Abstract
Background and Aims A series of studies have shown that temperature triggers the onset of xylogenesis of trees after winter dormancy. However, little is known about whether and how moisture availability influences xylogenesis in spring in drought-prone areas.
Methods Xylogenesis was monitored in five mature Qilian junipers (Juniperus przewalskii) by microcore sampling from 2009 to 2011 in a semi-arid area of the north-eastern Tibetan Plateau. A simple physical model of xylem cell production was developed and its sensitivity was analysed. The relationship between climate and growth was then evaluated, using weekly wood production data and climatic data from the study site.
Key Results Delayed onset of xylogenesis in 2010 corresponded to a negative standardized precipitation evapotranspiration index (SPEI) value and a continuous period without rainfall in early May. The main period of wood formation was in June and July, and drier conditions from May to July led to a smaller number of xylem cells. Dry conditions in July could cause early cessation of xylem differentiation. The final number of xylem cells was mainly determined by the average production rate rather than the duration of new cell production. Xylem growth showed a positive and significant response to precipitation, but not to temperature.
Conclusions Precipitation in late spring and summer can play a critical role in the onset of xylogenesis and xylem cell production. The delay in the initiation of xylogenesis under extremely dry conditions seems to be a stress-avoidance strategy against hydraulic failure. These findings could thus demonstrate an evolutionary adaptation of Qilian juniper to the extremely dry conditions of the north-eastern Tibetan Plateau.
Keywords: Semi-arid forest, Juniperus przewalskii, juniper, onset of xylogenesis, drought, standardized precipitation evapotranspiration index, SPEI, xylem production rate, wood formation, xylem differentiation
INTRODUCTION
In cold climates, tree phenology is primarily controlled by temperature (Körner, 2006; Rossi et al., 2008; Hanninen and Tanino, 2011). Experiments inducing artificial heating of the stem in evergreen conifers during the quiescent stage showed that it is possible to induce reactivation of cell division (Oribe et al., 2001; Gričar et al., 2006), demonstrating that temperature is a trigger for xylogenesis. The higher temperatures predicted for the near future are thus expected to advance xylem formation in spring (Rossi et al., 2011; King et al., 2013), although photoperiodic constraints could restrict the responses of some boreal and temperate species to climate warming (Körner and Basler, 2010). Most studies in temperate and cold climates have been performed in ecosystems in which snowmelt provides abundant water especially at the beginning of the growing seasons, precipitation is frequent in spring and summer, and water availability is not a limiting factor for xylem formation (Rathgeber et al., 2011; Rossi et al., 2011; Prislan et al., 2013). Little is thus known about the effect of precipitation on the onset of xylogenesis in cold drought-prone areas.
Drought stress can affect xylogenesis through its effects on the cambium and the developing xylem, given that xylem cell expansion is a turgor-driven process depending on cellular water uptake and solute accumulation (Kozlowski and Pallardy, 2002; Fonti et al., 2010). Before resumption of xylogenesis, trees must recover an adequate water balance, since turgor is an important requisite for plant growth (Kozlowski and Pallardy, 2002). Both cell division and expansion are sensitive to water potential (Abe et al., 2003; Fonti et al., 2010), and water deficit is the primary constraint for tree growth (Balducci et al., 2013; Vieira et al., 2014). However, the majority of that work has concerned genetics (Chaffey, 2002; Qiu et al., 2013). There is still little research into xylogenesis and its climatic control (Rossi et al., 2008; Li et al., 2013; Prislan et al., 2013). Tree growth responses to water scarcity are complex, involving adaptive changes and/or deleterious influences (Fonti et al., 2010; Gea-Izquierdo et al., 2012; Lautner, 2013). On the one hand, long and severe summer droughts may slow down or even stop cell division, leading to missing rings, as observed in semi-arid forests in northern China (Liang et al., 2006). On the other hand, cell division can be resumed after a cessation when moisture conditions improve, which produces intra-annual density fluctuations in tree rings (Cherubini et al., 2003; Campelo et al., 2007; De Luis et al., 2011) or distorted and collapsed cells (Abe et al., 2003; Arend and Fromm, 2007). In addition, water deficiency occurring in the second part of the growing season can cause an early cessation of cell division (Eilmann et al., 2011) or light ring formation (Liang and Eckstein, 2006). Based on these pieces of evidence, we speculate that precipitation prior to the growing season could play a key role in the onset of xylogenesis in arid and semi-arid areas.
However, to date, xylogenesis studies have not shown the influences of precipitation on its onset. In a cold environment in the southern boreal forest of Quebec, Canada, no effect of spring rehydration was observed on radial growth initiation in Picea mariana (Turcotte et al., 2009). Within inner Alpine dry valleys, tree water status early during the growing season determines the total annual above-ground growth (Swidrak et al., 2013). In Mediterranean climates, Camarero et al. (2010) observed that the climatic responses of Juniperus thurifera and Pinus halepensis changed during the year, with stem radius variations dependent on temperature during growth onset and on precipitation during the summer. Studies in dry inner Alpine valleys also found that early spring temperature rather than water availability controlled xylogenesis in drought-exposed Pinus sylvestris (Gruber et al., 2010; Swidrak et al., 2011). Drought treatment even caused earlier onset of the spring growing season in the Mediterranean shrub Erica multiflora (Bernal et al., 2011). Inner Alpine valleys with mean annual precipitation >500 mm and Mediterranean sites characterized by moist springs, according to the aforementioned studies, are probably not dry enough to show the impact of precipitation on the onset of xylogenesis. The semi-arid forests of the Tibetan Plateau, with their regular exposure to water-limited growth conditions, could therefore be an ideal ecosystem to test the proposed relationship between initiation of xylogenesis and moisture availability.
On the north-eastern Tibetan Plateau, close to the Gobi desert, the natural forests are dominated by Qilian juniper (Juniperus przewalskii). Except for a few sites at the upper timberline (Bräuning, 2001; Liu et al., 2005; Zhu et al., 2008), its growth is primarily limited by precipitation (Zhang et al., 2003; Shao et al., 2005; Liu et al., 2006; Yang et al., 2013). The high percentage of drought-induced missing rings in Qilian juniper also indicates that the dry environmental conditions are close to the physiological limit of this species (Shao et al., 2005; Liang et al., 2006). In addition, the low amount of snowmelt due to the dry winters makes sporadic spring rainfall more crucial for the growth of Qilian juniper. Although Qilian juniper is crucial for developing millennial tree ring chronologies in the area (Zhang et al., 2003; Sheppard et al., 2004; Shao et al., 2005), the driving force of wood formation affecting tree ring width is still unknown. Based on strong limiting effects of moisture deficit and high temperatures, related to increased evaporation in May and June on its growth (Zhang et al., 2003; Li et al., 2008; Zheng et al., 2008), we hypothesized that the onset of xylogenesis in Qilian juniper might be largely regulated by moisture availability in spring although the thermal conditions are favourable.
