Abstract
Air pollutants have been associated with increased diabetes in humans. We hypothesized that ozone would impair glucose homeostasis by altering insulin signaling and/or endoplasmic reticular (ER) stress in young and aged rats. One, 4, 12, and 24 month old Brown Norway (BN) rats were exposed to air or ozone, 0.25 or 1.0 ppm, 6 h/day for 2 days (acute) or 2 d/week for 13 weeks (subchronic). Additionally, 4 month old rats were exposed to air or 1.0 ppm ozone, 6 h/day for 1 or 2 days (time-course). Glucose tolerance tests (GTT) were performed immediately after exposure. Serum and tissue biomarkers were analyzed 18 h after final ozone for acute and subchronic studies, and immediately after each day of exposure in the time-course study. Age-related glucose intolerance and increases in metabolic biomarkers were apparent at baseline. Acute ozone caused hyperglycemia and glucose intolerance in rats of all ages. Ozone-induced glucose intolerance was reduced in rats exposed for 13 weeks. Acute, but not subchronic ozone increased α2-macroglobulin, adiponectin and osteopontin. Time-course analysis indicated glucose intolerance at days 1 and 2 (2> 1), and a recovery 18 h post ozone. Leptin increased day 1 and epinephrine at all times after ozone. Ozone tended to decrease phosphorylated insulin receptor substrate-1 in liver and adipose tissues. ER stress appeared to be the consequence of ozone induced acute metabolic impairment since transcriptional markers of ER stress increased only after 2 days of ozone. In conclusion, acute ozone exposure induces marked systemic metabolic impairments in BN rats of all ages, likely through sympathetic stimulation.
Keywords: Aging, Air pollution, Ozone, Metabolic syndrome, Serum biomarkers, Epinephrine
Introduction
Epidemiological studies have demonstrated a positive association between long-term-exposures to ambient air pollutants, namely particulate matter (PM) and ozone (O3), and incidence of cardiovascular diseases. Atherosclerosis as measured by increasing carotid intimal medial thickness has been associated with ambient PM (Adar et al., 2013; Brook and Rajagopalan, 2010; Gan et al., 2011; Künzli, 2013). The role of life-stage as a determinant of an individual's susceptibility to the cardiovascular health effects of air pollution is poorly defined. Because more than 100 million Americans live in areas of the US that do not meet EPA air quality standards for O3 (U.S. Environmental Protection Agency, 2012) and the age distribution within the US is rapidly shifting upwards, age-related susceptibility has important public health relevance. Advancing age heralds an increased likelihood of the appearance of cardiovascular risk factors, systemic inflammation, endothelial dysfunction, insulin resistance, metabolic syndrome, diabetes, the progression of atherosclerotic vascular disease and subsequent clinical events. Animal studies and new epidemiological studies are now beginning to provide some mechanistic insights.
Recently air pollution, especially PM, has been linked to increased incidence of metabolic syndrome (Brook et al., 2008; Chen and Schwartz, 2008; Liu et al., 2013; Sun et al., 2013). Long-term exposure to vehicular traffic, as estimated by proximity of residence to highways, is positively associated with increased insulin resistance in children (Thiering et al., 2013). Yet, none of these studies provide any information about the effects of ozone and the role of age as a determinant of cellular metabolic response in relation to PM or O3. To address this gap in knowledge, we sought to characterize the metabolic response to O3 exposure in Brown Norway (BN) rats and the influence of age. In contrast to Sprague–Dawley, Wistar and Fischer 344 rats that develop spontaneous or diet related diseases and obesity (Christian et al., 1998; Newby et al., 1990), the BN rat represents a model of normative human aging without development of such complications. To simulate healthy human aging, we used BN rats (Lipman et al., 1996) of different ages to examine metabolic effects of acute and subchronic O3 exposure.
There are two potential mechanisms by which exposure to air pollutants, such as ozone, can cause systemic metabolic effects. 1) Lung injury from pollutant exposure might cause systemic release of mediators, such as cytokines, oxidatively modified proteins and lipids, or vasoactive substances which can provoke metabolic response in distant organs (liver, muscle and adipose tissues). 2) Inhalation of pollutants (ozone) can stimulate C-fiber mediated neuronal response that through stress pathway produce metabolic effects in distant organs. Cardiovascular effects of air pollutants have been postulated to occur through autonomic stimulation and systemic mediators (Gackiere et al., 2011). Yet, systemic mediators or neurohumoral factors responsible for metabolic alterations have not been identified. It has been shown that chronic ambient PM exposure induces metabolic impairments and liver endoplasmic reticular stress (Laing et al., 2010; Özcan et al., 2004; Sun et al., 2009), however it is not known if O3 exposure also produces such effects in the liver, and if so, could produce ER stress. Moreover, it is not known whether air pollution-induced metabolic effects could lead to, or exacerbate, insulin resistance, obesity, and type2 diabetes in susceptible individuals. The primary goals of this study were to examine 1) if O3, a prototypic air pollutant, might induce metabolic alterations in glucose homeostasis in BN rats, 2) whether these alterations are associated with increased circulating cytokines or neurohormonal mediators of the stress response, and 3) whether there is greater susceptibility of very young versus very old rats relative to young adult animals, commonly used in air pollution and other toxicology research. We hypothesized that acute ozone exposure will cause impaired glucose homeostasis and liver endoplasmic reticular stress associated with increases in stress hormones and that these effects will be exacerbated in old rats due to their compromised metabolic processes. We also hypothesized that subchronic ozone exposure would result in exacerbated impairment in glucose homeostasis, which could potentially result in impaired insulin sensitivity. We examined acute and subchronic effects of O3 with a time-course evaluation for systemic metabolic alterations and liver ER stress in healthy aging BN rats.
Materials and methods
Animals
Male Brown Norway rats were purchased from (Charles River Laboratories, Kingston, NY, or Portage, MI). Except for 1 and 3 month old rats, retired breeders were purchased at 8–10 month age and allowed to age at our NHEERL, EPA facility. All rats were maintained in AAALAC approved facilities, housed individually in polycarbonate cages (25 cm × 15 cm × 50 cm) containing laboratory-grade pine shavings (Granville Mills, Creedmore, NC). Colony rooms were maintained at constant temperature (22 °C), humidity (50% RH) and a 12 h light, 12 h dark illumination-cycle. Access to food (Rodent Chow 5001: Ralston Purina Laboratories, St. Louis, MO) and tap water was available ad libitum. The Institutional Animal Care and Use Committee (U.S. EPA NHEERL) approved the animal research protocol.
Ozone generation and animal exposures
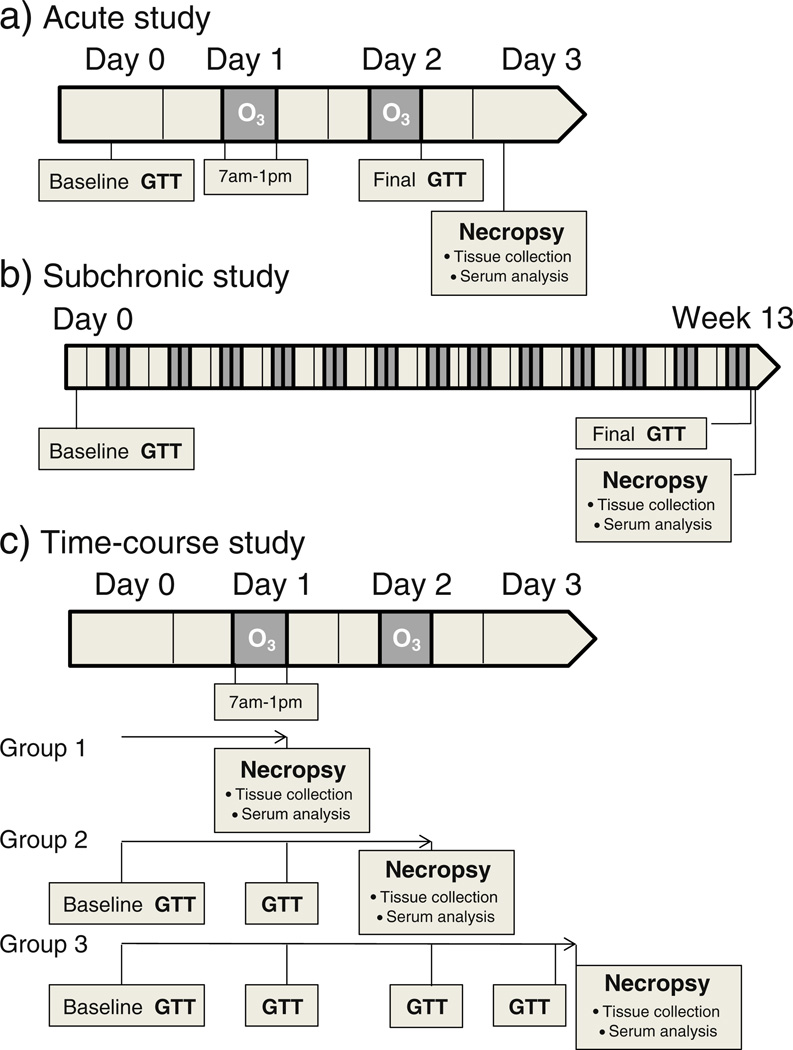
A silent arc discharge generator (OREC, Phoenix, AZ) generated O3 from oxygen. Mass flow controllers regulated the entry of O3 into the Rochester style “Hinners” chambers. Photometric O3 analyzers (API Model 400) monitored the O3 concentrations in the chambers. Three different types of exposure studies were conducted and are described as the “acute”, “subchronic” and “time-course” studies (Fig. 1). Exposure chamber conditions and actual ozone concentrations achieved are shown in Supplementary Material, Table 1. For the acute study, 1, 4, 12 and 24 month old BN rats (n=8–12/age group) were exposed for 6 h/day on 2 consecutive days to either FA (0 ppm) or O3 (0.25 ppm and 1.0 ppm) (Fig. 1a). For the subchronic study, 1, 9 and 21 month old BN rats (n=8–12/age group) were exposed to FA or O3 (0.25 or 1.0 ppm) for 6 h/day × 2 days/week over a 13 week period (referred to here by their ages at the end of the 13 week period: 4, 12, and 24 months) (Fig. 1b). For the time-course study, 4 month old BN rats (n =6) were exposed to FA or 1.0 ppm O3 6 h/day for 1 day or for two consecutive days. One group of rats was allowed to recover for 18 h after 2 days of O3 exposure (Fig. 1c).
