints. In addition, there is activity in the parotid glands and salivary glands of the lower jaw. This latter uptake could be suppressed by pretreatment with 1 to 2 mg of iv folate.
In RA, FolateScan was more sensitive in detecting swollen or swollen and/or painful joints than was the clinical examination (Table III). For example, in patients with RA who had at least one actively swollen joint, FolateScan detected a total of 375 active joints (joints with increased uptake) compared to a total of 195 swollen joints detected by clinical examination. The total number of swollen joints detected by both the scan and the examination was 94 among patients with active RA, while the total number of swollen joints not detected by the FolateScan was 101, and the total number of swollen joints detected by the scan but not by examination was 281 (Table III). Results were similar for patients with RA and inactive disease: the FolateScan detected a total of 82 active joints not detected by the physical examination. In general, these results were also similar when either swelling and/or pain were considered.
Table III.
Comparison of joints assessed as swollen by clinical examination to joint uptake on FolateScan.
| Study subjects | n. | Number of swollen joints as assessed by clinical examination |
Number of active joints as assessed by FolateScan |
Number of clinically swollen joints detected by FolateScan |
Number of clinically swollen joints not detected by FolateScan |
Excess number of active joints detected by FolateScan compared to clinical examination |
|---|---|---|---|---|---|---|
| Rheumatoid arthritis | 40 | 205 | 460 | 97 | 108 | 363 |
| 124 | 38 | 167 | 86 | |||
| Active | 26 | 195 | 375 | 94 | 101 | 281 |
| 102 | 38 | 157 | 64 | |||
| Inactive | 14 | 10 | 85 | 4 | 7 | 82 |
| 22 | 0 | 10 | 22 | |||
| Osteoarthritis | 6 | 4 | 44 | 2 | 2 | 42 |
| 4 | 1 | 3 | 3 |
values in each cell are total number of joints assessed as involved for each cohort.
Figures in bold include only joints with “marked” uptake on the FolateScan as active by scan.
In patients with OA, only 4 joints were considered swollen on physical examination, and the FolateScan detected 2 of these along with 42 other joints. When swelling and/or pain was considered, 46 joints were identified by physical examination and the FolateScan detected only 17 of these along with 27 other joints. FolateScan had a specificity of 100% for painful/swollen joints (Tables II and III). An example of the findings in a patient with OA is depicted in Figure 2. Among RA patients, the number of swollen joints by clinical exam was correlated with ESR (r=0.43; p=0.006) and C-rp (r=0.35; p=0.03). The number of actively involved joints by FolateScan was also correlated with ESR (r=0.47; p=0.002) and C-rp (r=0.36; p=0.02). There was no correlation between uptake in the joints and acute phase reactants in patients with OA, although the numbers of patients was small.
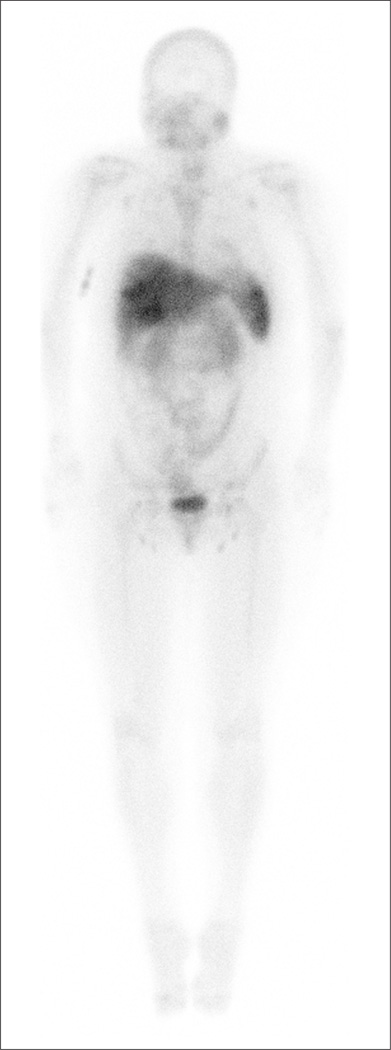
Fig. 2.
FolateScan in a patient with osteoarthritis of the knees, carpometacarpal joints of the thumbs, and distal interphalangeal joints of the hands, showing no increased uptake in the joints. FolateScan is avidly taken up in the spleen and liver in all subjects.
The primary analysis is based on any uptake of FolateScan in the joints. Because uptake was relatively weak in some joints, we performed a subanalysis of only joints with marked uptake to assess the effect on the scan performance. These results are presented in bold in the respective cells of Tables II and III, treating “mild” uptake as “none” for the scan. These analyses show a decrease in sensitivity for the FolateScan in the patients with RA (47% vs. 19% for swollen joints; Table II). There are not as many involved joints by the FolateScan compared to the clinical examination, because many of the clinically active joints are dismissed as uninvolved by the scan when this higher threshold is used. For the patients with OA, scan appeared more specific, and was often not as positive in painful OA joints, perhaps because they are not swollen (i.e. lack inflammation).