The objectives of this study were (1) to monitor the timing and dynamics of xylem differentiation in Qilian juniper exposed to drought at its lower distribution of the north-eastern Tibetan Plateau and (2) to analyse the climatic driving force for wood formation. Xylogenesis was monitored at weekly intervals during 2009–2011, in which dry conditions were different between months and years. By comparing the climatic parameters with xylem phenology, we tested the aforementioned hypothesis.
MATERIALS AND METHODS
Study site
The study was conducted in the north-eastern part of the Tibetan Plateau in an undisturbed forest of the Dulan area (36°00'N, 98°11'E). In this area, Qilian junipers are on average approx. 500 years old, with the oldest individuals being 1045 years old. The study site is located at 3850 m a.s.l. with a mean slope of 15 °, representing a lower limit of the altitudinal distribution of this species (Fig. 1). Tree ring analysis of 47 cores from 23 trees at the same site showed that missing rings account for 0·44 % of a total of 25 382 dated rings (Zheng et al., 2008).
Fig. 1.
(A) Location of the study site and the nearby Dulan meteorological station on the Tibetan Plateau (inset). (B) A view of the sampling site.
The climate is typically dry-continental, with relatively wet summers and dry winters. Climatic data (1954–2012) from the meteorological station at Dulan (36°18'N, 98°06'E, 3190 m a.s.l.), 32 km from the study site, showed that the average annual precipitation is 201·5 mm, of which 89 mm were recorded between June and July. On average, total precipitation for winter months (December to February) is only 12·6 mm. The annual mean temperature is 3·1 °C, with July (mean temperature of 15·0 °C) and January (−9·9 °C) being the warmest and coldest months, respectively.
Field sampling and tissue preparation
Five individuals of Qilian juniper (Juniperus przewalskii Komarov) were randomly selected. The average tree diameter at breast height was 50–60 cm and the average tree height was around 8 m. Microcores (2·5 mm in diameter and 20–25 mm in length) were collected from the stems at 1–1·3 m height by a Trephor at weekly intervals from April to October 2009, 2010 and 2011 (Rossi et al., 2006a). The microcores contained the previous three xylem tree rings, the developing current annual xylem ring, the cambial zone and adjacent phloem.
The microcores were fixed in formalin–ethanol–acetic acid (FEA) solution to avoid tissue deterioration. In the laboratory, each microcore was oriented by marking the transverse side with a pencil, dehydrated with a graded series of ethanol and d-limonene, and embedded in paraffin. Transverse sections of 9–12 µm thickness were cut with a Leica RM 2245 rotary microtome (Leica Microsystems, Wetzlar, Germany). The sections were stained with 3 % safranine (Merck, Darmstadt, Germany) and 0·5 % astra blue (Sigma-Aldrich, Steinheim, Germany), both in 95 % ethanol, and permanently fixed to the slides. Furthermore, all slides were examined with visible and polarized light under a Nikon Eclipse 800 light microscope and an NIS Elements BR3 image analysis system, and photographed with a DS-Fi1 digital camera attached to the microscope.
Histological observations
Tracheids were produced via cell divisions in the cambium. Cells in the cambial zone were characterized by thin cell walls and small radial diameters. Xylem differentiation occurred through two phases: enlargement and wall thickening. During enlargement, tracheids contained protoplast enclosed in thin primary cell walls and their radial diameter was at least twice that of a cambial cell (Deslauriers et al., 2003). During the secondary wall thickening phase, tracheids changed the colour of the cell wall from light blue to dark red under normal light due to cellulose and lignin deposition (Gričar et al., 2005). At the end of differentiation, tracheids were considered mature when the protoplast was lost and the walls were completely stained red.
The cambium consisted of three or four cells in the dormant period (Fig. 2). In spring, xylogenesis was considered to have started when at least one radial file of enlarging cells was observed (Rossi et al., 2008). When no new xylem cells were produced, cell division was considered to be finished. Xylogenesis was considered to be completed when the last cells in the wall thickening phase matured at the end of the growing period. For each tree and sampling, the total xylem cell number was determined by counting the number of cells in enlargement, wall thickening and mature phases along three radial files (Deslauriers et al., 2003).
Fig. 2.
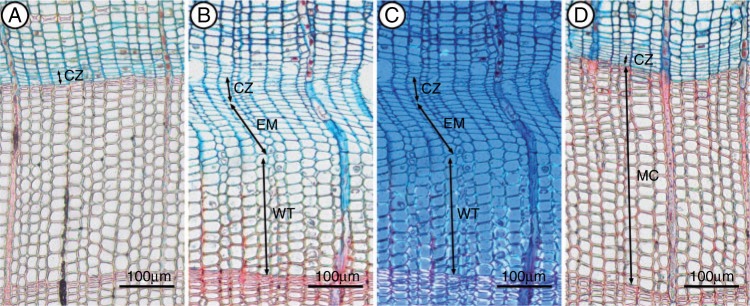
Phases of wood formation in Qilian juniper in 2011. (A) Cambial zone (CZ) on 29 April; no new xylem cell production observed; (B) enlarging (EM) and wall-thickening (WT) cells on 10 July; (C) enlarging (EM) and wall-thickening (WT) cells on 10 July, under polarized light; (D) the current xylem tree ring consists entirely of mature cells (MC) on 2 October.
Xylem phenology
For each tree, five phenophases were considered to assess the phenology of xylem development, including onset and end of cell enlargement, end of wall thickening and the duration of both cell enlargement and xylem differentiation. For each phenophase, differences among years were compared by analysis of variance (ANOVA) and Tukey’s test. Before using ANOVA, all critical dates were tested for normality and equality of variance (Quinn and Keough, 2002).
Xylem cell production
The dynamics of total xylem cell number were fitted with a Gompertz equation using the non-linear mixed model defined as:
where y is the total number of tracheids at time t, where t is the time expressed as day of the year, A is the upper asymptote, β is the x-axis placement parameter and κ is the rate of change parameter.