Fig. 1.
Schematics of the study design for acute, subchronic and time-course experiments. Timing of glucose tolerance test (GTT), O3 exposures, and necropsy/serum and tissue collection are shown for each sub-study. Post-exposure GTTs were done immediately following exposure whereas necropsy and tissue and serum collection were done either 18 h after exposure (acute, subchronic, and group 3 time-course study) or immediately following exposure (group 1 and 2 time-course study).
Glucose tolerance testing (GTT)
For animals undergoing subchronic FA or O3 exposure, GTT was performed 2–3 days prior to the first O3 exposure (baseline), and immediately after 2 consecutive days of exposure during week 1 and week 13, to obtain data for acute and subchronic exposures, respectively. For the time-course study, rats designated for the 18 h recovery group underwent GTT. In these rats, GTT was performed 2–3 days prior to O3 exposure, immediately after the 1st day of 6 h O3 exposure, immediately after the 2nd day of O3 exposure, and after an 18 h recovery period prior to necropsy. Rats were fasted for 8 h prior to glucose tolerance tests. In instances where GTT followed immediately after O3 exposure, rats were fasted during exposure periods. For the 18 h recovery period, food was removed 8–10 h prior to GTT and necropsy (overnight starting from 10 pm). Prior to glucose injections, baseline glucose levels were measured by pricking the distal surface of rats' tails with a sterile needle, to obtain ~1 µl of blood. A Bayer Contour glucose meter was used to determine blood glucose levels, using test strips, which require 0.6 µL whole blood. After the 1st measurement, rats were given an intraperitoneal injection of glucose solution with a dose of 2 g/kg/10 ml (20% d-glucose; 10 ml/kg). Measurement with the glucose meter was repeated every 30 min over the course of 2 h.
Necropsy and sample collection
For the acute and subchronic studies, rats were necropsied 18 h after the final O3 exposure. These rats had undergone GTT immediately after the final exposure on the day prior to necropsy and were not fasted prior to necropsy (Figs. 1a–b). For the time-course study, one group of rats did not undergo GTT and was necropsied immediately after the 1st 6 h O3 exposure. The 2nd group of rats underwent baseline GTT as well as GTT after the first day of exposure, and was necropsied immediately after 2nd day of O3 exposure. The 3rd group underwent GTT as indicated above and was necropsied 18 h post 2nd day O3 exposure (Fig. 1c). In each case, rats in the time-course study were fasted for 8–10 h prior to necropsy. Rats were weighed and anesthetized with an overdose of sodium pentobarbital (Virbac AH, Inc., Fort Worth, TX; 50–100 mg/kg, ip). Blood samples were collected through an abdominal aortic puncture directly into vaccutainers without coagulant for serum preparation. Tubes were centrifuged at 3500 ×g for 10 min and aliquots of serum were stored at −80 °C until analyzed. Heart and lung tissues were processed and preserved for a separate experiment. Liver, gracilis leg muscle, and abdominal adipose tissues were collected and frozen in liquid nitrogen for protein and RNA analysis.
Serum analysis
Rat-specific electrochemiluminescence assays (Meso Scale Discovery, Gaithersburg, MD) were used to measure A2M, α1-acid glycoprotein (AGP), adiponectin, glucagon, insulin, interleukin 6 (IL-6) and leptin in the serum (leptin was not analyzed in the acute or subchronic studies). In the time-course study, epinephrine was measured in undiluted serum using a rat specific ELISA kit (CUSA BIO, Wuhan, China). Total cholesterol (CHOL) and triglycerides (TRI) were measured in serum samples using kits from TECO Diagnostics (Anaheim, CA) while high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterols were measured with kits from Thermo Fisher Scientific, Inc. (Middletown, VA). Both types of kits were modified for use on the Konelab Arena 30 system (Thermo LabSystems, Espoo, Finland).
Analysis of tissue phosphoproteins
Tissue homogenates were prepared from liver, muscle, or adipose tissue disrupted with a probe homogenizer in lysis buffer containing protease and phosphatase inhibitors. The resulting homogenates were centrifuged at 13000 × g for 10 min at 4 °C. Liver, muscle, and adipose tissue extracts were measured for phospho-Akt, phospho-glycogen synthase kinase-3β, and phospho-insulin receptor substrate 1. All assays were performed using rat specific kits via the manufacturer's instructions (Meso Scale Discovery, Gaithersburg, MD). Supernatant protein levels were analyzed using a Coomassie Plus Protein Assay kit (Pierce, Rockford, IL). The assay was modified and adapted for use on the Konelab Arena 30 Clinical Analyzer (Thermo Chemical Lab Systems, Espoo, Finland).
RNA isolation and real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total liver and abdominal adipose tissue RNA was isolated from ~20 mg tissue each with a commercially available RNeasy mini kit (Qiagen, Valencia, CA) using silica gel membrane purification. RNA was resuspended in 40 µl RNAse free water. RNAse inhibitor was added and RNA yield was determined spectrophotometrically on a NanoDrop 1000 (Thermo Scientific, Wilmington, DE). Each RNA sample was diluted to a uniform concentration of 25 ng/ul and stored until RT-PCR was carried out. One-step real-time RT-PCR was done using the SuperScript III One-step RT-qPCR kit from Invitrogen (Grand Island, NY). All reactions were run in duplicate using 25 ng total RNA. 18S ribosomal RNA (18S) was run as an endogenous control for each sample separately. RT-PCR was conducted on an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Foster City, CA). RT-PCR conditions were as follows: 20 min at 53 °C for reverse transcription, 2 min at 95 °C for inactivation of reverse transcriptase, followed by 40 cycles of 15 s at 95 °C and 45 s at 60 °C. PCR for each transcript was run separately (parallel amplification). Primers were purchased from ABI as inventoried TaqMan Gene Expression Assays, each containing a 6-carboxy-fluorescein (FAM dye) label at the 5′ end. RT-PCR methods are detailed previously (Gordon et al., 2013). Data were analyzed using ABI sequence detection software (SDS), version 2.2. For each PCR plate, cycle threshold (Ct) was set to an order of magnitude above background. For each individual sample, target gene Ct was normalized to a control (18S) Ct to account for variability in starting RNA amount. Expression of each exposure group was quantified as fold difference over FA control, at the corresponding timepoint.
Statistical analysis
Acute and subchronic study markers and GTT were analyzed using a two-way analysis of variance (ANOVA). The two independent variables were age and exposure. The time-course GTT was analyzed by a two-way repeated measures MANOVA (multivariate ANOVA). The two independent variables were day and exposure. Time-course study biomarker measurements were analyzed using a one-way analysis of variance (ANOVA). The independent variable was formed by combining the three variables: day, exposure, and sacrifice time.
For acute and subchronic studies (biomarkers and other endpoints) ANOVA was used. In all cases, if the ANOVA assumptions were not satisfied by the data, a transformation was applied to that data and then, if the assumptions were satisfied, the ANOVA was performed on the transformed data. If the original data and the transformed data failed to address the violations of the assumptions, a distribution free method was employed. Pair wise comparisons were performed as subtests of the overall ANOVA. The nominal Type I error rate (α) was set at 0.05. No adjustments were made for multiple comparisons. While this results in an increased overall Type I error, the Type II error rate (β) remained in control.
