Abstract
Using the Xiphophorus fish melanoma model we show a strong male bias for cutaneous malignant melanoma, consistent with that seen in the human population. To examine underlying factors, we exposed adult X. couchianus fish to a single, sub-lethal dose of UVB and measured circulating sex steroid hormones and expression of associated hormone receptor genes over a 24 hour period. We found that a single exposure had profound effects on circulating levels of steroid hormones with significant decreases for all free sex steroids at 6 and 24 h and increases in conjugated 2-estradiol and 11-ketotestosterone at 6 and 24 h, respectively. Whereas ARα expression increased in male and female skin, neither ARβ nor either of the ER’s showed significant responses to UVB in either sex. The rapid response of male androgens and their receptors in the skin after UVB irradiation implicates hormones in the male-bias of skin cancer and suggests that the photoendocrine response immediately after UV exposure may be relevant to melanomagenesis.
Keywords: androgen receptor, estrogen receptor, melanoma, testosterone, ultraviolet-B, Xiphophorus
Introduction
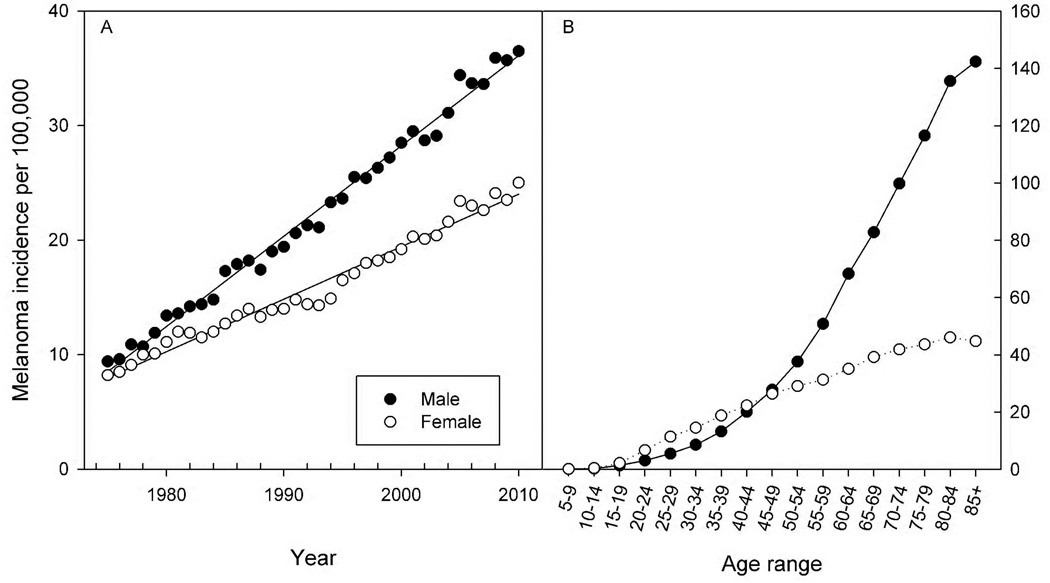
Cutaneous malignant melanoma (CMM) is one of the few cancers increasing in prevalence as well as mortality (NCI, 2012). The SEER (Surveillance Epidemiology and End Result; http://seer.cancer.gov/statistics/) incident rates for CMM in the U.S. from 1975 to 2010 show comparable levels of CMM in the late 1970’s but a significant divergence between the sexes during the past three decades (Fig. 1A). Regression analysis of the SEER data indicate that the annual rate of increase in CMM was 0.8 in males and 0.46 in females per 100,000 individuals, or nearly a two-fold greater rise in male compared to female incidence over this time period. The age-dependence for CMM is striking with slightly higher rates in women aged 20–40 and progressively higher rates in males after 45–50 years of age (Fig. 1B). The underlying contributors to the overall increase in melanoma are probably several-fold but likely include increased exposure to ultraviolet-B radiation (UVB; 290–320 nm) during recreational sunbathing and indoor tanning (Zhang et al., 2012) as well as increased detection of putative melanoma through increased public awareness and screening. The causes for the sex differences in CMM are less clear. Recreational and cosmetic exposures to natural and artificial UVR have increased significantly in adolescents and young adults in recent years and may correlate with the increase in CMM in 20–40 year old females (Dore and Chignol, 2012). Over the past 30 years, the male rate of CMM in the human population has been about 2-times that of female CMM resulting in a sizeable disparity by 2010 (Fig. 1A). As evidenced in Fig. 1B most of this difference results from dramatically higher CMM rates in males compared to females age 50–85.
Fig. 1.
SEER data for melanoma incidence in the U.S. population. The melanoma incidence per 100,000 individuals is shown for males (●) and females (○) in the U.S. In Panel A annual rates are shown from 1975 through 2010; in Panel B rates are shown as a function of age. Data were obtained from http://seer.cancer.gov/statistics/.
Sex differences in CMM revealed by the SEER data combined with a long history of published findings suggest that hormones may play a role in the etiology of CMM. Androgens and estrogens exert their biological effects through corresponding receptors ARα, ARβ, ERα, and ERβ, all of which act as ligand-dependent transcription factors and are members of a nuclear receptor superfamily. The importance of hormone signaling in the pathogenesis of prostate and breast cancer as well as the effectiveness of anti-hormone therapy for these types of cancer is well documented. In contrast, the role hormones play in melanoma susceptibility and malignancy has remained controversial due to contradictory findings of laboratory and clinical studies. For example, whereas estradiol stimulates tumor growth and metastasis in B16 melanoma cells (Lopez et al., 1978), decreased mRNA expression levels of ERα and ERβ are found in biopsies from more invasive human melanomas (de Giorgi et al., 2009).