From the estimated parameters of the Gompertz function, the date of the inflection point (tp), the corresponding maximum production rate (rmax) and the average production rate (rm) were computed according to Rathgeber et al. (2011) as:
We propose expressing the final number of tracheids as a function of average production rate and cell production duration:
where Ncell is the final number of xylem cells calculated by the median of the total number of tracheids after the cessation of cell production and Δt is the duration of cell division defined as the duration of cell enlargement. To determine the specific contribution of the rate and duration to the total number of tracheids, a sensitivity analysis of the model was performed (Rathgeber et al., 2011).
All of the parameters above were calculated for each tree. To compare the tracheid production among years, the final number of xylem cells (Ncell), the critical dates of the maximum production rate (tp) and the average production rate (rm) were assessed by ANOVA and Tukey’s test. Before ANOVA, all data were tested for normality and equality of variance (Quinn and Keough, 2002).
Climate data
To evaluate climatic conditions before wood formation, degree-day sum and total precipitation from twarming (see below) to the onset of xylogenesis were calculated. Degree-day sum is an index representing the effect of temperature and was calculated by a modified sine wave model based on daily maximum and minimum temperatures integrating the area of the curve above 5 °C (Seo et al., 2008). The threshold of 5 °C was based on the study of Rossi et al. (2008). The date on which a daily mean temperature warmer than 5 °C had been recorded for five consecutive days was defined as twarming. The variability of data was assessed by the coefficient of variation (CV), defined as the ratio between the standard deviation and the mean. By standardizing the measure of dispersion, the CV allows an adequate comparison of the variability among measurements with different means.
To quantify drought severity, the standardized precipitation evapotranspiration index (SPEI) was calculated, using the half-monthly difference between precipitation and potential evapotranspiration for the 1955–2011 period. The SPEI, based on precipitation and temperature data, enables evaluation of both water surplus and deficit on different time scales (Vicente-Serrano et al., 2010). We used the SPEI package from the R freeware program (http://cran.r-project.org) to calculate the SPEI. A period of drought was characterized by a negative SPEI value.
Daily precipitation and mean temperature were considered to describe climate during growing seasons. We chose four climatic variables as potential explanatory variables for xylem growth: minimum, mean and maximum temperature, and precipitation.
Climate–growth relationships
We used Pearson coefficients to evaluate the relationship between xylem growth and climatic variables (Mudelsee et al., 2003). To remove the endogenous growth trend, xylem growth was measured by a growth index, which was calculated by dividing the number of tracheids produced during the week by the values predicted by the Gompertz model. Taking into account delayed responses, the potential explanatory variables were averaged (temperature) or summed (precipitation) weekly from 1 to 10 d before each sampling date. Given the effects of temporal autocorrelation, correlation coefficients were calculated by both sets of data of first-order difference.
RESULTS
Xylem phenology
Delayed onset and short duration of new xylem cell production were found in 2010 compared with the other two years (Table 1). The first cells in the enlargement phase, corresponding to the onset of xylogenesis, were detected on 4 and 11 June [day of the year (DOY) 155 and 162] in 2009 and 2011, respectively. The onset of xylogenesis in 2010, however, was observed as late as on 29 June (DOY 180), about 3 weeks later than in 2009 and 2011. The cessation of cell enlargement occurred in the first half of August (DOY 213–225), with slight differences among the years (Table 1). The duration of new xylem production in 2010 was only 46 d, much shorter than the 59 d in 2009 and 61 d in 2011, although cell enlargement in 2010 was finished on 13 August (DOY 225), the latest among the three years (Table 1).
Table 1.
Mean ± s.d. of onset and end of cell enlargement, end of wall thickening and the duration of both cell enlargement and xylem differentiation during 2009–2011
| 2009 | 2010 | 2011 | F | P | |
|---|---|---|---|---|---|
| Onset of cell enlargement (DOY) | 155 ± 4b | 180 ± 7 a | 162 ± 8b | 19·21 | <0·001 |
| End of cell enlargement (DOY) | 213 ± 5b | 225 ± 6a | 222 ± 4ab | 7·22 | 0·009 |
| End of wall thickening (DOY) | 246 ± 4a | 243 ± 4a | 232 ± 2b | 22·72 | <0·001 |
| Duration of cell enlargement (d) | 59 ± 4a | 46 ± 8b | 61 ± 5a | 8·026 | 0·006 |
| Duration of xylem differentiation (d) | 92 ± 6a | 64 ± 6b | 71 ± 3b | 30·32 | <0·001 |
Results from ANOVA are reported as F-values and probability (P).
Values bearing the same letters within rows are not statistically different at 0·05 probability (Tukey test).
DOY, day of the year.
The last cells in the wall thickening phase, corresponding to the end of xylem differentiation, were detected between the end of August and the beginning of September (DOY 232–246) (Table 1). However, a 2 week earlier cessation was observed in 2011 compared with those in 2009 and 2010. As a result, the period of xylem differentiation in 2009 was 92 d, much longer than the 64 d in 2010 and 71 d in 2011 (Table 1).
Xylem cell production
During the three study years, >65 % of tracheids were produced in June and July. The final number of tracheids produced in 2011 was only 10·3, significantly lower than in 2009 and 2010 (Table 2). Based on modelled dynamics of xylem formation, the maximum production rate (rmax) occurred on 26 and 27 June (DOY 177 and 178) in 2009 and 2011, and on 7 July (DOY 188) in 2010. However, the average production rate (rm) in 2011 was only 0·10 cells d−1, which was much less than the 0·23 in 2009 and 0·29 in 2010 (Table 2).
Table 2.
Median ± median deviation of the final number of xylem cells (Ncell), mean ± s.d. of the inflection point (tp) and the average production rate (rm) during 2009–2011
| 2009 | 2010 | 2011 | F | P | |
|---|---|---|---|---|---|
| Ncell (number of cells) | 18·7 ± 3·1a | 18·8 ± 3·5a | 10·3 ± 1·6b | 14·527 | 0·001 |
| tp (DOY) | 177 ± 1b | 188 ± 1a | 178 ± 1b | 205·79 | <0·001 |
| rm (cells per day) | 0·23 ± 0·03b | 0·29 ± 0·02a | 0·10 ± 0·01c | 101·07 | <0·001 |
Results from ANOVA are reported as F-values and probability (P).