Sigma Stat 3.5 was used to compare age effects in GTT baseline data. The 4 month group was compared with all other age groups using a one way ANOVA followed by Dunnett's or Dunn's post hoc comparison for significance at p=0.05. The data for each timepoint were analyzed independently. Epinephrine data were analyzed using two-way ANOVA followed by Duncan's multiple range test.
Results
Age and ozone effects on baseline blood glucose and glucose tolerance
Age effects
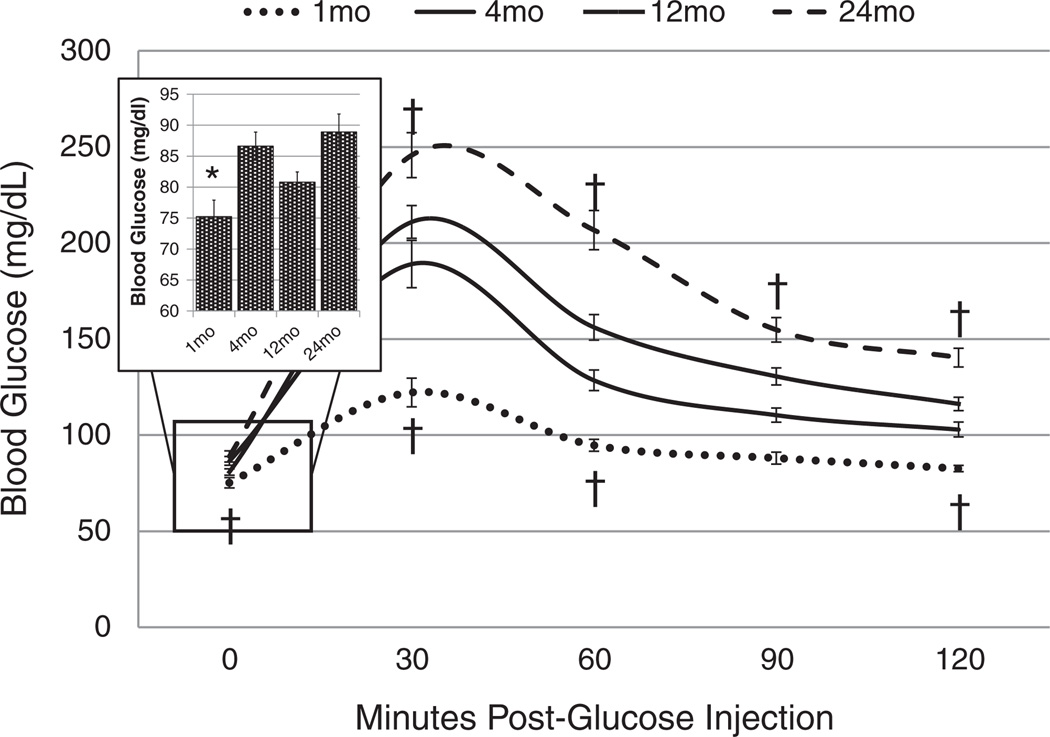
Baseline age-related differences in glucose tolerance were observed prior to exposure. Fasting glucose measured prior to O3 exposure was lowest in 1 month old rats when compared to other age groups (Fig. 2 insert). Additionally, clearance of intraperitoneally injected glucose was fastest in 1 month old rats and slowest in 24 month old rats. Compared to 4 month old rats, 24 month old rats had elevated blood glucose at all post-glucose injection timepoints during GTT (Fig. 2).
Fig. 2.
Glucose tolerance test (GTT) comparison of 1, 4, 12, and 24 month old BN rats prior to the start of O3 exposure. Inset graph shows fasting blood glucose levels for all ages. Each value is the mean blood glucose measurement ± S.E. of 18 to 21 rats. Mean values of blood glucose at each timepoint are compared for age-factor, relative to 4 month old rats († = p < 0.05 †† = p < 0.01). 0 ppm indicates FA exposure.
Ozone effects
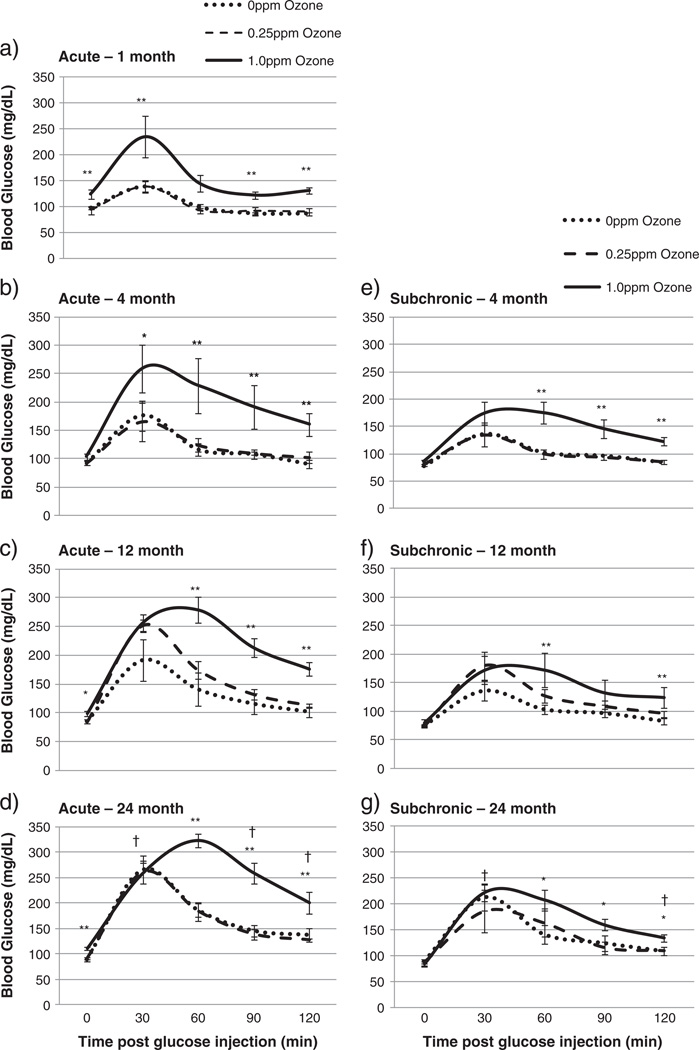
O3 exposure resulted in marked glucose intolerance in all three studies when measured immediately after exposure. Figs. 3a–d show glucose tolerance after 2 consecutive days of O3 exposure for all four age groups in the acute study. One, 12, and 24 month old rats showed elevated fasting blood glucose after 1 ppm O3 exposure. Rats in all age groups exposed to 1 ppm O3 had impaired clearance of glucose, relative to FA groups.
Fig. 3.
Glucose tolerance test (GTT) comparison of BN rats after acute or subchronic exposure to FA or O3, performed immediately after O3 exposure. Each value is the mean blood glucose measurement ±S.E. of 4 to 10 rats. The individual plots show acute GTT results; a) 1 month b) 4 month c) 12 month d) 24 month; and subchronic GTT results, e) 4 month f) 12 month g) 24 month. Mean values of blood glucose at each timepoint are compared for age-factor, relative to 4 month old rats († = p < 0.05 ††=p < 0.01). Significant exposure effects are indicated relative to FA exposed group (* = p < 0.05 ** = p < 0.01). 0 ppm indicates FA exposure.
In rats subchronically exposed to O3 over 13 weeks, glucose tolerance remained impaired, but to a lesser degree than in the acute exposures (Figs. 3e–g). All age groups exposed to 1.0 ppm O3 had elevated blood glucose at the end of GTT, relative to FA exposed rats, but fasting blood glucose was not elevated by ozone in any age group when compared to FA exposed rats.
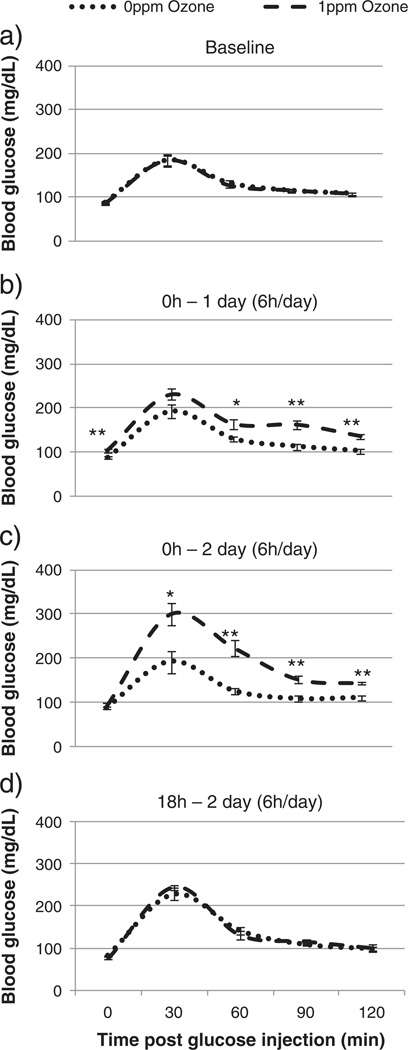
GTT was performed immediately after the final O3 exposure in the acute and subchronic aging studies. However, tissue analysis in these studies was done the following day, 18 h after final exposure. To determine whether the O3 effects on tissues coincided with glucose intolerance immediately after each day of O3 and after 18 h recovery, a time-course study was conducted using 4 month old BN rats. Time-course analysis showed that in 4 month old rats, O3 induced hyperglycemia on day 1 but not on day 2 (no hyperglycemia was seen after 2 days in 4 month old rats even in the acute study). Glucose intolerance began on day 1 of acute 1 ppm O3 exposure and increased on day 2 of exposure (Figs. 4b–c). At 18 h after exposure, glucose tolerance returned to baseline levels, equivalent to glucose tolerance in the FA exposed group (Fig. 4d).