FolateScan uptake in extra-articular organs of patients with and without RA is presented in Table IV. FolateScan appeared to be safe, with bruising at the injection site being the only recorded side effect. No other adverse reactions were reported by any subjects.
Table IV.
FolateScan uptake in extra-articular organs.
| Patients with active rheumatoid arthritis (n=26) |
Patients with inactive rheumatoid arthritis (n=14) |
Healthy control subjects (n=5) |
Osteoarthritis (n=6) |
|
|---|---|---|---|---|
| Lung | 1 (4%) | 0 (0%) | 0 | 0 |
| Kidney | 26 (100%) | 12 (85%) | 5 (100%) | 6 (100%) |
| Skin | 0 (0%) | 0 (0%) | 0 | 0 (100%) |
| Brain | 0 (0%) | 0 (0%) | 0 | 0 (0%) |
| Large bowel | 16 (62%) | 8 (57%) | 5 (100%) | 4 (67%) |
| Small bowel | 16 (62%) | 7 (50%) | 4 (60%) | 5 (83%) |
| Liver | 25 (96%) | 14 (100%) | 5 (100%) | 6 (100%) |
| Spleen | 19 (73%) | 10 (68%) | 4 (75%) | 6 (100%) |
Discussion
FolateScan, technetium Tc 99m EC20, is a folate-targeted imaging agent which we hypothesized may have a potential application as a diagnostic test to detect inflammation in the synovium and extraarticular tissues. This increased uptake is in large part related to the increased numbers of macrophages seen in inflammatory conditions such as RA and other diseases.
Pre-clinical studies of RA imaging in both mice with collagen-induced arthritis and in rats with adjuvant-induced arthritis yielded results similar to those see in humans. Inflamed and/or swollen joints displayed significant uptake of EC20, and the livers of arthritic (but not healthy) rodents also demonstrated retention of the imaging agent (6, 9). Further, analyses of the cell type responsible for EC20 accumulation in inflamed tissues revealed exclusive uptake by activated macrophages in these rodent models (28).
The primary objectives of this study were to assess the safety and articular uptake of EC20 during active joint inflammation in patients with RA. In our study, FolateScan appeared to have relatively high specificity for detection of actively involved joints in patients with RA, with modest sensitivity. There was a striking difference in the uptake of FolateScan compared to joints assessed as active by clinical examination, suggesting either that the scan is overly sensitive for the detection of inflamed joints, or that the conventional clunial examination underestimates the true extent of joint activity in patients seen in the clinic. This would have important clinical implications, as it is well appreciated that in the face of even apparently clinically quiescent disease, continued low grade, even clinically undetectable inflammatory disease activity may lead to joint damage with the resultant consequences for joint function and patient well-being. A method of detecting such disease activity could advance the control of disease, justifying continued or more aggressive management strategies for such patients.
Most or all subjects including the normal controls and those with RA and OA had increased uptake in liver, spleen, kidney, and small and large bowel, likely limiting its utility for evaluation of inflammatory organ involvement. FolateScan is a relatively minimally invasive procedure which has a good safety profile. It has the potential to provide real-time information which is more complete than the clinical assessment, at least with respect to inflammatory arthritis. A methodological limitation of the study is that the clinical examination was used as the comparator standard, although it may not be sensitive enough to serve this purpose. Other potential comparators could include use of magnetic resonance imaging (MRI), ultrasonograpy (US) or synovial biopsy, but these have limitations in terms of validity, practicality and/or patient acceptance, whereby MRI is emerging as a potentially useful technique for evaluation of joint activity and damage (29, 30). The FolateScan technique allows imaging of all joints in a single session, and is less time consuming than MRI or US. In this study, the clinical examination of the joints was used as the standard for comparison of joint involvement, since this, and not MRI, is the standard of practice in the clinic and in clinical trials, and because funds were not available for MRI. Future studies of FolateScan in RA could include serial examination before and after therapeutic intervention to provide further evidence of the utility of the technique for assessing active arthritis as well as comparison to MRI.
Because depletion of macrophages greatly reduces folate receptor and abolishes uptake of EC20, it is conceivable that folic acid could be exploited to target therapeutic agents to sites of inflammatory autoimmune disease enriched in activated macrophages (7, 8). Further studies will serve to define the potential role and usefulness of FolateScan as a diagnostic tool to assess active inflammation, including comparisons with other techniques such as MRI and US and cost considerations, and to identify patients that might respond to folate-targeted therapies.