Values bearing the same letters within rows are not statistically different at 0·05 probability (Tukey test).
DOY, day of the year.
A simple physical model using total Ncell, as well as average rate (rm) and duration (Δt) of new xylem cell production, gave satisfactory results, with r2 of 0·91 (F = 136·3, P < 0·01, n = 15) (Fig. 3). Sensitivity analysis of the model showed that a low rm resulted in low Ncell, with nearly no effect of Δt (Fig. 3). Average or high values of Ncell were mainly influenced by rm. Generally, Ncell was more sensitive to rm than to Δt variation.
Fig. 3.
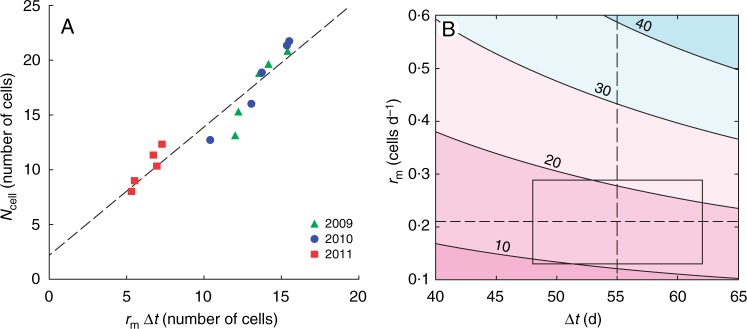
(A) The simple physical model of the total number of xylem cells (Ncell), the average rate (rm) and the duration (Δt) of cell production. The dashed line represents the regression: Ncell = 2·175 + 1·172rmΔt, r2 = 0·913, P < 0·01, n = 15. (B) Sensitivity analysis of the physical model. The dashed lines represent the means, and the frame delimits the area of the mean ± s.d.
Climate condition during the growing season
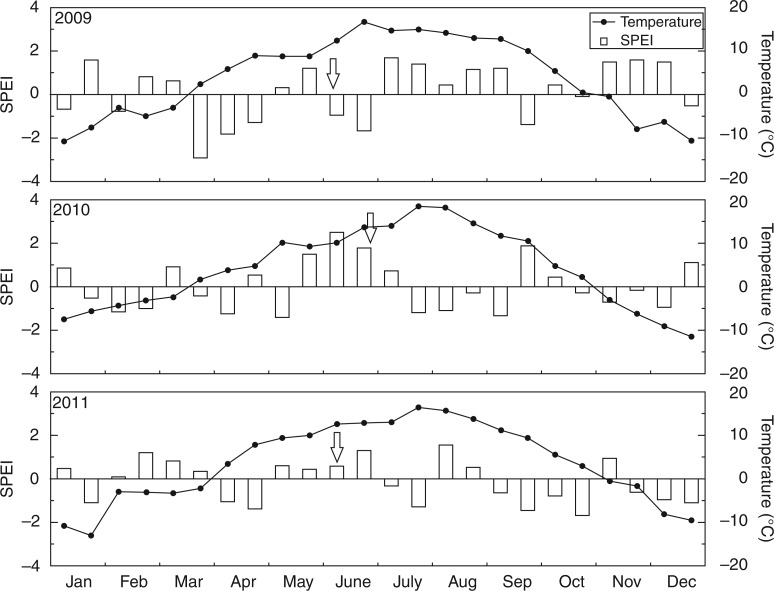
During the three study years, the mean temperature on a scale of half a month was >5 °C by the end of April (Fig. 4). However, the sign of the SPEIs in the first half of May was not consistent among years. A negative SPEI value in the first half of May in 2010 indicated a continuously dry period, which coincided with the 3 week delayed onset of xylogenesis in 2010 (Fig. 4). In 2009 and 2011, however, sporadic rainfall was observed during the same period (Fig. 5). Nonetheless, due to abundant rainfall in the first half of June (Fig. 5), the precipitation sum prior to wood formation in 2010 was larger than in 2009 and 2011, with a CV value of 75·3 % (Table 3). It should be mentioned that the onset of xylogenesis in 2010 occurred 22 d after a heavy precipitation event (43·5 mm on June 4). In addition, the degree-day sums before wood formation in 2009 and 2011 were 291·6 and 301·9, respectively, much less than the 442·5 in 2010 with a CV value of 24·4 % (Table 3). Furthermore, the mean temperature in May was 8·7 °C in 2009, but 9·6 °C in 2010 and 2011.
Fig. 4.
The standardized precipitation evapotranspiration index (SPEI) and the mean temperature on a scale of half a month during 2009–2011. Arrows indicate the onset of xylogenesis.
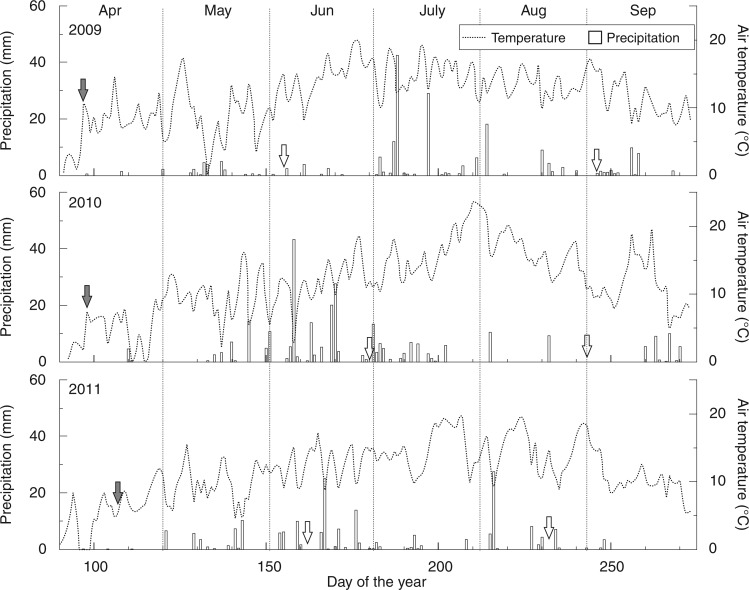
Fig. 5.
Daily precipitation sum (bars) and mean air temperature (dotted lines) from April through September during 2009–2011. Filled arrows indicate the date (twarming), i.e. the fifth day, when daily mean temperature warmer than 5 °C was recorded for five consecutive days. Open arrows indicate the onset and the end of xylem differentiation.