However, both estrogens and androgens can regulate proliferation and differentiation of epidermal cells, functional activity of dermal fibroblasts, wound healing and the immune response in the skin (Reeve, et al., 2009). More pertinent to this study, there is evidence that androgens and estrogens can influence the proliferation of melanocytes and melanogenesis (McLeod et al., 1994; Tadokoro et al., 1997), such as hyperpigmentation associated with increased estrogen levels during pregnancy and its enhancement by sun exposure (Osterlind, 1992). However, there has been considerable dispute over the role of androgens and estrogens in melanoma causation and treatment. Discrepancies between human biopsies and in vitro systems (i.e., melanocyte cell lines) have not yet clarified the issues (de Giorgi et al., 2009; Lopez et al., 1978)
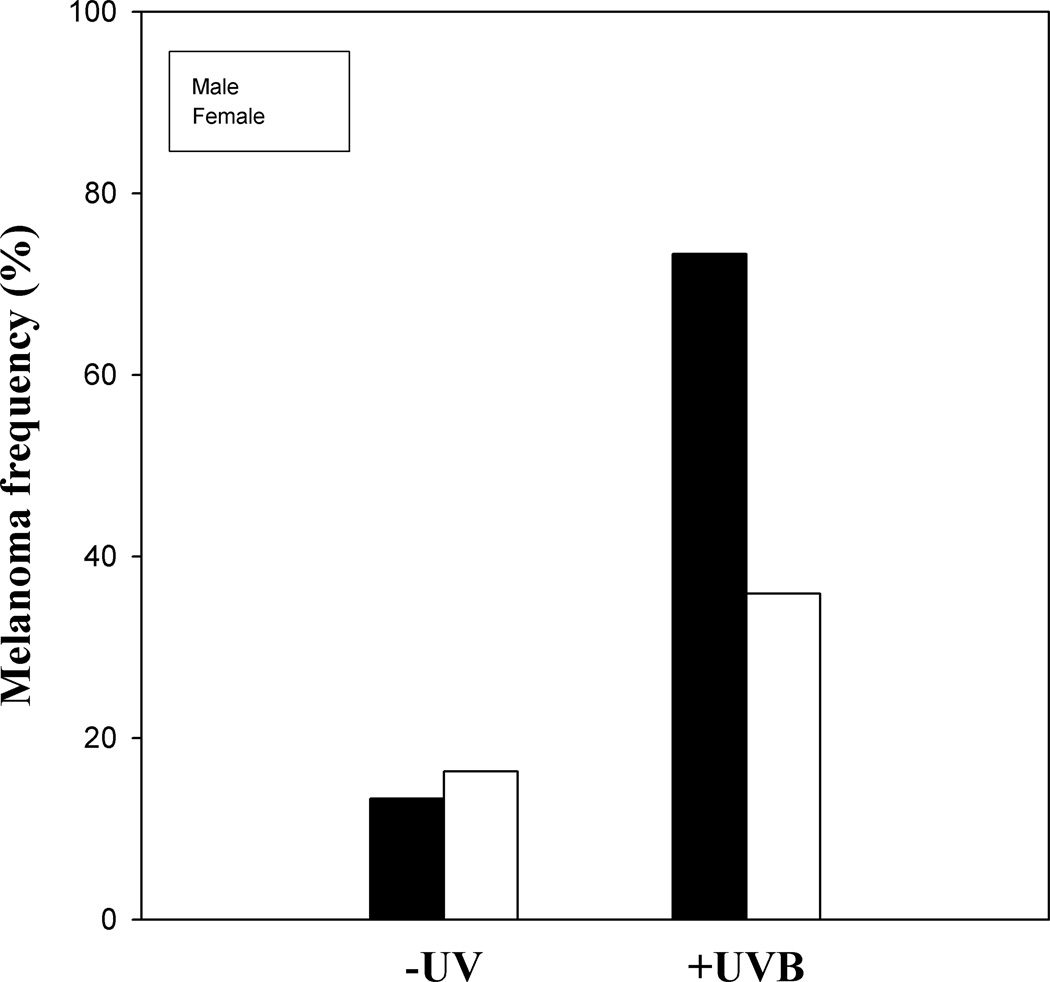
When the data from a large published UVB tumor study (Mitchell et al., 2010) were separated by sex we found a very significant difference in the melanoma frequencies between males and females in the irradiated fish but not in unirradiated fish (Fig. 2). Specifically, we observed 16.3% of the females (n = 129) and 13.3% of the males (n = 29) with melanomas in the absence of UVR and 35.9% of the females (n = 117) and 73.3% of the males (n = 27) with melanomas after a 5-day neonatal treatment with UVB radiation. In other words, we see a two-fold higher incidence of melanoma in males compared to females in response to UVB irradiation but no significant difference between males and females in spontaneous melanoma frequencies (-UV). These data suggest that UVB radiation affects males and females differently in this Xiphophorus melanoma model. The observed sex-bias for melanoma suggests that mechanisms underlying UVB induced melanoma should include pathways that differ between male and female fish.
Fig. 2.
Sex bias of UV-induced melanoma in an experimental model. Neonatal exposure to UVB radiation induces a significantly greater frequency of melanomas in males compared to females in the Xiphophorus backcross hybrids. Melanoma frequencies in male (black bars) and female (open bars) fish irradiated at 5–10 days post-parturition with 6.8 kJ/m2 narrow band UVB radiation and scored at 12–14 months of age. Original combined sex data were published in Mitchell et al. (2010).
A male-bias is also observed for squamous cell carcinoma in SKH-1 hairless mice exposed to chronic UVB (3×/week) (Thomas-Ahner et al., 2007). The authors showed that male mouse skin had higher frequencies of cutaneous oxidative DNA damage and lower antioxidant levels compared to female skin 48 h after acute UVB exposure. Differences in anti-oxidant defenses were held responsible for the sex bias for SCC in the mice. It is possible that the male-bias toward CMM in Xiphophorus is determined by a similar mechanism, the caveat being that the mouse model relies on long-term chronic UVB exposure for tumor initiation and progression. In the absence of chronic UVR, a similar mechanism for CMM in Xiphophorus would necessarily rely on mutations derived from the cumulative oxidative damage produced by acute exposure of neonates to UVB and endogenous oxidative damage produced over the lifetime of the organism (Mitchell and Fernandez, 2011). In addition, we and others have shown that direct, non-oxidative, UVB-induced DNA damage is responsible for all observed CMM in the fish model. Hence we believe it unlikely that sex differences in the oxidative response in fish determine the sex-bias of CMM as they do for SCC in the hairless mouse model. In response to these concerns, we designed an experiment to examine whether a single acute UVB exposure could affect oxidative damage levels, circulating levels of sex and stress steroid hormones and expression of sex steroid receptors in the skin of X. couchianus and help explain the sex-bias of UVB-induced melanoma in fish. The endocrine response to UVR has not been investigated and it is our hope that data from acute UVB administration regimens may lead to further insight into the sex-bias of melanoma.
The high degree of heterozygosity of interspecific Xiphophorus fish hybrids has made this non-mammalian model a valuable resource for the molecular analysis of gene regulation of tumorigenesis. The Poeciliid fish genus Xiphophorus includes 28 species of swordtails and platyfishes from Mexico and Central America. Recent work in genetics, genomics and proteomics has given this model the technical sophistication requisite for state-of-the-art inquiry into the etiology and biochemistry of CMM (for reviews see Meierjohann and Schartl, 2006; Walter et al., 2006; Mitchell et al., 2007; Patton et al., 2010). With a comprehensive gene linkage map, microsatellite markers, EST databases and recent completion of the Xiphophorus genome sequence, the model has the tools to explore the molecular genetics of melanoma. Backcross hybrids susceptible to melanoma formation inherit a sex-linked oncogene, Xmrk (Xiphophorus melanoma receptor kinase). Xmrk is the fish homologue of the human EGFR gene and a functional receptor tyrosine kinase which is highly active in malignant melanoma. Melanoma development is determined, at least in part, by activation and overexpression of Xmrk, which triggers a variety of signal transduction pathways, identical to those in human melanomagenesis, resulting in altered cell cycle control.