Fig. 4.
Glucose tolerance test (GTT) comparison of BN rats after exposure to FA or O3 in the time-course study. Each value is the mean blood glucose measurement ± S.E. of 4 to 10 rats. Mean values of blood glucose at each timepoint are compared for significant exposure effects relative to FA exposed group (* = p < 0.05 ** = p < 0.01). 0 ppm indicates FA exposure.
Serum lipids
Age effects
Serum triglycerides and cholesterols were compared for age-related differences relative to 4 month old FA exposed rats (acute and subchronic studies). Significant differences existed in levels of serum lipids among the examined age groups. Elevated total cholesterol, HDL, LDL, and triglycerides were noted in 1 month old rats relative to 4 month old rats (Table 1). Twenty-four month old rats had elevated serum total cholesterol and triglycerides in both acute and subchronic study groups when compared to 4 month old rats. Additionally, 24 month old rats had higher HDL cholesterol than 4 month old rats in the subchronic study, but this trend did not reach significance in the acute study.
Table 1.
Comparison of serum lipids in BN rats after acute and subchronic exposure to FA or O3.
| Age | O3 | CHOL mg/dl | HDL CHOL mg/dl | LDL CHOL mg/dl | TRIG mg/dl |
|---|---|---|---|---|---|
| Acute | |||||
| 1 month | 0 ppm | 71.61 ± 4.09†† | 26.49 ± 1.4†† | 16.36 ± 0.96†† | 25.47 ± 1.72† |
| 0.25 ppm | 73.29 ± 4.17 | 26.41 ± 1.11 | 16.15 ± 0.59 | 22.91 ± 1.79 | |
| 1 ppm | 76.22 ± 2.95 | 23.89 ± 1.33 | 16.89 ± 1.98 | 25.87 ± 3.67 | |
| 4 months | 0 ppm | 51.92 ± 1.86 | 19.64 ± 0.41 | 11.16 ± 0.57 | 40.68 ± 5.88 |
| 0.25 ppm | 54.46 ± 1.44 | 19.88 ± 0.65 | 11.7 ± 0.57 | 42.78 ± 4.72 | |
| 1 ppm | 56.05 ± 1.33 | 18.71 ± 0.75 | 11.08 ± 0.26 | 37.37 ± 5.04 | |
| 12 months | 0 ppm | 55.89 ± 3.38 | 18.25 ± 0.36 | 10.43 ± 0.98 | 50.74 ± 10.75 |
| 0.25 ppm | 59.58 ± 2.26 | 20.48 ± 0.83 | 11.56 ± 0.48 | 47.7 ± 2.16 | |
| 1 ppm | 58.43 ± 1.5 | 21.55 ± 0.37** | 9.25 ± 0.39 | 49.27 ± 3.44 | |
| 24 months | 0 ppm | 71.58 ± 2.95†† | 20.25 ± 0.75 | 11.63 ± 1.00 | 65.12 ± 4.83†† |
| 0.25 ppm | 69.38 ± 1.91 | 19.33 ± 1.3 | 10.91 ± 0.57 | 61.36 ± 3.61 | |
| 1 ppm | 71.89 ± 1.93 | 20.93 ± 0.57 | 10.18 ± 0.68 | 89.02 ± 12.26 | |
| Subchronic | |||||
| 4 months | 0 ppm | 45.66 ± 1.13 | 19.17 ± 0.57 | 10.03 ± 0.55 | 39.9 ± 3.33 |
| 0.25 ppm | 47.33 ± 2.42 | 20.66 ± 0.62 | 10.2 ± 0.78 | 39.09 ± 2.66 | |
| 1 ppm | 44.71 ± 2.37 | 19.3 ± 0.61 | 9.88 ± 0.53 | 34.56 ± 4.04 | |
| 12 months | 0 ppm | 51.16 ± 2.51 | 19.05 ± 0.56 | 11.32 ± 0.42 | 52.73 ± 5.97 |
| 0.25 ppm | 50.54 ± 1.73 | 20.76 ± 0.90 | 9.59 ± 0.33 | 40.31 ± 4.91 | |
| 1 ppm | 57.25 ± 1.13 | 22.05 ± 0.45** | 9.97 ± 0.43 | 46.98 ± 4.81 | |
| 24 months | 0 ppm | 66.65 ± 3.70†† | 22.94 ± 0.98†† | 10.71 ± 1.12 | 95.31 ± 15.33†† |
| 0.25 ppm | 61.69 ± 3.25 | 20.14 ± 1.07* | 9.95 ± 0.57 | 62.82 ± 7.10* | |
| 1 ppm | 66.38 ± 3.03 | 21.91 ± 0.69 | 10.82 ± 0.25 | 78.02 ± 2.87 |
Serum samples were collected during necropsy 18 h after final acute or subchronic exposure and analyzed as described in methods. Values represent non-fasting serum levels.
Abbreviations: total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL CHOL), low-density lipoprotein cholesterol (LDL CHOL), and triglycerides (TRIG).
Each value is the mean ± S.E. of 5–12 rats/group.
Mean values of each serum component are compared for age-factor relative to 4 month old rats († = p < 0.05 †† = p < 0.01).
Significant exposure effects are indicated relative to FA exposed group (* = p < 0.05 ** = p < 0.01).
0 ppm indicates FA exposure.
Ozone effects
O3 at 1 ppm increased serum HDL cholesterol of rats 12 months old in both the acute and subchronic studies (Table 1). These were no other significant O3 exposure-induced changes in total cholesterol or LDL cholesterol in any other age groups. Time-course analysis of 4 month old rats exposed to 1 ppm O3 showed no significant changes in any of the serum lipids tested (Data not shown).
Serum biomarkers of inflammation, acute phase response and metabolic syndrome
Age effects
Age-related increases were seen in IL-6, insulin, A2M, and adiponectin, while GLP-1 and glucagon were decreased with increasing age (Table 2). In 1, 4, 12 and 24 month old rats (FA exposed, acute study), AGP showed no clear age-related trends, except for elevated levels in 1 month old rats relative to the 4 month old group. In the subchronic study, FA exposed rats (4, 12, and 24 month old) appeared to follow the same general age-related trends in serum markers, with increases of insulin, A2M, and adiponectin.
Table 2.
Comparison of serum biomarkers in BN rats after acute and subchronic exposure to FA or O3.