Table 3.
Climate conditions before xylem differentiation are described by degree-day sum and total precipitation from twarming to the onset of xylogenesis, where twarming is the date when daily mean temperature warmer than 5 °C was recorded for five consecutive days
| 2009 | 2010 | 2011 | CV | |
|---|---|---|---|---|
| Degree-day sum | 291·6 | 442·5 | 301·9 | 24·4 % |
| Precipitation (mm) | 48·2 | 178·2 | 60·1 | 75·3 % |
CV, coefficient of variation.
The main growing season (June–July) in 2011 was characterized by relatively low precipitation (Fig. 5). The rainfall during this period was only 95 mm in 2011, being much less than the 116·9 mm in 2009 and 185·4 mm in 2010. In particular, precipitation in July was only 14·5 mm in 2011, compared with 106·8 mm and 43·2 mm in 2009 and 2010, respectively. Extremely dry conditions in July were associated with an earlier ending of xylogenesis in 2011.
Climate–growth relationships
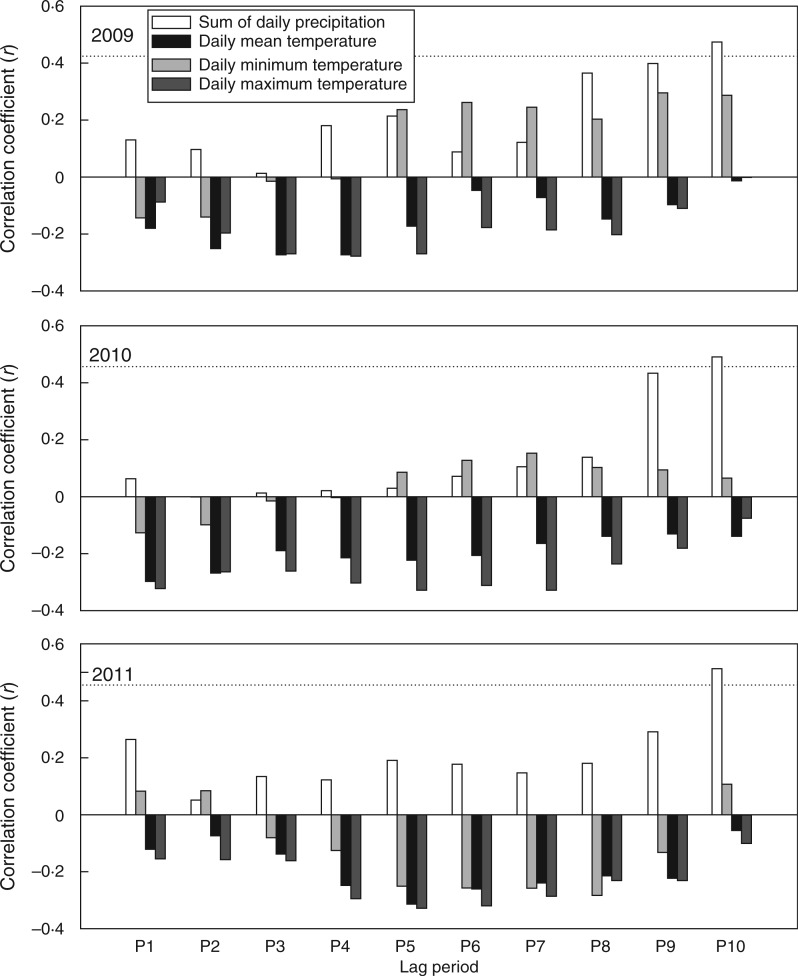
Xylem growth showed positive and significant responses to precipitation at a time lag of 10 d (P10 in Fig. 6). A negative but not significant correlation was observed between xylem growth and daily maximum temperatures. A similar situation was also recorded for daily mean temperatures. However, xylem growth responded differently to daily minimum temperatures between years: it showed positive responses to daily minimum temperatures in 2009 and 2010, whereas the opposite pattern occurred in 2011.
Fig. 6.
Pearson correlation coefficients (r) between xylem growth and climatic variables during 2009–2011. P1– P10 represents the 7 d period that is shifted backwards from 1 d (P1) to 10 d (P10) before the sampling date. The dotted lines indicate the 0·05 significance level.
DISCUSSION
Impact of environment on the onset of xylogenesis
There is a considerable interest among biologists and dendroecologists in the environmental factors driving xylogenesis, because the timing of the onset of xylogenesis determines the amount and quality of xylem produced (Begum et al., 2013). Generally, temperature has been identified as the dominant environmental signal triggering tree phenology (Körner, 2006). A threshold temperature was shown to be decisive for xylogenesis in conifers of cold climates (Rossi et al., 2008), whereas Seo et al. (2008) demonstrated that one or more factors other than, or in addition to, heat sum determines the onset of wood formation in Scots pine in northern Finland. In the present study, the onset of xylogenesis in 2010 significantly differed from that of the other two years. From the similar values of degree-day sums before xylogenesis in 2009 and 2011, we suggest that temperature has a limiting effect on the onset of xylogenesis (Table 3). This is also evident from the mean air temperature above 5 °C before the onset of xylogenesis in the three study years. Wood formation only started when suitable thermal conditions were met. In 2010, however, the degree-day sums from twarming to 4 and 11 June (the onset dates of xylogenesis in 2009 and 2011, respectively) was 250·0 and 290·9, which was not much less than in 2009 and 2011. Additionally, a high CV also shows highly discrete distribution. Much higher degree-day sums before wood formation and later onset of xylogenesis in 2010 than in 2009 and 2011 (Tables 1 and 3) suggest that other climatic variables, in addition to temperature, can also have an over-riding effect on the initiation of xylogenesis.