Materials and Methods
Animals
Fish used in this study were originally obtained from the Xiphophorus Genetic Stock Center located at Texas State University in San Marcos, TX, USA and have been maintained in our facility since 2000. The original X. couchianus stock was collected in 1961 from the Huasteca canyon (Nuevo Leon, Mexico). This stock is highly inbred, currently in its 100+ generation of full sibling inbreeding. This species was selected because it is a parental component of the hybrid backcross used in the melanoma experiments shown in Fig. 2 and because the logistics of this collaborative experiment between MD Anderson-Smithville and the University of Alabama, Tuscaloosa required a large number of fish during a fairly short window of time for which our large colony was ideally suited. More detailed descriptions of the genus, its zoogeography and hybrid crossing schemes are available at www.xiphophorus.org.
Endocrine response to UVR
For the hormone studies we used 30 males and 30 females of X. couchianus at 4–5 months of age. Each fish was physically and visually isolated from other individuals for 2 weeks prior to treatment in 2.5 gal tanks under a 12h light: 12h dark photoperiod to stabilize and equilibrate established hormone levels and reduce circadian variation. Adult fish were exposed to a single acute dose of 6.4 kJ/m2 UVB (as described in Mitchell et al., 2010) and then returned to their original aquaria. At 1.5, 6 and 24 h fish were transferred to 400 mL beakers containing 150 mL aquarium water for 30 min during which time hormones diffused across the gills into the surrounding water (Scott et al., 2008). Groups of 6 fish were used for each of the different time points and then sacrificed immediately after hormone collection for dissection and additional studies (e.g., gene expression). This water-borne hormone collection technique provides an accurate measure of circulating hormones in many fish species (Scott et al., 2008), including livebearers (Archard et al., 2012; Gabor and Contreras, 2012). Immediately after the 30 min hormone collection period fish were sacrificed and eyes, skin and muscle were dissected and flash frozen for qPCR analysis of hormone receptor expression. Water samples containing hormones were refrigerated overnight and processed the following day. Two controls were generated; (1) sham irradiated fish designated as 0 h or -UV and (2) a water control sample without fish.
Hormone extraction
Hormones were extracted from the water samples using C18 solid phase extraction columns (Lichrolut RP-18, 500 mg, 3.0 mL; Merck) fitted to a 12-port manifold (Earley et al., 2006; Rodgers et al., 2006). Columns were primed using two consecutive washes with 2 mL HPLC grade methanol (MeOH) followed by two consecutive washes with 2 mL distilled water. Water samples collected from each fish were drawn into the columns using a vacuum pump. Salts were removed with two consecutive 2 mL washes of distilled water and the columns were frozen and shipped to Dr. Ryan Earley at the University of Alabama, Tuscaloosa for further processing. Freeze storage of water samples and columns does not affect steroid concentrations (Ellis et al., 2004). The columns were thawed and purged with two consecutive 2 mL washes of distilled water. Free steroid hormone, which diffuses across the gills of the fish and reflects circulating hormone levels (Scott et al., 2008), was eluted from the columns into 12 × 75 mm (6 mL) borosilicate tubes by two consecutive 2 mL washes with ethyl acetate. Sulfated and glucuronidated conjugated fractions, which are present in urine and bile and likely estimate the fish’s endocrine status several hours prior to sampling (Scott et al., 2008), were eluted into new 12 × 75 mm (6 mL) borosilicate tubes by two consecutive 2 mL washes with HPLC grade MeOH. The 4 mL of eluted solvent (ethyl acetate or methanol) was evaporated with a gentle stream of nitrogen (0.7 bar), through an evaporating manifold in a 38 °C water bath. The resulting hormone residue was re-suspended in 840 µL of enzyme-immunoassay (EIA) buffer supplied with the appropriate kit (see below), and the samples were stored at −20 °C until assayed. We report the hormone data as release rates (pg/g/h).
Enzyme-immunoassay (EIA)
Kits (Cayman Chemicals Inc.) were used for all hormones: cortisol, 11-ketotestosterone (KT), testosterone (T), and estradiol (E2). Briefly, 50 µL of each sample was pipetted into 96-well plates followed by 50 µL of acetylcholinesterase tracer and 50 µL of antiserum. Cortisol and KT plates were incubated overnight (18 h) on an orbital shaker at 4°C; T plates were incubated for 2 h on an orbital shaker at room temperature; E2 plates were incubated for 1 h on an orbital shaker at room temperature. The plates were then washed 5× with wash buffer (provided with the kits), blotted dry, and 200 µL of Ellman’s reagent added to each well. The plate was wrapped in aluminum foil and placed on an orbital shaker for 60–120 min depending on the assay. Plates were read at 405 nm on a BioTek ELX800 UV microplate reader. All antisera are reported by Cayman Chemical Inc. to have 100% specificity for the focal steroid hormone. Assays were validated by assessing parallelism of a serially diluted X. couchianus pool with the kit standard curves, and by assessing quantitative recovery from X. couchianus pooled samples spiked with known concentrations of each hormone and passed over the Sep-Pak C18 columns. Quality control data are shown in Supplemental Materials.
Real-time quantitative PCR (qPCR)
Levels of ARα, ARβ, ERα and ERβ mRNA were examined by real-time quantitative reverse transcriptase PCR on an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems) by the TaqMan® primer and probe system. Primers and probes were designed using File Builder 3.1 software to a unique region of the genes (Tables S1 and S2). For qPCR analysis of transcript levels, 1 µg of RNA isolated from the skin and eye of six fish per treatment group were reverse transcribed to cDNA by random priming using the Applied Biosystems High-Capacity cDNA Archive Kit (Applied Biosystems) in 50 µL reactions. For qPCR, 2 µl of cDNA were used per assay in a reaction volume of 20 µl and each sample was analyzed in duplicate. Serial dilutions of RNA from control (-UV) epidermis were used for the construction of relative standard curves for each assay. Samples were normalized to 18S RNA and all qPCR assays were analyzed based on the relative quantification or ΔΔCt method. The ddCt method implements the 2−ΔΔCT algorithm and is commonly used to estimate the relative expression of target genes in different samples. In all qPCR analyses, control samples processed without reverse transcriptase, as well as no template control samples were included to verify a lack of contaminating genomic DNA in the sample preparations.