| Acute | |||||
| Biomarker | O3 | 1mo | 4mo | 12mo | 24mo |
| IL-6 | 0 ppm | 27.43 ± 4.34†† | 48.55 ± 1.79 | 50.8 ± 3.75 | 55.1 ± 1.94 |
| (pg/ml) | 1 ppm | 34.94 ± 3.71 | 45.95 ± 2.52 | 54.05 ± 4.38 | 63.68 ± 2.75 |
| Insulin | 0 ppm | 284.53 ± 52.29†† | 576.57 ± 60.92 | 939.76 ± 185.97 | 902.45 ± 69.85† |
| (pg/ml) | 1 ppm | 264.72 ± 16.79 | 451.34 ± 53.34 | 1047.63 ± 246.34 | 1136.95 ± 161.26 |
| GLP-1 | 0 ppm | 29.29 ± 3.06 | 25.76 ± 1.39 | 22.07 ± 2.38 | 18.93 ± 1.27†† |
| (pg/ml) | 1 ppm | 30.53 ± 1.38 | 27.7 ± 1.2 | 23.36 ± 1.32 | 20.17 ± 1.29 |
| Glucagon | 0 ppm | 304.17 ± 18.96†† | 200.9 ± 22.52 | 123.56 ± 25.45† | 110.69 ± 7.35†† |
| (pg/ml) | 1 ppm | 322.2 ± 30.43 | 203.75 ± 15.86 | 145.58 ± 19.62 | 111.68 ± 11.02 |
| AGP | 0 ppm | 79.07 ± 18.63 | 136.16 ± 19.55 | 140.19 ± 13.88 | 232.74 ± 61.11 |
| (ng/ml) | 1 ppm | 429.63 ± 186.06** | 144.38 ± 18.11 | 134.66 ± 8.17 | 237.46 ± 33.81 |
| A2M | 0 ppm | 35.9 ± 2.12 | 15.04 ± 2.43 | 10.86 ± 2.19 | 16.14 ± 9.26 |
| (ng/ml) | 1 ppm | 114.9 ± 46.05** | 10.92 ± 1 | 7.62 ± 0.3 | 7.47 ± 0.56 |
| Adiponectin | 0 ppm | 3578 ± 653 | 5529 ± 616 | 5679 ± 447 | 8337 ± 1648† |
| (ng/ml) | 1 ppm | 13,066 ± 4586** | 5801 ± 571 | 5515 ± 263 | 8596 ± 939 |
| Subchronic | |||||
| Biomarker | O3 | – | 4mo | 12mo | 24mo |
| IL-6 | 0 ppm | – | 48.67 ± 1.82 | 55.53 ± 4.7 | 55.66 ± 3.3 |
| (pg/ml) | 1 ppm | – | 53.89 ± 2.17 | 54.08 ± 3.66 | 62.59 ± 3.49 |
| Insulin | 0 ppm | – | 686.92 ± 45.01 | 1176.69 ± 75.59†† | 1436.6 ± 192.78†† |
| (pg/ml) | 1 ppm | – | 558.64 ± 74.71 | 1068.22 ± 159.55 | 1356.59 ± 88.96 |
| GLP-1 | 0 ppm | – | 24.75 ± 1.86 | 20.84 ± 1.35 | 20.67 ± 2.37 |
| (pg/ml) | 1 ppm | – | 25.41 ± 1.14 | 22.51 ± 1.48 | 20.15 ± 1.75 |
| Glucagon | 0 ppm | – | 163.61 ± 20.65 | 117.12 ± 15.14 | 113.56 ± 32.19 |
| (pg/ml) | 1 ppm | – | 166.48 ± 11.08 | 130.66 ± 17.57 | 123.02 ± 22.3 |
| AGP | 0 ppm | – | 128.28 ± 12.02 | 130.4 ± 11.38 | 250.19 ± 62.17†† |
| (ng/ml) | 1 ppm | – | 106 ± 3.73 | 132.71 ± 8.15 | 255.96 ± 46.82 |
| A2M | 0 ppm | – | 11.55 ± 0.97 | 10.31 ± 1.36 | 12.08 ± 3.62 |
| (ng/ml) | 1 ppm | – | 10.28 ± 0.55 | 8.95 ± 0.64 | 8.9 ± 0.69 |
| Adiponectin | 0 ppm | – | 5297 ± 385 | 5371.46 ± 371 | 8814 ± 1729†† |
| (ng/ml) | 1 ppm | – | 4567 ± 127 | 5452 ± 265 | 9081 ± 1276 |
Serum samples were collected during necropsy 18 h after final acute or subchronic exposure and analyzed as described in methods. Values represent non-fasting serum levels.
Abbreviations: interleukin 6 (IL-6), Glucagon-like peptide-1 (GLP-1), α1-acid glycoprotein (AGP), α2-macroglobulin (A2M).
Each value is the mean ± S.E. of 5–12 rats per group.
Mean values of each serum marker are compared for age-factor relative to 4mo animals († = p < 0.05 †† = p < 0.01).
Significant exposure effects are indicated relative to FA exposed group (* = p < 0.05 ** = p < 0.01).
0 ppm indicates FA exposure.
Ozone effects
With respect to O3 exposure, serum proteins in rats 1 month old were the most affected. Increased levels of the acute phase response protein A2M and adiponectin were observed in 1 month old rats exposed acutely to 1 ppm O3 (Table 2) compared to FA control. There were no O3-related effects in any of the serum markers analyzed in subchronically exposed rats. Serum markers in the time-course analysis using 4 month old rats showed O3 exposure-related changes. Acute phase proteins A2M and AGP were increased on the 2nd day of O3 exposure, but returned to baseline at 18 h of recovery after 2 days of O3 (the timepoint that corresponds with serum collection for the acute and subchronic analyses) (Fig. 5). Leptin was increased after the 1st day of O3 exposure which coincided with ozone induced hyperglycemia but not after the 2nd day or 18 h recovery (Fig. 5). MCP-1 was lower after the 1st day of O3 exposure and GLP-1 was not changed (Supplementary Material, Table 2). Serum IL-6 was not changed by O3 during the time-course study (Fig. 5). Insulin, while not significantly changed, appeared to be lowered in rats exposed to 1 ppm O3 on the 2nd day. Lipocalin appeared to be increased during day 1 and 2 of O3 exposure, although not significantly (Fig. 5).
Fig. 5.
Comparison of serum biomarkers in BN rats exposed to FA or O3 in the time-course study. Serum was collected during necropsy immediately after 1 day or 2 days O3 exposure, or 18 h after 2 days O3 exposure and analyzed as described in methods. Control values may vary due to diurnal variation and the injection of glucose in glucose tolerance tests for animals in the 2 day 18 h timepoint group. Abbreviations: interleukin 6 (IL-6), α1-acid glycoprotein (AGP), α2-macroglobulin (A2M). Each value is the mean ± S.E. of 6 rats. Mean values of markers are compared for significant exposure effects relative to FA exposed group (* = p < 0.05 ** = p < 0.01). 0 ppm indicates FA exposure.
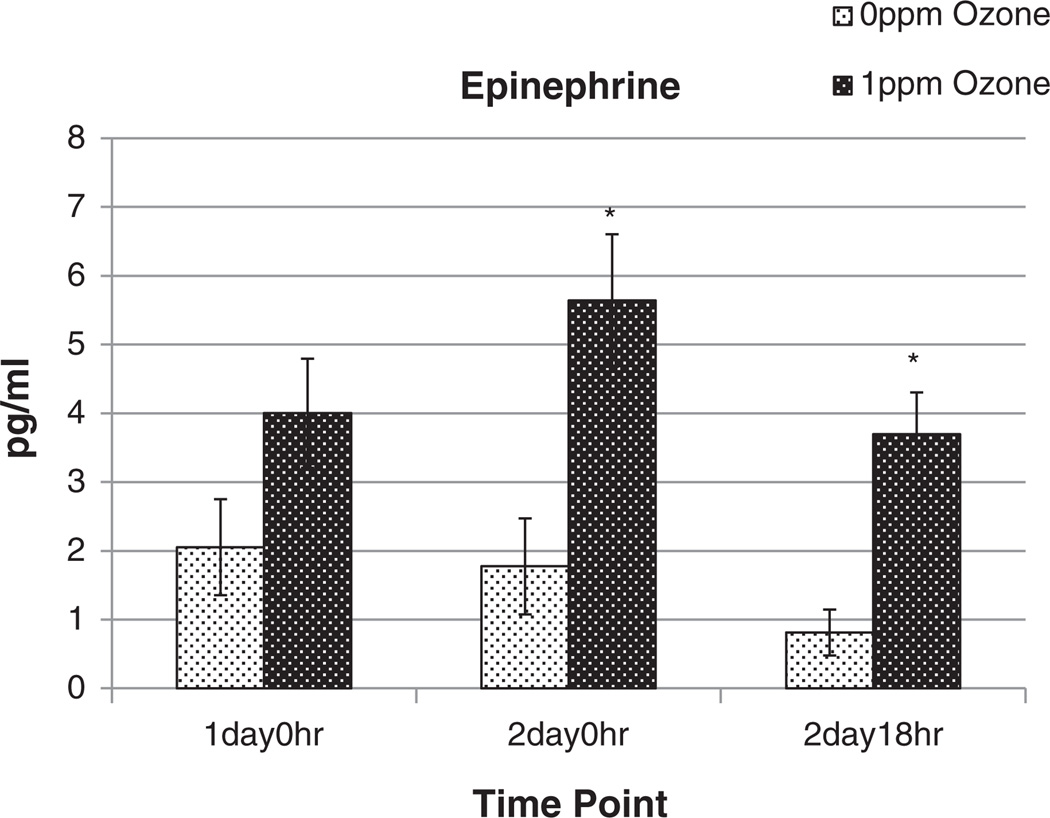
Serum epinephrine levels were analyzed in the time-course study to determine the possible contribution of sympathetic stimulation. Epinephrine, a neurohumoral regulator of the sympathetic nervous system, was increased markedly in serum after 1 ppm O3 exposure, on the 2nd day of exposure and remained elevated at 18 h recovery, relative to the FA group (Fig. 6). A trend of epinephrine increase was noted also on the first day of O3 exposure (Fig. 6).
Fig. 6.
Comparison of serum epinephrine in BN rats exposed to FA or O3 in the time course study. Serum was collected during necropsy immediately after 1 day or 2 days O3 exposure, or 18 h after 2 days O3 exposure and analyzed for epinephrine. Control values may vary due to diurnal variation and the injection of glucose during glucose tolerance testing for animals in the 2 day 18 h timepoint group. Each value is the mean ± S.E. of 4–6 rats. Mean values of markers are compared for significant exposure effects relative to FA exposed group (* = p < 0.05). 0 ppm indicates FA exposure.