Precipitation in the first half of May can also have a major influence on the onset of xylogenesis in Qilian juniper, as exemplified by the fact that the delayed onset in 2010 corresponded to extremely dry conditions in early May (Fig. 5). High temperatures in May 2010 amplified the impact of drought by evapotranspiration. Furthermore, in the three years, only the SPEI of early May in 2010 was negative, which reinforces the importance of precipitation in this period for the onset of xylogenesis. In spite of a heavy precipitation event in early June, it still took 22 d until the onset of xylogenesis in 2010. In Scots pine, water for enlargement of the first tracheids is stored in the stem to withstand drought (Swidrak et al., 2011). However, this adaptation mechanism does not seem to work well under prolonged drought, as observed in pines that suffered high mortality (Oberhuber, 2001; Bigler et al., 2006). In our study area, the climate is characterized by extremely dry winters. Total precipitation for winter months (December to February) from 1954 to 2012 is only 12·6 mm on average, so it is not possible for Qilian juniper to use water from snowmelt for the initiation of cell division. As a consequence, precipitation in late spring has a key influence on the onset of xylogenesis. Due to a potential relationship between primary growth (bud-burst, foliage and shoot) and secondary growth (Huang et al., 2014), dry conditions in late spring may delay primary growth and hence xylogenesis. The drought-induced delay might be an evolutionary strategy to adapt to a dry climate and avoid hydraulic failure, as well as to decrease the risk of tree mortality. All this has probably been decisive for the survival of some Qilian junipers for >1000 years under a dry climate.
In addition to temperature and precipitation, xylogenesis can also be influenced by photoperiod. The photoperiodic constraint to growth was verified by the finding that a shorter photoperiod delayed bud burst in Norway spruce (Partanen et al., 1998), as well as that maximum daily growth rates in conifers in cold environments occurred around the time of maximum day-length (Rossi et al., 2006b). However, photoperiodic regulation of maximum growth rate seems to be less common in semi-arid forests. Gruber et al. (2010) suggested that competition in carbon allocation hastened the timing of maximum cell production, which was observed approx. 4–6 weeks before the summer solstice in Pinus sylvestris under drought stress. In the present study, the dates of the maximum production in 2009 and 2011 were close to the summer solstice, whereas the culmination in 2010 occurred on 7 July (DOY 188), about 2 weeks after the summer solstice (Table 2). The delayed timing of the maximum production rate in 2010 seems to be the consequence of the drought-induced delay of the onset of xylogenesis.
Impact of drought on the duration of xylem differentiation
The duration of xylem differentiation, affected by the onset of cell enlargement and completion of wall thickening, was significantly higher in 2009 than in 2010 and 2011. Xylem differentiation in 2011 had an earlier cessation than in 2009 and 2010. During the 2011 growing season, Qilian junipers experienced extreme drought in July (Fig. 5). We therefore suggest that arid conditions in July may lead to an early cessation of wood formation. The total precipitation in August 2011 was 56 mm, much higher than the 38·1 mm and 19·9 mm in 2009 and 2010, but this was probably not enough to extend the period of xylem differentiation in 2011. Similar results were also found in dry inner Alpine valleys (Eilmann et al., 2011) and in a Mediterranean climate (De Luis et al., 2011; Vieira et al., 2014).
However, a short period of wood formation does not mean a low number of tracheids. Despite a short growth period in 2010, the trees formed almost the same number of tracheids as in 2009. Although the duration of wood formation in 2011 was approximately as long as that in 2010, the amount of tracheids produced in 2011 was only half of that in 2010. This is in accordance with the result of Dunn et al. (2007) in black spruce, but contrasts with the results showing that a short growth period corresponds to a narrow growth ring (Vaganov et al., 1999; Deslauriers et al., 2003) and that a higher number of tracheids produced could often be related to a prolonged growing period (Gričar et al., 2005; Thibeault-Martel et al., 2008). Meanwhile, our sensitivity analysis showed that the final number of xylem cells (Ncell) was mainly determined by the rate rather than the duration of xylem cell production (Fig. 3B). Rossi et al. (2014) found that most of the variability in xylem growth of black spruce can be explained by the duration of cell production. Differences in local climatic conditions are a possible explanation for this discrepancy. Compared with the cold and humid weather conditions in other studies, the limited and sporadic rainfall in our study area can more probably lead to drought-induced reduction in soil moisture. When soil moisture drops below the threshold, stomatal closure occurs. This is followed by decreased water and CO2 fluxes. As a consequence, photosynthetic efficiency and therefore radial growth is reduced (McDowell et al., 2013). Thus, under the negative influence of drought, the actual growing period becomes shorter.
Impact of precipitation on xylem growth
In semi-arid areas, dry conditions prior to and during the early growing period showed limiting effects on tree growth. A recent study using controlled soil moisture and temperature also showed that a water deficit prior to the growing season can cause a 2–4 week delay in completely restoring cambial activity of P. mariana saplings, resulting in the formation of a narrow xylem tree ring (Balducci et al., 2013). As suggested by this study, a narrow ring may be formed mainly due to a delayed onset of xylem formation.
Xylem growth in Qilian juniper proved to be primarily controlled by precipitation, although high temperatures may increase water stress by evapotranspiration, thus affecting xylem growth. This was evident in the negative but not significant correlation between xylem growth and daily maximum and mean temperatures (Fig. 6). In addition, given that most tracheids were produced in June and July, precipitation immediately before (in May) and during this period is a determinant of the amount of wood produced. This is consistent with studies carried out on tree ring chronologies collected from trees located at our study site (Zheng et al., 2008) and nearby sites (Zhang et al., 2003; Shao et al., 2005; Yang et al., 2013), as well as studies on junipers of other semi-arid areas (e.g. Sass-Klaassen et al., 2008). It has also been reported that drought can affect cell production and the final number of cells in a tree ring (Camarero et al., 2010; Balducci et al., 2013; Vieira et al., 2014). Precipitation thus strongly limits xylem growth, which may relate to a decline in cell division when exposed to drought (Savidge, 2001; Abe et al., 2003).
Conclusions
To the best of our knowledge, the present study showed for the first time that precipitation in the early growing season can be a critical trigger of xylogenesis when the thermal conditions are favourable, which allowed our hypothesis to be accepted. The delay in the initiation of xylogenesis under extremely dry conditions seems to be a stress avoidance strategy against hydraulic failure. This strategy may decrease the risk of tree mortality and, as a consequence, enable Qilian juniper to live >1000 years in a typically dry-continental climate. Precipitation affects not only the onset of xylogenesis but also cell division in Qilian juniper. Cell production was greatly influenced by the average production rate rather than the duration of new xylem cell production in our dry site, being different from research conclusions for other tree species from other cold and humid areas. Rising temperatures since the late 1970s have increased aridity in many continental regions of the globe, exposing semi-arid forests in particular to additional stress (Dai et al., 2013). Such drought stress may postpone the initiation of xylogenesis, reduce cell production or contribute to radial growth decline, as is the case for semi-arid forests in Inner Asia (Dulamsuren et al., 2010; Liu et al., 2013). Although this study advanced the important message about necessary factors in controlling the initiation of xylogenesis in semi-arid areas, long-term monitoring is necessary to detect potential thresholds in precipitation or soil moisture for the onset of xylogenesis.