Quantification of 8-oxodeoxyguanosine
DNA was purified from skin tissues from 4 different adult female and 4 different male X. couchianus fish using a non-phenol-based extraction procedure (Qiagen, Inc.). Total DNA was digested and dephosphorylated by nuclease P1 (Wako Chemicals) followed by alkaline phosphatase (Promega). The digested DNA was analyzed by reversed phase HPLC using a C18 analytical column (Beckman, Ultrasphere ODS, 5µm, 4.5 × 250 mm) coupled to a photodiode array detector (PDA, SPD-M10A, Shimadzu) with nucleoside quantitation using UV absorption at 254 nm. In-line with the UV detector, an electrochemical (EC) detector (CoulArray, ESA) set at 290 mV was used to measure levels of 8-oxodeoxyguanosine. The results are expressed as the number of 8-oxodG lesions in 105 deoxyguanosine (dG) bases: or in 4 × 105 total bases.
Statistics
The differences between the means of the response variables (i.e., all hormone and gene expression data) were compared using two-sample t-tests with p-values corrected by sequential Dunn-Sidak adjustments to account for compounding Type I error. The statistical package used was the latest version of R (3.0.1) available at http://cran.us.r-project.org.
Results
Sex differences in constitutive oxidative damage frequencies
To determine if the male-bias for CMM induction in the Xiphophorus backcross hybrid was similar to that observed for SCC induction in hairless mice, we analyzed the difference in the male and female response to oxidative stress. At the sublethal doses required for this experiment, UVB induces extremely low levels of 8-oxodeoxyguanosine 8-oxodG (Mitchell et al., 2002). However, 8-oxodG is produced as a byproduct of normal metabolism and is considered an important lesion contributing to CMM progression. We reasoned that, should significant sex differences in the oxidative response exist, the net, constitutive level of 8-oxodG in the skin would be different in males and females with males showing higher levels of oxidative damage. We measured constitutive levels of the oxidative damage marker 8-oxodG in untreated male and female X. couchianus skin. We found no significant difference using a two-sample t-test (P=0.381). Specifically, untreated male fish skin had 4.9 8-oxodG × 105 dG (SD=1.9; n=4) and female fish had 4.4 8-oxodG × 105 dG (SD=2.0; n=4).
Acute UVB effects on sex hormones
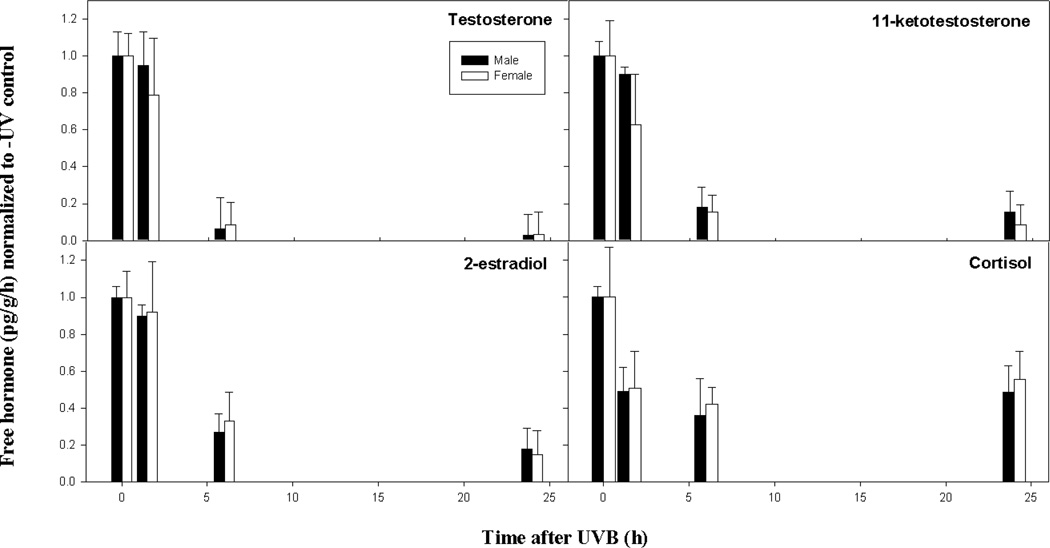
To begin to understand a role for sex hormones in CMM and, more specifically, in a whole organism response to UVB, we measured release rates of free and conjugated testosterone (T), 11-ketotestosterone (KT), estradiol (E2) and cortisol in unexposed fish and release rates of these same hormones at 1.5, 6 and 24 h post-irradiation (Figs. 3 and 4). Free steroid hormones diffuse across the gills of the fish and reflect circulating hormone levels (Scott et al., 2008). Although not the most abundant, KT is generally considered the more potent male androgen in teleost fish (Margiotta-Casaluci and Sumpter, 2011). Relative release rates of free hormones indicated that T, KT and E2 were qualitatively higher in male fish compared to female fish in the absence of UVR (Table S3). Release rates of free cortisol were higher in male fish compared to females and release rates of all four conjugated hormones were higher in males (Table S4).
Fig. 3.
The time course for the free, circulating hormone response in adult male and female X. couchianus fish exposed to acute UVB. Steroid hormones shown include testosterone (Panel A), 11-ketotestosterone (Panel B), 2-estradiol (Panel C) and cortisol (Panel D). The mean and standard error of the mean (SEM) were calculated from raw data (pg/g/h) for each condition (n = 6) and normalized to the –UV control. The normalized error was determined as SEM/mean. Legend shows male (black bars) and female (open bars) responses.
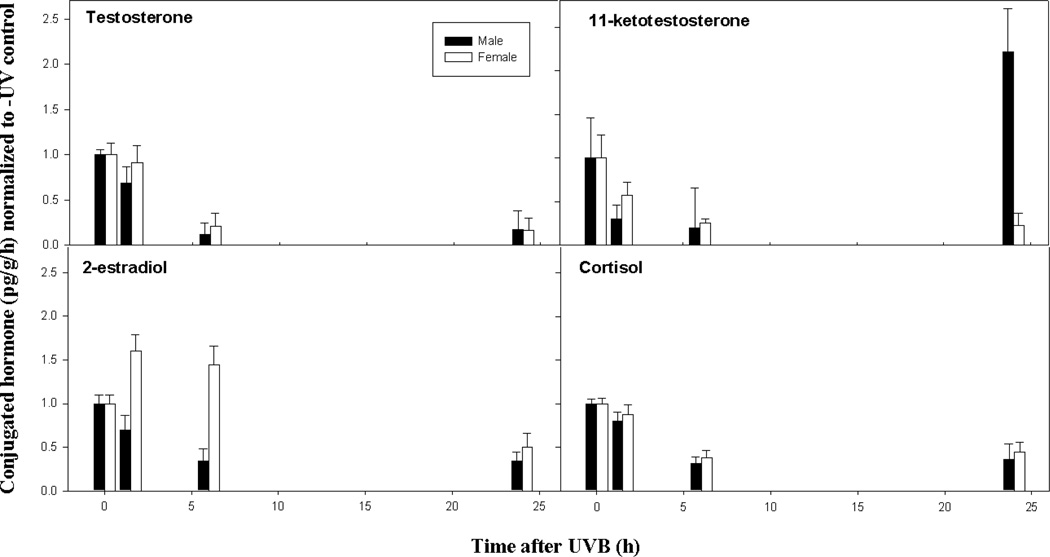
Fig. 4.