Tissue levels of phosphoproteins involved in insulin signaling
Age effects
Proteins phosphorylated during insulin signaling were measured in liver and adipose tissues to determine whether there were underlying age-related differences (in FA exposed rats) in insulin sensitivity. Liver tissue from control rats (FA exposed–acute study) showed decreased levels of phosphorylated insulin receptor substrate (pIRS1) as animals aged, with 24 month old rats having less than half the amount found in 4 month old rats (Supplementary Material, Table 3). Similar but less apparent trends were noted in the subchronic study (Supplementary Material, Table 4). The levels of two other phosphoproteins involved in insulin signaling, phosphorylated AKT (pAKT) and phosphorylated glycogen synthase kinase 3β (pGSK-3β), did not show age-related variation in the liver of FA exposed rats in the acute study. In the subchronic study, 24 month old rats had higher levels of liver pAKT and pGSK-3β than corresponding 4 month old rats (Supplementary Material, Table 4). Adipose tissue GSK-3β was lower in 24 month old rats when compared to 4 month old rats in the acute study (Supplementary Material, Table 5). Additionally, 12 and 24 month old FA exposed rats had lower levels of adipose tissue pIRS1 relative to the 4 month old group (Supplementary Material, Table 5, 6), suggesting reduced insulin signaling.
Ozone effects
Although no significant O3-induced effects on pIRS1, pGSK or pAKT levels were noted in liver or adipose tissues following acute exposure (Supplementary Material, Table 4), in the subchronic study, pAKT decreased significantly in liver tissue from 24 month old rats exposed to 1 ppm O3 (Supplementary Material, Table 5). No significant O3-induced changes were noted in other phospho-protein markers from adipose tissue in acute and subchronically exposed rats (Supplementary Material, Table 5, 6). Time-course analysis of phosphoprotein markers in liver, adipose, and muscle tissue of 4 month old rats exposed to 1 ppm O3 for 1 or 2 days showed no significant changes in any of the markers except an increase in muscle pAKT on the 1st day of O3 exposure (Supplementary Material, Table 7).
Liver gene expression for ER stress markers
Age effects
Among several markers examined, baseline mRNA expression levels of the ER-stress gene Eif2α appeared to be higher in the liver of 1 month old rats, relative to 4 month old young adult controls in the acute study (Supplementary Material, Table 8). No other ER-stress related gene markers were significantly different between age groups in the acute or subchronic studies (Supplementary Material, Table 9).
Ozone effects
In the acute study, 2-day exposure to 1 ppm O3 increased liver BiP (Hspa5), as well as α1-acid glycoprotein (Orsm1) expression in 1 month old rats. CHOP (Ddit3) expression increased in 4 month old rats and Eif2α and Crtc2 mRNA increased in livers of 24 month old rats after 1 ppm O3 exposure (Supplementary Material, Table 8). Subchronic exposure to 1 ppm O3 increased Crebzf, Eif2α and Crtc2 expression in the livers of 4 month old rats (Supplementary Material, Table 9). With time-course analysis, ER stress markers BiP (Hspa5) and CHOP (Ddit3) showed increased mRNA expression in liver tissue after O3 exposure in the 18 h recovery group (Table 3). Other markers tested, Atf6b, Eif2α, and Eif2αk3 also showed changes at 18 h after 2 day O3 exposure (Table 3). In general, acute and subchronic exposure induced liver ER stress markers, and these changes were reflected in later time-points in the time course study.
Table 3.
Relative biomarker mRNA expression (fold relative to respective air group) in liver tissue following exposure of BN rats to FA or O3 in the time-course study.
| Time-course study | |||||||
|---|---|---|---|---|---|---|---|
| mRNA | 1d–0 h | 2d–0 h | 2d–18 h | ||||
| 0 ppm | 1 ppm | 0 ppm | 1 ppm | 0 ppm | 1 ppm | ||
| Liver | Orm1 | 1.03 ± 0.12 | 0.94 ± 0.15 | 1.02 ± 0.08 | 1.58 ± 0.32* | 1.03 ± 0.11 | 1.17 ± 0.10 |
| Hspa5 | 1.06 ± 0.15 | 0.56 ± 0.04* | 1.02 ± 0.09 | 0.67 ± 0.10** | 1.02 ± 0.10 | 2.15 ± 0.28** | |
| Atf6b | 1.00 ± 0.04 | 0.82 ± 0.04** | 1.01 ± 0.05 | 0.73 ± 0.04** | 1.01 ± 0.06 | 1.19 ± 0.04** | |
| Eif2a | 1.00 ± 0.03 | 0.80 ± 0.03** | 1.00 ± 0.04 | 0.85 ± 0.06* | 1.00 ± 0.03 | 1.17 ± 0.05* | |
| Eif2ak3 | 1.02 ± 0.08 | – | 1.01 ± 0.06 | 0.56 ± 0.02** | 1.01 ± 0.06 | 1.4 ± 0.11** | |
| Pdk4 | 1.12 ± 0.25 | 0.81 ± 0.07 | 1.09 ± 0.17 | 0.56 ± 0.22 | 1.01 ± 0.05 | 1.55 ± 0.26 | |
| G6cp | 1.12 ± 0.25 | 0.64 ± 0.07* | 1.06 ± 0.15 | 0.88 ± 0.05 | 1.05 ± 0.12 | 0.93 ± 0.10 | |
| Gys2 | 1.01 ± 0.07 | 0.73 ± 0.03* | 1.03 ± 0.11 | 0.7 ± 0.10** | 1.00 ± 0.03 | 1.25 ± 0.10** | |
| Slc2a2 | 1.01 ± 0.05 | 0.87 ± 0.02 | 1.02 ± 0.08 | 0.84 ± 0.07 | 1.02 ± 0.09 | 0.99 ± 0.04 | |
| Crtc2 | 1.00 ± 0.05 | 0.97 ± 0.02 | 1.01 ± 0.08 | 1.01 ± 0.07 | 1.01 ± 0.09 | 1.14 ± 0.05 | |
| Ddit3 | 1.05 ± 0.14 | 1.05 ± 0.16 | 1.05 ± 0.16 | 0.96 ± 0.19 | 1.03 ± 0.12 | 1.6 ± 0.22* | |
| Adipose | Slc2a4 | 1.03 ± 0.12 | 0.91 ± 0.12 | 1.04 ± 0.15 | 0.96 ± 0.15 | 1.04 ± 0.14 | 0.79 ± 0.13 |
| Fabp4 | 1.05 ± 0.15 | 0.92 ± 0.09 | 1.04 ± 0.12 | 1.65 ± 0.21** | 1.04 ± 0.13 | 1.20 ± 0.17 | |
Tissues were collected during necropsy after 1 day or 2 days O3 exposure, or 18 h after 2 days O3 exposure. mRNA was extracted and analyzed as described in methods.
Abbreviations: AGP (Orsm1), heat shock 70kDa protein 5 (Hspa5), CAMP responsive element binding protein-like 1 (Atf6b), eukaryotic translation initiation factor 2A (Eif2α), eukaryotic translation initiation factor 2A kinase 3 (Eif2αk3), pyruvate dehydrogenase kinase isozyme 4, mitochondrial (Pdk4), glucose-6-phosphatase, catalytic (G6pc), glycogen synthase 2, liver (Gys2), solute carrier family 2 (facilitated glucose transporter), member 2 (hepatic) (Slc2a2), cAMP-response element-binding protein regulated transcription co-activator 2 (Crtc2), DNA-damage inducible transcript 3 (Ddit3), solute carrier family 2 (facilitated glucose transporter), member 4 (adipose) (Slc2a4), fatty acid binding protein 4 (Fabp4).
Each value is the mean fold change (relative to mean value of time-matched air group) ± S.E. of at least 6 rats.
Mean values of markers are compared for significant exposure effects relative to FA exposed group (* = p < 0.05 ** = p < 0.01).
0 ppm indicates FA exposure.
Gene expression of metabolic markers
Age effects
Expression levels of genes involved with glucose homeostasis and insulin signaling were examined in liver and adipose tissues. Age-related differences were apparent in the expression levels of several markers when FA exposed animals of different ages were compared. In the acute study, 1 month old rats expressed more liver Irs2 and Pdha1 than 4 month old rats (Supplementary Material, Table 8). Fatty acid binding protein (Fabp4) was also highly expressed in the liver of 1 month relative to 4 month old rats. In the subchronic study, Pparg in the liver of 12 month old rats was significantly increased when compared to 4 month old rats (Supplementary Material, Table 9).
Ozone effects
Following acute exposure to 1 ppm O3, liver Fabp4 mRNA expression increased in 12 month old rats relative to their age-matched FA controls. Crtc2, a gene involved with glucagon signaling as well as an ER stress response pathway, increased after 1 ppm O3 exposure in 24 month old rats. The expression of other metabolic genes, such as G6pc, Irs2, and Pparg was unchanged after acute O3 exposure in rats of all ages (Supplementary Material, Table 7). After subchronic exposure to 1 ppm O3, Irs2, Pck1 and Crtc2 expressions were increased in 4 month old rats relative to FA controls (Supplementary Material, Table 9).