ACKNOWLEDGEMENTS
We thank Macairangjia and Chengye He for weekly microcore sampling, Dr Nigel Chaffey and two reviewers for useful comments and suggestions, and Martin Cregeen for language editing. This work was supported by the National Natural Science Foundation of China (41171161) and the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB03030402). International co-operation was supported by the bilateral project between China and Slovenia (BI-CN/09-11-012) and COST Action (FP1106, STReESS).
LITERATURE CITED
- Abe H, Nakai T, Utsumi Y, Kagawa A. 2003. Temporal water deficit and wood formation in Cryptomeria japonica. Tree Physiology 23: 859–863. [DOI] [PubMed] [Google Scholar]
- Arend M, Fromm J. 2007. Seasonal change in the drought response of wood cell development in poplar. Tree Physiology 27: 985–992. [DOI] [PubMed] [Google Scholar]
- Balducci L, Deslauriers A, Giovannelli A, Rossi S, Rathgeber CB. 2013. Effects of temperature and water deficit on cambial activity and woody ring features in Picea mariana saplings. Tree Physiology 33: 1006–1017. [DOI] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R. 2013. Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiologia Plantarum 147: 46–54. [DOI] [PubMed] [Google Scholar]
- Bernal M, Estiarte M, Peñuelas J. 2011. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biology 13: 252–257. [DOI] [PubMed] [Google Scholar]
- Bigler C, Bräker OU, Bugmann H, Dobbertin M, Rigling A. 2006. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems 9: 330–343. [Google Scholar]
- Bräuning A. 2001. Climate history of the Tibetan Plateau during the last 1000 years derived from a network of Juniper chronologies. Dendrochronologia 19: 127–137. [Google Scholar]
- Camarero JJ, Olano JM, Parras A. 2010. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytologist 185: 471–480. [DOI] [PubMed] [Google Scholar]
- Campelo F, Nabais C, Freitas H, Gutiérrez E. 2007. Climatic significance of tree-ring width and intra-annual density fluctuations in Pinus pinea from a dry Mediterranean area in Portugal. Annals of Forest Science 64: 229–238. [Google Scholar]
- Chaffey N. 2002. Why is there so little research into the cell biology of the secondary vascular system of trees? New Phytologist 153: 213–223. [Google Scholar]
- Cherubini P, Gartner BL, Tognetti R, Bräker OU, Schoch W, Innes JL. 2003. Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biological Reviews of the Cambridge Philosophical Society 78: 119–148. [DOI] [PubMed] [Google Scholar]
- Dai A. 2013. Increasing drought under global warming in observations and models. Nature Climate Change 3: 52–58. [Google Scholar]
- De Luis M, Novak K, Raventós J, Gričar J, Prislan P, Čufar K. 2011. Climate factors promoting intra-annual density fluctuations in Aleppo pine (Pinus halepensis) from semiarid sites. Dendrochronologia 29: 163–169. [Google Scholar]
- Deslauriers A, Morin H, Begin Y. 2003. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Canadian Journal of Forest Research 33: 190–200. [Google Scholar]
- Dulamsuren C, Hauck M, Leuschner C. 2010. Recent drought stress leads to growth reductions in Larix sibirica in the western Khentey, Mongolia. Global Change Biology 16: 3024–3035. [Google Scholar]
- Dunn AL, Barford CC, Wofsy SC, Goulden ML, Daube BC. 2007. A long-term record of carbon exchange in a boreal black spruce forest: means, responses to interannual variability, and decadal trends. Global Change Biology 13: 577–590. [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Graf Pannatier E, Rigling A. 2011. Drought alters timing, quantity, and quality of wood formation in Scots pine. Journal of Experimental Botany 62: 2763–2771. [DOI] [PubMed] [Google Scholar]
- Fonti P, von Arx G, Garcia-Gonzalez I, et al. 2010. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytologist 185: 42–53. [DOI] [PubMed] [Google Scholar]
- Gea-Izquierdo G, Fonti P, Cherubini P, Martin-Benito D, Chaar H, Canellas I. 2012. Xylem hydraulic adjustment and growth response of Quercus canariensis Willd. to climatic variability. Tree Physiology 32: 401–413. [DOI] [PubMed] [Google Scholar]
- Gričar J, Čufar K, Oven P, Schmitt U. 2005. Differentiation of terminal latewood tracheids in silver fir trees during autumn. Annals of Botany 95: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Oven P. 2006. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Science and Technology 41: 463–475. [Google Scholar]
- Gruber A, Strobl S, Veit B, Oberhuber W. 2010. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiology 30: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanninen H, Tanino K. 2011. Tree seasonality in a warming climate. Trends in Plant Science 16: 412–416. [DOI] [PubMed] [Google Scholar]
- Huang J, Deslauriers A, Rossi S. 2014. Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytologist 203: 831–841. [DOI] [PubMed] [Google Scholar]
- King GM, Gugerli F, Fonti P, Frank DC. 2013. Tree growth response along an elevational gradient: climate or genetics? Oecologia 173: 1587–1600. [DOI] [PubMed] [Google Scholar]
- Körner C. 2006. Significance of temperature in plant life. In: Morison JIL, Morecroft MD, eds. Plant growth and climate change. Oxford: Blackwell, 48–69. [Google Scholar]
- Körner C, Basler D. 2010. Phenology under global warming. Science 327: 1461–1462. [DOI] [PubMed] [Google Scholar]
- Kozlowski TT, Pallardy SG. 2002. Acclimation and adaptive responses of woody plants to environmental stresses. Botanical Review 68: 270–334. [Google Scholar]
- Lautner S. 2013. Wood formation under drought stress and salinity. In: Fromm J, ed. Cellular aspects of wood formation. Berlin: Springer, 187–202. [Google Scholar]
- Li J, Cook ER, D’Arrigo R, et al. 2008. Common tree growth anomalies over the northeastern Tibetan Plateau during the last six centuries: implications for regional moisture change. Global Change Biology 14: 2096–2107. [Google Scholar]
- Li X, Liang E, Gricar J, Prislan P, Rossi S, Cufar K. 2013. Age-dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiology 33: 48–56. [DOI] [PubMed] [Google Scholar]
- Liang E, Eckstein D. 2006. Light rings in Chinese pine (Pinus tabulaeformis) in semiarid areas of north China and their palaeo-climatological potential. New Phytologist 171: 783–791. [DOI] [PubMed] [Google Scholar]