The time course for the conjugated hormone response in adult male and female X. couchianus fish exposed to acute UVB. Steroid hormones shown include testosterone (Panel A), 11-ketotestosterone (Panel B), 2-estradiol (Panel C) and cortisol (Panel D). The mean and standard error of the mean (SEM) were calculated from raw data (pg/g/h) for each condition (n = 6) and normalized to the –UV control. The normalized error was determined as SEM/mean. Legend shows male (black bars) and female (open bars) responses.
The effects of acute UVB on circulating (free) hormones are shown in Fig. 3 and Table S3. Free T, KT, and E2, as well as cortisol, showed very similar patterns in their response to UVB in both males and females. No significant differences were observed between the –UV control and 1.5 h samples for male T, KT and E2 or for female T and E2. A marginally significant reduction was, however, observed in circulating KT in female fish at 1.5 h post-irradiation relative to the -UV (0 h) control. At 6 and 24 h after UVB exposure, significant reductions relative to the –UV (0h) control and 1.5 h periods were observed for all three free sex hormones in both males and females (see P-values in Table S5); no further differences were observed between 6 and 24 h. Free cortisol showed a significant decrease from 0 h to 1.5 h for males and a marginally significant decrease at 1.5 h for females; levels at 6 and 24 h were not significantly different from 1.5 h in both sexes.
An important step in steroid catabolism involves the formation of hydrophilic molecules (i.e., sulphate and glucuronide conjugates) to allow excretion. Most excretory products are in a conjugated form and sulphated and glucuronidated conjugated fractions, which are present in urine and bile, respectively, are likely estimates of the endocrine status of the fish several hours prior to sampling (Scott et al., 2008). The effects of acute UVB on release rates of conjugated hormones are shown in Fig. 4 and Table S4. The patterns of conjugated hormones in response to UVB are different from those observed for free hormones, particularly when comparing males and females. In males, the conjugated fractions of T, KT and E2 showed marginally significant declines within 1.5 h (see Table S6 for P-values). In females the early UV response was much less pronounced than in males; at 1.5 h there was an apparent but non-significant increase in conjugated E2. By 6 h post-UV there were further decreases in conjugated T, KT, E2 and cortisol in males and similar decreases in females, with the exception of E2, which remained at a high level. By 24 h all of the conjugated hormones in both male and female fish were significantly reduced with the exception of KT in males that showed a very significant increase compared to earlier time points.
The UV response observed in free and conjugated hormones was different. The free hormone response in both males and females showed a general decline in all of the hormones analyzed. In contrast, the conjugated hormone response showed two striking exceptions to this pattern; (1) KT significantly increased over control levels at 24 h post-UV in males but not females and (2) release rates of conjugated E2 in females showed an early and sustained increase up to at least 6 h after UV in contrast to males, which showed a significant decline during this time period.
Acute UVB effects on sex hormone receptors
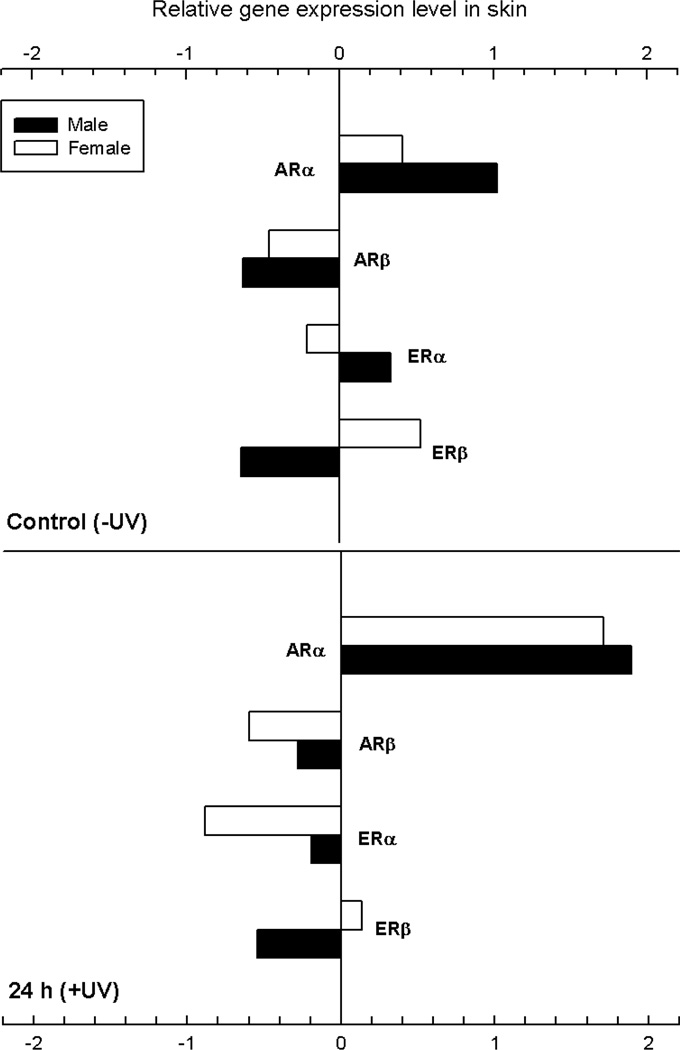
Effects of UVB irradiation on androgen and estrogen receptor gene expression in the skin of adult male and female X. couchianus were determined using quantitative real-time PCR (qPCR). Androgen and estrogen receptor gene expression levels were measured in the skin of unexposed fish and at 24 h after exposure to a single dose of 6.8 kJ/m2 UVB radiation. All data were normalized to expression in the eye and average fold-differences for relative gene expression were calculated. With the exception of ARα, none of the hormone receptors examined, including ARβ, ERα and ERβ, showed significant changes in expression 24 h post-irradiation (see Table S7). We did observe significant differences between constitutive levels of ARα in males and females (Fig. 5, top panel) as well as induction of ARα 24 h after exposure to a single, acute dose of UVB. At 24 h post-UV normalized ARα levels were indistinguishable between male and female animals.