In time-course analysis, after the 1st and/or 2nd day of exposure to 1 ppm O3, significant inhibition of G6pc, HSPA5, ATF6, Eif2α, Eif2αk3, and Gys2 was noted (Table 3). G6pc, the catalytic unit of glucose 6 phosphatase, is transcriptionally regulated and converts glycogen stores into free glucose in the process of gluconeogenesis, suggesting increased circulating glucose might not have resulted from increased gluconeogenesis at very early time-point. Fabp4 mRNA expression, measured in white adipose tissues, was induced in rats immediately after the 2nd day of O3 exposure, but this increase was reversed after 18 h of recovery. In the time-course study (Table 4), one day of O3 exposure inhibited glycogen synthase (Gys2) mRNA expression (protein involved in glycogen synthesis).
Discussion
In this study we show that, acute O3 exposure produced profound metabolic effects in rats of all ages characterized by acute hyperglycemia, impaired glucose tolerance, and increased circulating leptin, but not the pro-inflammatory cytokine, IL-6. These effects accompanied a trend of decreased insulin as well as liver and adipose phospho IRS-1, suggesting modest insulin insensitivity. Importantly, the time-course study showed marked increases in circulating epinephrine at all times suggesting the contribution of sympathetic neurohormonal activation. Our data show that ER stress was a consequence of initial hyperglycemia and glucose intolerance based on initial inhibition after a single 6-hour O3 but subsequent induction of ER stress responsive genes after 2 days of O3 exposure in time-course experiment. Increased ER stress was also supported by the induction of ER stress genes after O3 exposure in the acute and subchronic studies. These metabolic effects, induced by O3 exposure, occurred in all age groups with no apparent O3-age interaction. Modest exacerbation of glucose intolerance was seen in senescent 24 month old rats relative to 4 month old rats. Furthermore, some degree of attenuation of metabolic effects was noted after 13 weeks of episodic O3 exposures in all age groups. Our studies show that O3 exposure causes metabolic effects in rats of all ages likely by initial sympathetic stimulation followed by diminution of these effects during the adaptation phase. These metabolic effects could increase the susceptibility of those with underlying metabolic disorders such as diabetes and obesity.
Our data demonstrate that like in humans (Puntmann et al., 2011), age influences metabolic risk factors in healthy, aging BN rats. These include age-dependent increases in glucose intolerance, circulating lipids, inflammatory proteins, and insulin, together with decreases in glucagon. Also age-related, changes in several tissue phosphoproteins and genes were suggestive of insulin resistance. BN rats have been used as a model for healthy aging because they are not predisposed to obesity or age-related pathologies (Lipman et al., 1996). We show that, as in the case of humans (Kmiec, 2010), even in normally aging rats, circulating insulin and glucose intolerance continue to increase starting from 1 month to 24 months of age. The clearance rate of glucose from the blood might be reflective of age-related decreases in the rate of uptake and metabolism of glucose by the muscle and brain. Biomarkers associated with insulin resistance and decreases in glucagon in 24 month old rats are suggestive of glucose intolerance and insulin resistance at this age. As noted in aging humans (Flannery et al., 2012), BN rats also show old age-related hyperlipidemia. The hypercholesterolemia in very young rats (1 month old) was in concordance with increased cholesterol in juveniles (Kit et al., 2012) and might reflect an active metabolic state of peripheral tissues and growth related movement of lipids.
Our data show that acute O3 exposure induces profound metabolic changes, characterized by short-lived hyperglycemia and glucose intolerance, which are rapidly reversible after termination of exposure. A number of potential mechanisms can explain impaired metabolism after O3 exposure. O3 is known tomodulate the release of neurohumoral factors (Gackiere et al., 2011), oxidize lipoproteins and membrane proteins (Pulfer et al., 2005), and induce lung injury with attendant release of pro-inflammatory mediators, all of which could influence peripheral and systemic metabolism. In order to assess the potential contribution of various mechanisms, an elaborate analysis was performed for neurohormones, acute phase response proteins, and biomarkers of insulin regulation in blood and insulin signaling in tissues.
Although increased circulating cytokines have been postulated to cause a systemic response after PM exposure (Tsai et al., 2012), our data do not support the contribution of cytokines such as IL-6 in O3-induced metabolic impairment. Lipocalin levels were increased after acute O3 exposure but this increase was only noted after 2 days, again suggesting that this is not likely the cause of acute hyperglycemia and glucose intolerance. Similarly, increases in circulating acute phase proteins were noted later, after a 2-day O3 exposure, and therefore, the acute phase response might not be involved in O3-induced impairment of glucose homeostasis. Because acute phase proteins AGP and A2M are made primarily in the liver (Blackburn, 1994), O3's impact on the liver over a longer time period cannot be excluded.
ER stress has been shown to be involved in the dysregulation of glucose homeostasis (Özcan et al., 2004). Moreover, increased ER stress has also been linked to air pollution (Laing et al., 2010). Therefore, we initially wanted to examine whether O3 caused liver ER stress and if this ER stress was associated with impaired glucose homeostasis. Examination of transcriptional targets of ER stress in all three studies indicated that O3 caused modest ER stress after a 2-day exposure following an initial inhibition of genes responsive to ER stress after 1-day exposure. The data for increased ER stress from the acute and subchronic studies support the conclusion that ER stress was induced following 2 day ozone exposure. ER stress is produced by accumulation of unfolded proteins (unfolded protein response) causing decreased protein translation and increased production of protein chaperones that remove unfolded proteins (Muoio and Newgard, 2004). This process helps restore cellular homeostasis, and in severe stress conditions, induces the apoptosis pathway (Hummasti and Hotamisligil, 2010). Liver ER stress can be produced by conditions of hypoxia, glucose deprivation, changes in redox status, and changes in calcium homeostasis (Muoio and Newgard, 2004). Because glucose intolerance preceded effects on liver markers of ER stress, we believe that acute O3-induced metabolic impairment is not caused by liver ER stress and that liver ER stress might be caused by initial systemic metabolic response.
Time- and dose-dependent neuronal activation in the dorsolateral regions of the nucleus tractus solitarius and in interconnected central structures such as the caudal ventrolateral medulla, the parabrachial nucleus, the central nucleus of the amygdala and the paraventricular hypothalamic nucleus has been shown after acute O3 exposure (Gackiere et al., 2011) and linked to the activation of catecholeminergic mechanisms (Cottet-Emard et al., 1997; Genc et al., 2012). In our time-course experiment O3 exposure sharply increased circulating epinephrine at all time-points suggesting a potential contribution of sympathetic stimulation mediated by neuronal activation in O3-induced metabolic impairment. It is of interest to determine which brain region might be responsible for this metabolic impact. We are currently investigating the role of sympathetic activation on O3-induced metabolic effects in different organs such as liver, adipose tissues, muscle, and if this O3 effect can promote or contribute to obesity and diabetes.
Because the metabolic effects of O3 are likely mediated by neurohumoral activation, the role of nociceptive stimulation and acute neuroreflux mechanisms will need to be examined in relation to metabolic impairments. Acute hyperglycemia was noted in the time-course experiment immediately after exposure on day 1, when glucose intolerance was also readily apparent, and this was accompanied by a trend of increased leptin on day 1 but not day 2. This is contrary to the decrease in leptin expected during fasting (Dubuc et al., 1998), which occurred in our studies during exposure/prior to necropsy. Leptin has been proposed as an indicator of energy balance, and hyperleptinemia is associated with diet-induced obesity (Brennan and Mantzoros, 2006; Margetic et al., 2002). The presence of increased leptin after initial O3 exposure indicates hormonal involvement in the metabolic response to O3 exposure, however this hypothalamic control of leptin release which coincided with hyperglycemia was short lived and likely independent of glucose intolerance response suggesting that the acute sympathetic stimulation might concurrently induce homeostatic metabolic mechanisms.
Subchronic O3 exposure resulted in milder glucose intolerance than that measured after acute exposure, suggesting an adaptive response to repeated episodic exposure. The adaptive response, as determined by reduced O3-induced inflammatory (JÖRres et al., 2000) and functional pulmonary changes in man (Schelegle et al., 2003), has been previously characterized. Rodents are also known to exhibit adaptation upon repeated O3 exposure (Kirschvink et al., 2002; van Bree et al., 2002). We show here that metabolic effects on glucose homeostasis are likely responsive to similar adaptive mechanisms. Because these systemic metabolic changes are accompanied by decreases in body temperature and bradycardia in rodents (Watkinson et al., 2001), further characterization of this response and how this might relate to humans that lack this response (Watkinson et al., 2001) will need to be examined.