Aknowledgements
The authors would like to thank our study coordinators Jane Jaquith and Terry Brinkman for their organizational abilities and humanistic talent in making this study possible.
The study agent, FolateScan™, and technical support were provided by Endocyte Inc. The study was funded by the Gund Foundation of the Mayo Clinic.
Footnotes
Conflict of interest: Dr. Matteson is a member of the scientific advisory board for inflammatory diseases for Endocyte Inc.; Drs. Low, Messmann and Morgenstern are employees of Endocyte Inc.; the other co-authors have declared no competing interests.
References
- 1.Kinne RW, Brauer R, Stuhlmuller B, Palombo-kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tak PP, Smeets TJ, Daha RR, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 4.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:15–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 5.Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999;26:717–719. [PubMed] [Google Scholar]
- 6.Turk MJ, Breur GJ, Widmer WR, et al. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002;46:1947–1955. doi: 10.1002/art.10405. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima-matsushita N, Homma T, Yu S. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1609–1616. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;29:1205–1217. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Turk MJ, Waters DJ, Low PS. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004;213:165–172. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Shen F, Ross JF, Wang X, Ratnam M. Identification of a novel folate receptor, a truncated receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry. 1994;33:1209–1215. doi: 10.1021/bi00171a021. [DOI] [PubMed] [Google Scholar]
- 11.Garin-chesa P, Campbell I, Saigo PE, Lewis JL, JR, Old LJ, Rettig WJ. Trophoblast and ovarian cancer antigen LK26: sensitivity and specificity in immunopathology and molecular identification as a folate-binding protein. Am J Pathology. 1993;142:557–567. [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Cancer. 1994;73:2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani LT, Miotti S, MÉnard S, et al. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies Mov18 and Mov19. Eur J Cancer. 1994;3:363–369. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 14.Mattes MJ, Major PP, Goldenberg DM, Dion AS, Hutter RVP, Klein KM. Patterns of antigen distribution in human carcinomas. Cancer Research Suppl. 1990;50:880S. [PubMed] [Google Scholar]
- 15.Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Research. 1992;52:3396–3401. [PubMed] [Google Scholar]
- 16.Siegel BA, Dehdashti F, Mutch DG, et al. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical results. J Nucl Med. 2003;44:700–707. [PubMed] [Google Scholar]
- 17.Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005;94:2135–2146. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 18.Low PS, Henne WA, Doorneweer DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008 doi: 10.1021/ar7000815. in press. [DOI] [PubMed] [Google Scholar]
- 19.Leamon CP, Parker MA, Vlahov IR, et al. Synthesis and biological evaluation of EC20: a new folate-derived,99mTc-based radio-pharmaceutical. Bioconjugate Chem. 2002;13:1200–1210. doi: 10.1021/bc0200430. [DOI] [PubMed] [Google Scholar]
- 20.Reddy JA, Xu LC, Parker N, Vetzel M, Leamon CP. Preclinical evaluation of (99m)Tc-EC20 for imaging folate receptor-positive tumors. J Nucl Med. 2004;45:857–866. [PubMed] [Google Scholar]
- 21.Wang S, Luo J, Lantrip DA, et al. Design and synthesis of [111In]DTPA-folate for use as a tumor-targeted radiopharmaceutical. Bioconjug Chem. 1997;8:673–679. doi: 10.1021/bc9701297. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RE, Siegel BA, Edell SL, et al. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J Nucl Med. 2008;49:899–906. doi: 10.2967/jnumed.107.049478. [DOI] [PubMed] [Google Scholar]
- 23.Chen WT, Mahmood U, Weissleder R, Tung CH. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res Ther. 2005;7:R310–R317. doi: 10.1186/ar1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo ML, Van’t hof MA, Kuper HH, Van leeuwen MA, Van de putte LB, Van riel PL. Modified disease activity scores that include twenty-eight joint counts. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 26.Scott DL, Antoni C, Choy EH, Van riel PCLM. Joint counts in routine practice. Rheumatology. 2003;42:919–923. doi: 10.1093/rheumatology/keg235. [DOI] [PubMed] [Google Scholar]
- 27.Efron B. Regional Conference Series in Applied Mathematics 38. Philadelphia: SIAM; 1982. The jackknife, the bootstrap and other resampling plans. [Google Scholar]
- 28.Paulos CM, Varghese B, Widmer R, Breur GJ, Vlashi E, Low PS. Folate-targeted immunotherapy effectively treats established adjuvant and collagen-induced arthritis. Arthritis Res Ther. 2006;8:R77. doi: 10.1186/ar1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mcqueen F, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999;58:156–163. doi: 10.1136/ard.58.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durez P, Malghem J, Toukap AN, et al. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 2007;56:3919–3327. doi: 10.1002/art.23055. [DOI] [PubMed] [Google Scholar]