Fig. 5.
Effects of UVB irradiation on androgen and estrogen receptor gene expression in the skin of male and female X. couchianus. Data were measured by qPCR in unexposed fish and at 24 h after exposure to a single dose of 6.8 kJ/m2 UVB radiation.. All data were normalized to the eye and average fold-differences for relative gene expression were calculated from the average ddCT values.
Discussion
Consistent with the human (SEER) data, we show a significant sex-bias for UVB-induced cutaneous malignant melanoma in a hybrid fish melanoma model with male fish displaying ~2-fold greater frequency than female fish at the time of sacrifice and biopsy (i.e., 12–14 months). We know that this result is dependent on exposure of neonates to acute short-term UVB radiation. Although childhood exposure is considered a major risk factor in the human population this is the first study with experimental evidence supporting this idea. As with most experimental animal models, a major advantage of the fish is that the UV exposure is precisely controlled and limited to a 5-day period shortly after birth. This is in sharp contrast to the extreme variability in UV exposure history in the human population. Given the limited neonatal UVB exposure and absence of any additional UVR, it is reasonable to suspect that sex differences in hormone production and/or signaling at the time of, or immediately following, irradiation are responsible for the sex-bias observed in melanoma frequency. The fact that spontaneous melanomas do not show sex-bias in this backcross hybrid suggests that events contributing to tumor initiation and progression in the absence of UV are the same in males and females.
As mentioned, sex-bias for melanoma in fish is similar to that observed for squamous cell carcinoma in SKH-1 mice (Thomas-Ahner et al., 2007). In this study it was determined that sex differences in the oxidative stress response associated with chronic irradiation was presumed to differentially affect SCC progression during chronic UVB exposure. In the absence of chronic exposure in Xiphophorus, CMM progression would necessarily rely entirely on endogenous oxidative damage (Mitchell and Fernandez, 2011). We therefore presumed that if sex differences in the oxidative response (i.e., induction and repair) existed in the fish, then different levels of constitutive endogenous oxidative damage should be observed in males and females. Sex differences in oxidative damage levels were not observed. Furthermore, data from other fish studies support this conclusion. For example, the goodeid fish Girardinichthys viviparus shows similar levels of superoxide dismutase and catalase activities in both sexes after exposure to polychlorinated biphenyls (Vega-Lopez et al., 2007). Hence, it probable that sex differences in the oxidative stress response in Xiphophorus do not play a role in melanoma formation as they presumably do in SCC in the mouse model.
Since the sex-bias for melanomas was observed only in the UVB irradiated population and not in the unirradiated control animals, it follows that the UV response is different in male and female neonates. Mechanisms underlying UVB induced melanoma should thus include pathways that differ between males and females at a young age. Ideally, we would have preferred to study the acute response of sex hormones in 5–10 day old neonates, rather than adults. Unfortunately, 5–10 day old fry measure < 6 mm in length and levels of steroid hormones are presumably low and varied. In addition, the backcross hybrids show considerable genetic diversity compared to the parental fish, compromising data interpretation. Because the effects of acute UV on hormone levels in whole organisms has never been determined, we choose to perform our studies on adult fish, fully aware of the caveat that the neonate and adult responses could be very different due to developmental stage and hormone status. However, our results indicate a dramatic response to UV in adult fish that could, potentially, be recapitulated in neonates and add some insight into the sex-bias of melanoma both in fish and humans.
The SEER data show a striking difference in melanoma frequency between men and women after age 50 (Fig. 1B). It may not be coincidental that both male and female sex hormones begin to decline at this age and it is therefore not unreasonable to believe that melanomagenesis in males and females may be related to a decline in testosterone and estrogen. How this actually works is a matter for speculation but suggests that testosterone may inhibit melanoma formation in younger men (<50) but loses its effectiveness when circulating concentrations drop below a certain level. Since the fish in the tumorigenesis studies were exposed to UV only as neonates, it is probable that whatever mechanism(s) drives the sex difference in melanoma formation occurs at the time of irradiation. It is even possible that constitutive or induced high testosterone levels in early life may prime male melanoma susceptibility later in life. If differences in the sex hormone landscape alone were contributing to melanoma in the absence of UVR then sex-differences in tumor frequency should also be observed in the spontaneous rates as well, which they are not.
The case for steroid hormones in the etiology of UVB-induced melanoma is strong but early in its development. Epidemiological data suggest that testosterone may inhibit melanoma formation. However, testosterone inhibitors (i.e., flutamide) have been used to treat CMM with some, albeit inconsistent, success. As referenced above, androgens and estrogens can influence the proliferation of melanocytes and melanogenesis in humans. Evidence from Xiphophorus supports the idea that hormones and, in particular androgens, may be involved in melanomagenesis. For instance, Xiphophorus melanomas can be induced in the absence of UV by testosterone mediation (Schartl et al., 1982) and are most prevalent in dominant males that have higher levels of circulating androgens (Hannes and Franck, 1983; Schartl et al., 1995). In addition, aggression and dominance in Xiphophorus is positively correlated with whole body and serum testosterone levels (Hannes, 1986), which have been implicated as contributing factors to melanoma susceptibility. Replication of these studies using KT in place of testosterone could implicate KT as a potentially important steroid in melanomagenesis.
Steroid hormones act by binding to nuclear receptors that function as ligand-activated transcription factors. In mammalian skin these transcription factors control a variety of functions, including hair growth, sebaceous gland function, proliferation and differentiation of epidermal cells (including melanocytes), wound healing and activity of dermal fibroblasts and immune cells in the skin (Slominski and Wortsman, 2000). We observe a near complete reduction in free testosterone and estradiol release rates within the first 24 h post-exposure to UVB, suggesting that these hormones are essentially lost from circulation due to some active mechanism such as receptor binding, steroid binding to globulin or metabolic clearance (see Mommsen et al., 1999; Scott et al., 2005; Bobe et al., 2010). Indeed, receptor and steroid binding globulin characteristics (e.g., dissociation constants and binding capacities), and rates of steroid conjugation and metabolism all show flexibility in response to such things as stressors and major life history events (e.g., Pottinger et al., 1992; Laidley and Thomas, 1997; Hobby et al., 2000; Tveiten et al., 2000; Dean et al., 2003). Thus, it stands to reason that UVB exposure influences, either directly or indirectly, one or more of these mechanisms. Steroid binding globulins are of particular interest because they limit the bioavailability of hormones, and show particular affinity for androgens (Bobe et al., 2010; Hammond, 2011). Active clearance of the hormone, or any mechanism that reduces or prevents sex steroid signaling, could represent an “adaptive” response to UV, particularly if the actions of these hormones trigger pathological states (e.g., aberrant melanocyte proliferation, tumor), as mentioned above. This idea is further supported by the high variance measured in conjugated KT at 24 h in male fish and suggests that the photoendocrine response, particularly for 11-KT, is complex and may be determined by unknown environmental, genetic or epigenetic factors. It may not be coincidental that a significant decrease in sex hormone-binding globulin is observed during progression in male, but not female, melanoma patients (Miller et al., 1997). A role for sunlight in these observations is not known.