- Liang E, Liu X, Yuan Y, et al. 2006. The 1920s drought recorded by tree rings and historical documents in the semi-arid and arid areas of northern China. Climatic Change 79: 403–432. [Google Scholar]
- Liu H, Williams PA, Allen CD, et al. 2013. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Global Change Biology 19: 2500–2510. [DOI] [PubMed] [Google Scholar]
- Liu X, Qin D, Shao X, Chen T, Ren J. 2005. Temperature variations recovered from tree-rings in the middle Qilian Mountain over the last millennium. Science in China Series D-Earth Sciences 48: 521–529. [Google Scholar]
- Liu Y, An Z, Ma H, et al. 2006. Precipitation variation in the northeastern Tibetan Plateau recorded by the tree rings since 850 AD and its relevance to the Northern Hemisphere temperature. Science in China Series D-Earth Sciences 49: 408–420. [Google Scholar]
- McDowell NG, Fisher RA, Xu CG, et al. 2013. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytologist 200: 304–321. [DOI] [PubMed] [Google Scholar]
- Mudelsee M. 2003. Estimating Pearson’s correlation coefficient with bootstrap confidence interval from serial dependent time series. Mathematical Geology 35: 651–665. [Google Scholar]
- Oberhuber W. 2001. The role of climate in the mortality of Scots pine (Pinus sylvestris L.) exposed to soil dryness. Dendrochronologia 19: 45–55. [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. 2001. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta 212: 684–691. [DOI] [PubMed] [Google Scholar]
- Partanen J, Koski V, Hanninen H. 1998. Effects of photoperiod and temperature on the timing of bud burst in Norway spruce (Picea abies). Tree Physiology 18: 811–816. [DOI] [PubMed] [Google Scholar]
- Prislan P, Čufar K, Koch G, Schmitt U, Gričar J. 2013. Review of cellular and subcellular changes in the cambium. IAWA Journal 34: 391–407. [Google Scholar]
- Qiu Z, Wan L, Chen T, et al. 2013. The regulation of cambial activity in Chinese fir (Cunninghamia lanceolata) involves extensive transcriptome remodeling. New Phytologist 199: 708–719. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. 2002. Experimemtal design and data analysis for biologists. Cambridge: Cambrige University Press, 173–207. [Google Scholar]
- Rathgeber CB, Rossi S, Bontemps JD. 2011. Cambial activity related to tree size in a mature silver-fir plantation. Annals of Botany 108: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. 2006a. Trephor: a new tool for sampling microcores from tree stems. IAWA Journal 27: 89–97. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, et al. 2006b. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytologist 170: 301–310. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Griçar J, et al. 2008. Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography 17: 696–707. [Google Scholar]
- Rossi S, Morin H, Deslauriers A, Plourde P-Y. 2011. Predicting xylem phenology in black spruce under climate warming. Global Change Biology 17: 614–625. [Google Scholar]
- Rossi S, Girard MJ, Morin H. 2014. Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Global Change Biology 20: 2261–2271. [DOI] [PubMed] [Google Scholar]
- Sass-Klaassen U, Couralet C, Sahle Y, Sterck FJ. 2008. Juniper from Ethiopia contains a large-scale precipitation signal. International Journal of Plant Sciences 169: 1057–1065. [Google Scholar]
- Savidge R. 2001. Intrinsic regulation of cambial growth. Journal of Plant Growth Regulation 20: 52–77. [Google Scholar]
- Seo JW, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. 2008. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiology 28: 105–112. [DOI] [PubMed] [Google Scholar]
- Shao X, Huang L, Liu H, Liang E, Fang X, Wang L. 2005. Reconstruction of precipitation variation from tree rings in recent 1000 years in Delingha, Qinghai. Science in China Series D-Earth Sciences 48: 939–949. [Google Scholar]
- Sheppard PR, Tarasov PE, Graumlich LJ, et al. 2004. Annual precipitation since 515 BC reconstructed from living and fossil juniper growth of northeastern Qinghai Province, China. Climate Dynamics 23: 869–881. [Google Scholar]
- Swidrak I, Gruber A, Kofler W, Oberhuber W. 2011. Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiology 31: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidrak I, Schuster R, Oberhuber W. 2013. Comparing growth phenology of co-occurring deciduous and evergreen conifers exposed to drought. Flora 208: 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeault-Martel M, Krause C, Morin H, Rossi S. 2008. Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Annals of Botany 102: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte A, Morin H, Krause C, Deslauriers A, Thibeault-Martel M. 2009. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agricultural and Forest Meteorology 149: 1403–1409. [Google Scholar]
- Vaganov E, Hughes M, Kirdyanov A, Schweingruber F, Silkin P. 1999. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400: 149–151. [Google Scholar]
- Vicente-Serrano SM, Begueria S, Lopez-Moreno JI. 2010. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. Journal of Climate 23: 1696–1718. [Google Scholar]
- Vieira J, Rossi S, Campelo F, Freitas H, Nabais C. 2014. Xylogenesis of Pinus pinaster under a Mediterranean climate. Annals of Forest Science 71: 71–80. [Google Scholar]
- Yang B, He M, Melvin TM, Zhao Y, Briffa KR. 2013. Climate control on tree growth at the upper and lower treelines: a case study in the Qilian Mountains, Tibetan Plateau. PLoS One 8: e69065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cheng G, Yao T, Kang X, Huang J. 2003. A 2,326-year tree-ring record of climate variability on the northeastern Qinghai-Tibetan Plateau. Geophysical Research Letters 30: 1739–1742. [Google Scholar]
- Zheng Y, Liang E, Zhu H, Shao X. 2008. Response of radial growth of Qilian juniper to climatic change under different habitats. Journal of Beijing Forestry University 30: 7–12. [Google Scholar]
- Zhu H, Zheng Y, Shao X, Liu X, Xu Y, Liang E. 2008. Millennial temperature reconstruction based on tree-ring widths of Qilian juniper from Wulan, Qinghai Province, China. Chinese Science Bulletin 53: 3914–3920. [Google Scholar]