Metabolic syndrome in humans at an advanced age is likely impacted by genetic and physiological factors (Eckel et al., 2005). Accordingly, we postulated that older BN rats will be impacted to a greater extent than young adult rats by O3. We noted that while O3 effects were seen in both young and old rats, the clearance of glucose was only slightly exacerbated after O3 exposure in senescent rats with already impaired glucose clearance at baseline, despite the presence of other age-related metabolic changes. Our data suggests that old rats undergoing healthy aging might not be markedly more sensitive to acute O3 exposure due to well preserved adaptive mechanisms. It is also likely that other pollutants such as PM might produce more chronic impact on metabolic processes as has been reported (Sun et al., 2013) and that these effects are likely exacerbated in old rats. The precise nature of how senescent rats will be impacted metabolically by O3 relative to young rats will need to be examined with emphases on neurohumoral mechanisms, sympathetic regulation, and neuronal plasticity at an old age.
In conclusion, we report that O3 produces acute metabolic alterations characterized by impaired glucose homeostasis and modest impairment of peripheral insulin sensitivity regardless of age. These metabolic alterations are not likely mediated by circulating cytokines, acute phase proteins or liver ER stress. Rather, neurohumoral sympathetic modulation might be important in metabolic effects in rodents. Although advanced age is associated with impaired glucose homeostasis and likely insulin resistance, O3 metabolic effects are only slightly exacerbated in geriatric rats. Further, acute metabolic impairments are rapidly reversible after the termination of exposure and repeated episodic exposure results in diminution of O3's metabolic effects. If and how these metabolic impairments seen in rats contribute to obesity and diabetes, and relate to human metabolic syndrome will require further study.
Supplementary Material
Acknowledgments
We thank Drs. Andrew Ghio, Michael Madden and Barbara Buckley for their critical review of the manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2013.09.029.
Footnotes
Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, Budoff M, Jacobs DR, Jr, Barr RG, Watson K, Kaufman JD. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn WD., Jr Validity of acute phase proteins as markers of disease activity. J. Rheumatol. Suppl. 1994;42:9–13. [PubMed] [Google Scholar]
- Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr. Atheroscler. Rep. 2010;12:291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- Brook RDMD, Jerrett MP, Brook JRP, Bard RLMA, Finkelstein MMMDP. The relationship between diabetes mellitus and traffic-related air pollution. J. Occup. Environ. Med. 2008;50:32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- Chen JC, Schwartz J. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ. Health Perspect. 2008;116:612–617. doi: 10.1289/ehp.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian MS, Hoberman AM, Johnson MD, Brown WR, Bucci TJ. Effect of dietary optimization on growth, survival, tumor incidences and clinical pathology parameters in CD Sprague–Dawley and Fischer-344 rats: a 104-week study. Drug Chem. Toxicol. 1998;21:97–117. doi: 10.3109/01480549809017854. [DOI] [PubMed] [Google Scholar]
- Cottet-Emard JM, Dalmaz Y, Pequignot J, Peyrin L, Pequignot JM. Long-term exposure to ozone alters peripheral and central catecholamine activity in rats. Pflugers Arch. 1997;433:744–749. doi: 10.1007/s004240050340. [DOI] [PubMed] [Google Scholar]
- Dubuc GR, Phinney SD, Stern JS, Havel PJ. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism. 1998;47:429–434. doi: 10.1016/s0026-0495(98)90055-5. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Flannery C, Dufour S, Rabol R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes. 2012;61:2711–2717. doi: 10.2337/db12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackiere F, Saliba L, Baude A, Bosler O, Strube C. Ozone inhalation activates stress-responsive regions of the CNS. J. Neurochem. 2011;117:961–972. doi: 10.1111/j.1471-4159.2011.07267.x. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Koehoorn M, Davies HW, Demers PA, Tamburic L, Brauer M. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ. Health Perspect. 2011;119:501–507. doi: 10.1289/ehp.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012;2012:782462. doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Jarema KA, Lehmann JR, Ledbetter AD, Schladweiler MC, Schmid JE, Ward WO, Kodavanti UP, Nyska A, MacPhail RC. Susceptibility of adult and senescent Brown Norway rats to repeated ozone exposure: an assessment of behavior, serum biochemistry and cardiopulmonary function. Inhal. Toxicol. 2013;25:141–159. doi: 10.3109/08958378.2013.764946. [DOI] [PubMed] [Google Scholar]
- Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 2010;107:579–591. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- JÖRres RA, Holz O, Zachgo W, Timm P, Koschyk S, MÜLler B, Grimminger F, Seeger W, Kelly FJ, Dunster C, Frischer T, Lubec G, Waschewski M, Niendorf A, Magnussen H. The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am. J. Respir. Crit. Care Med. 2000;161:1855–1861. doi: 10.1164/ajrccm.161.6.9908102. [DOI] [PubMed] [Google Scholar]
- Kirschvink N, Fievez L, Bureau F, Degand G, Maghuin-Rogister G, Smith N, Art T, Lekeux P. Adaptation to multiday ozone exposure is associated with a sustained increase of bronchoalveolar uric acid. Free Radic. Res. 2002;36:23–32. doi: 10.1080/10715760210169. [DOI] [PubMed] [Google Scholar]
- Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden C. Trends in serum lipids among US youths aged 6 to 19 years, 1988–2010. JAMA. 2012;308:591–600. doi: 10.1001/jama.2012.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec Z. Central control of food intake in aging. Interdiscip. Top. Gerontol. 2010;37:37–50. doi: 10.1159/000319993. [DOI] [PubMed] [Google Scholar]
- Künzli N. Air pollution and atherosclerosis: new evidence to support air quality policies. PLoS Med. 2013;10:e1001432. doi: 10.1371/journal.pmed.1001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing S, Wang G, Briazova T, Zhang C, Wang A, Zheng Z, Gow A, Chen AF, Rajagopalan S, Chen LC, Sun Q, Zhang K. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am. J. Physiol. Cell Physiol. 2010;299:C736–C749. doi: 10.1152/ajpcell.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J. Gerontol. A Biol. Sci. Med. Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 2013;41:361–373. doi: 10.1177/0192623312464531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg G, Hill R. Leptin: a review of its peripheral actions and interactions. Int. J. Obes. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Insulin resistance takes a trip through the ER. Science. 2004;306:425–426. doi: 10.1126/science.1104680. [DOI] [PubMed] [Google Scholar]
- Newby FD, DiGirolamo M, Cotsonis GA, Kutner MH. Model of spontaneous obesity in aging male Wistar rats. Am. J. Physiol. 1990;259:R1117–R1125. doi: 10.1152/ajpregu.1990.259.6.R1117. [DOI] [PubMed] [Google Scholar]
- Özcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Özdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Pulfer MK, Taube C, Gelfand E, Murphy RC. Ozone exposure in vivo and formation of biologically active oxysterols in the lung. J. Pharmacol. Exp. Ther. 2005;312:256–264. doi: 10.1124/jpet.104.073437. [DOI] [PubMed] [Google Scholar]
- Puntmann VO, Taylor PC, Mayr M. Coupling vascular and myocardial inflammatory injury into a common phenotype of cardiovascular dysfunction: systemic inflammation and aging—a mini-review. Gerontology. 2011;57:295–303. doi: 10.1159/000316577. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Walby WF, Alfaro MF, Wong VJ, Putney L, Stovall MY, Sterner-Kock A, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation attenuates airway injury/repair and release of substance P, but not adaptation. Toxicol. Appl. Pharmacol. 2003;186:127–142. doi: 10.1016/s0041-008x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Mukherjee B, Brook RD, Gatts GA, Yang F, Sun Q, Brook JR, Fan Z, Rajagopalan S. Air-pollution and cardiometabolic diseases (AIRCMD): a prospective study investigating the impact of air pollution exposure and propensity for type II diabetes. Sci. Total Environ. 2013;448:72–78. doi: 10.1016/j.scitotenv.2012.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, von Berg A, Koletzko S, Bauer CP, Heinrich J. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56:1696–1704. doi: 10.1007/s00125-013-2925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai DH, Amyai N, Marques-Vidal P, Wang JL, Riediker M, Mooser V, Paccaud F, Waeber G, Vollenweider P, Bochud M. Effects of particulate matter on inflammatory markers in the general adult population. Part. Fibre Toxicol. 2012;9:24. doi: 10.1186/1743-8977-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. The Green Book Nonattainment Areas for Criteria Pollutants. 2012 http://www.epa.gov/oar/oaqps/greenbk/ancl2.html.
- van Bree L, Dormans JA, Koren HS, Devlin RB, Rombout PJ. Attenuation and recovery of pulmonary injury in rats following short-term, repeated daily exposure to ozone. Inhal. Toxicol. 2002;14:883–900. doi: 10.1080/08958370290084674. [DOI] [PubMed] [Google Scholar]
- Watkinson WP, Campen MJ, Nolan JP, Costa DL. Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ. Health Perspect. 2001;109(Suppl. 4):539–546. doi: 10.1289/ehp.01109s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.