Male skin shows significantly higher constitutive levels of ARα compared to female skin (Fig. 5). Furthermore, in contrast to the decrease in circulating androgens, we see a significant up-regulation of ARα in the skin of both female and male fish at 24 h. This observation is not consistent with the autoregulation of nuclear receptors (Bagamasbad and Denver, 2011) and suggests that other mechanisms may be involved in upregulating ARα. The consequences of AR upregulation in fish skin (or in melanoma) are not known, however ARs are expressed in epidermal keratinocytes, melanocytes, and dermal fibroblasts as well as resident and transient cells of the skin immune system (Choudhry et al., 1992; Kimura et al., 1995); ERs have been detected at lower frequencies in these cells. ARα was also upregulated in a melanoma found on a wild-caught X. nezahuacoyotl compared to non-melanized and melanized skin from the same animal (data not shown). AR phosphorylates MAPK (mitogen-activated protein kinase) and CREB (cAMP response element binding protein) (Fix et al., 2004), activates PIK3/Akt and shows direct interaction with EGF receptors (Bonaccorsi et al., 2008). With the exception of CREB, these proteins/pathways have been identified as downstream events necessary for the transformed phenotype initiated in the Xiphophorus melanoma model (Meierjohann and Schartl, 2006) as well as in human melanoma.
In summary, we show, for the first time, that a single acute exposure to an erythemal dose of UVB radiation has profound effects on the short-term hormone profiles and hormone receptor expression patterns in an experimental model in vivo. In Xiphophorus, melanomas can be induced by testosterone (Schartl et al., 1982) and are most prevalent in aggressive, dominant males that have higher levels of circulating androgens (Hannes and Franck, 1983; Schartl et al., 1995). Our studies suggest that the photoendocrine response may be involved in the etiology of melanoma and that perturbation of hormonal status may be a key factor in tumorigenesis, particularly in males. The EPA defines an endocrine disruptor as an “exogenous agent that interferes with the normal function of endogenous hormones responsible for the maintenance of homeostasis and the regulation of developmental processes”. In this study we show that UVB behaves like an endocrine disruptor. Similar to endocrine disrupting chemicals, UVB poses the greatest risk to organisms during prenatal and early postnatal development with adverse consequences appearing much later in life. The hormone data presented here expand potential mechanisms that could be involved in the initiation of sunlight-induced melanoma and reinforce the idea that sun avoidance through behavior (avoidance) and chemoprevention (sunscreen use) is central to combatting this critical public health concern.
Supplementary Material
Significance.
The profound effect of solar ultraviolet radiation on the neuroendocrinology of the skin is a novel and surprising result that implicates sex steroids in melanoma etiology and may help to explain the observed sex-bias in melanoma in the human population.
Acknowledgments
This work was supported by NCI grant CA11367, NIEHS Center grant ES07784 and NCI training grant CA009480.
Footnotes
I declare that the authors have no competing interests as defined by the NIEHS, or other interests that might be perceived to influence the results and/or discussion reported in this article.
References
- Archard GA, Earley RL, Hanninen AF, Braithwaite VA. Correlated behavior and stress physiology in fish exposed to different levels of predation pressure. Functional Ecology. 2012;26:637–645. [Google Scholar]
- Bagamasbad P, Denver RJ. Mechanisms and significance of nuclear receptor auto- and cross-regulation. General and comparative endocrinology. 2011;170:3–17. doi: 10.1016/j.ygcen.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe J, Guiguen Y, Fostier A. Diversity and biological significance of sex hormone-binding globulin in fish, an evolutionary perspective. Molecular and Cellular Endocrinology. 2010;316:66–78. doi: 10.1016/j.mce.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi L, Nosi D, Quercioli F, Formigli L, Zecchi S, Maggi M, Forti G, Baldi E. Prostate cancer: a model of integration of genomic and non-genomic effects of the androgen receptor in cell lines model. Steroids. 2008;73:1030–1037. doi: 10.1016/j.steroids.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Choudhry R, Hodgins MB, Van Der Kwast TH, Brinkmann AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol. 1992;133:467–475. doi: 10.1677/joe.0.1330467. [DOI] [PubMed] [Google Scholar]
- Dean DB, Whitlow ZW, Borski RJ. Glucocorticoid receptor upregulation during seawater adaptation in a euryhaline teleost, the tilapia (Oreochromis mossambicus) General and Comparative Endocrinology. 2003;132:112–118. doi: 10.1016/s0016-6480(03)00053-4. [DOI] [PubMed] [Google Scholar]
- De Giorgi V, Mavilia C, Massi D, Gozzini A, Aragona P, Tanini A, Sestini S, Paglierani M, Boddi V, Brandi ML, et al. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol. 2009;145:30–36. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- Dore JF, Chignol MC. Tanning salons and skin cancer. Photochem Photobiol Sci. 2012;11:30–37. doi: 10.1039/c1pp05186e. [DOI] [PubMed] [Google Scholar]
- Earley RL, Edwards JT, Aseem O, Felton K, Blumer LS, Karom M, Grober MS. Social interactions tune aggression and stress responsiveness in a territorial cichlid fish (Archocentrus nigrofasciatus) Physiol Behav. 2006;88:353–363. doi: 10.1016/j.physbeh.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Ellis T, James JD, Stewart C, Scott AP. A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. Journal of Fish Biology. 2004;65:1233–1252. [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Contreras A. Measuring water-borne cortisol in Poecilia latipinna:is the process stressful, can stress be minimized and is cortisol correlated with sex steroid release rates? Journal of fish biology. 2012;81:1327–1339. doi: 10.1111/j.1095-8649.2012.03411.x. [DOI] [PubMed] [Google Scholar]
- Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85:431–441. doi: 10.1095/biolreprod.111.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannes RP. Blood and whole-body androgen levels of male swordtails correlated with aggression measures in a standard-opponent test. Aggressive Behavior. 1986;12:249–254. [Google Scholar]
- Hannes RP, Franck D. The effect of social isolation on androgen and corticosteroid levels in a cichlid fish (Haplochromis burtoni) and in swordtails (Xiphophorus helleri) Horm Behav. 1983;17:292–301. doi: 10.1016/0018-506x(83)90028-4. [DOI] [PubMed] [Google Scholar]
- Hobby AC, Pankhurst NW, Haddy JA. The effect of short term confinement stress on binding characteristics of sex steroid binding protein (SBP) in female black bream (Acanthopagrus buthcheri) and rainbow trout (Oncorhynchus mykiss) Comparative Biochemistry and Physiology Part A. 2000;125:85–94. doi: 10.1016/s1095-6433(99)00156-7. [DOI] [PubMed] [Google Scholar]
- Kimura M, Tomita Y, Watanabe H, Sato S, Abo T. Androgen regulation of intra-and extra-thymic T cells and its effect on sex differences in the immune system. Int J Androl. 1995;18:127–136. doi: 10.1111/j.1365-2605.1995.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Laidley CW, Thomas P. Changes in plasma sex steroid-binding protein levels associated with ovarian recrudescence in the spotted seatrout (Cynoscion nebulosus) Biology of Reproduction. 1997;56:931–937. doi: 10.1095/biolreprod56.4.931. [DOI] [PubMed] [Google Scholar]
- Lopez RE, Bhakoo H, Paolini NS, Rosen F, Holyoke ED, Goldrosen MH. Effect of estrogen on the growth of B-16 melanoma. Surg Forum. 1978;29:153–154. [PubMed] [Google Scholar]
- Margiotta-Casaluci L, Sumpter JP. 5alpha-Dihydrotestosterone is a potent androgen in the fathead minnow (Pimephales promelas) General and comparative endocrinology. 2011;171:309–318. doi: 10.1016/j.ygcen.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Mcleod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9–14. doi: 10.1016/0960-0760(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Meierjohann S, Schartl M. From Mendelian to molecular genetics: the Xiphophorus melanoma model. Trends Genet. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Miller JG, Gee J, Price A, Garbe C, Wagner M, Mac Neil S. Investigation of oestrogen receptors, sex steroids and soluble adhesion molecules in the progression of malignant melanoma. Melanoma Res. 1997;7:197–208. doi: 10.1097/00008390-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA. Different types of DNA damage play different roles in the etiology of sunlight-induced melanoma. Pigment Cell Melanoma Res. 2011;24:119–124. doi: 10.1111/j.1755-148X.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA, Nairn RS, Garcia R, Paniker L, Trono D, Thames HD, Gimenez-Conti I. Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model. Proc Natl Acad Sci U S A. 2010;107:9329–9334. doi: 10.1073/pnas.1000324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Meador J, Paniker L, Gasparutto D, Jeffrey WH, Cadet J. Development and application of a novel immunoassay for measuring oxidative DNA damage in the environment. Photochem Photobiol. 2002 Mar;75(3):257–265. doi: 10.1562/0031-8655(2002)075<0257:daaoan>2.0.co;2. 2002. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Paniker L, Sanchez G, Trono D, Nairn R. The etiology of sunlight-induced melanoma in Xiphophorus hybrid fish. Mol Carcinog. 2007;46:679–684. doi: 10.1002/mc.20341. [DOI] [PubMed] [Google Scholar]
- Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Reviews in Fish Biology and Fisheries. 1999;9:211–268. [Google Scholar]
- NCI Surveillance, Epidemiology, and End Results Program (SEER) 2012 http://seer.cancer.gov/
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Osterlind A. Hormonal and reproductive factors in melanoma risk. Clin Dermatol. 1992;10:75–78. doi: 10.1016/0738-081x(92)90060-c. [DOI] [PubMed] [Google Scholar]
- Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010;23:314–337. doi: 10.1111/j.1755-148X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottinger TG, Moran TA, Cranwell PA. The biliary accumulation of corticosteroids in rainbow trout, Oncorhynchus mykiss during acute and chronic stress. Fish Physiology and Biochemistry. 1992;10:55–66. doi: 10.1007/BF00004654. [DOI] [PubMed] [Google Scholar]
- Reeve VE, Allanson M, Cho JL, Arun SJ, Domanski D. Interdependence between heme oxygenase-1 induction and estrogen-receptor-beta signaling mediates photoimmune protection by UVA radiation in mice. J Invest Dermatol. 2009;129:2702–2710. doi: 10.1038/jid.2009.121. [DOI] [PubMed] [Google Scholar]
- Rodgers EW, Earley RL, Grober MS. Elevated 11-ketotestosterone during paternal behavior in the Bluebanded goby (Lythrypnus dalli) Horm Behav. 2006;49:610–614. doi: 10.1016/j.yhbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Schartl A, Malitschek B, Kazianis S, Borowsky R, Schartl M. Spontaneous melanoma formation in nonhybrid Xiphophorus. Cancer Res. 1995;55:159–165. [PubMed] [Google Scholar]
- Schartl A, Schartl M, Anders F. Promotion and regression of neoplasia by testosterone-promoted cell differentiation in Xiphophorus and Girardinus. Carcinog Compr Surv. 1982;7:427–434. [PubMed] [Google Scholar]
- Scott AP, Pinillos ML, Huertas M. The rate of uptake of sex steroids from water by Tinca tinca is influenced by their affinity for sex steroid binding protein in plasma. Journal of Fish Biology. 2005;67:182–200. [Google Scholar]
- Scott AP, Hirschenhauser K, Bender N, Oliveira R, Earley RL, Sebire M, Ellis T, Pavlidis M, Hubbard PC, Huertas M, et al. Non-invasive measurement of steroids in fish-holding water: important considerations when applying the procedure to behavior studies. Behaviour. 2008;145:1307–1328. [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;5:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Itami S, Hosokawa K, Terashi H, Takayasu S. Human genital melanocytes as androgen target cells. J Invest Dermatol. 1997;109:513–517. doi: 10.1111/1523-1747.ep12336630. [DOI] [PubMed] [Google Scholar]
- Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer research. 2007;67:3468–3474. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- Tveiten H, Scott AP, Johnsen HK. Plasma-sulfated C21-steroids increase during the periovulatory period in female common wolffish and are influenced by temperature during vitellogenesis. General and Comparative Endocrinology. 2000;117:464–473. doi: 10.1006/gcen.1999.7433. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez A, Galar-Martinez M, Jimenez-Orozco FA, Garcia-Latorre E, Dominguez-Lopez ML. Gender related differences in the oxidative stress response to PCB exposure in an endangered goodeid fish (Girardinichthys viviparus) Comp Biochem Physiol A Mol Integr Physiol. 2007;146:672–678. doi: 10.1016/j.cbpa.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Walter RB, Ju Z, Martinez A, Amemiya C, Samollow PB. Genomic resources for Xiphophorus research. Zebrafish. 2006;3:11–22. doi: 10.1089/zeb.2006.3.11. [DOI] [PubMed] [Google Scholar]
- Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30:1588–1593. doi: 10.1200/JCO.2011.39.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.