Abstract
Background and Aims Barley (Hordeum vulgare) double mutants Hv-Hd/tw2, formed by hybridization, are characterized by inherited phenotypic instability and by several new features, such as bract/leaf-like structures, long naked gaps in the spike, and a wide spectrum of variations in the basic and ectopic flowers, which are absent in single mutants. Several of these features resemble those of mutations in auxin distribution, and thus the aim of this study was to determine whether auxin imbalances are related to phenotypic variations and instability. The effects of auxin inhibitors and 2,4-D (2,4-dichlorophenoxyacetic acid) on variation in basic and ectopic flowers were therefore examined, together with the effects of 2,4-D on spike structure.
Methods The character of phenotypic instability and the effects of auxin inhibitors and 2,4-D were compared in callus cultures and intact plants of single homeotic Hv-tw2 and Hv-Hooded/Kap (in the BKn3 gene) mutants and alternative double mutant lines: offspring from individual plants in distal hybrid generations (F9–F10) that all had the same BKn3 allele as determined by DNA sequencing. For intact plants, two auxin inhibitors, 9-hydroxyfluorene-9-carboxylic acid (HFCA) and p-chlorophenoxyisobutyric acid (PCIB), were used.
Key Results Callus growth and flower/spike structures of the Hv-tw2 mutant differed in their responses to HFCA and PCIB. An increase in normal basic flowers after exposure to auxin inhibitors and a decrease in their frequencies caused by 2,4-D were observed, and there were also modifications in the spectra of ectopic flowers, especially those with sexual organs, but the effects depended on the genotype. Exposure to 2,4-D decreased the frequency of short gaps and lodicule transformations in Hv-tw2 and of long naked gaps in double mutants.
Conclusions The effects of auxin inhibitors and 2,4-D suggest that ectopic auxin maxima or deficiencies arise in various regions of the inflorescence/flower primordia. Based on the phenotypic instability observed, definite trends in the development of ectopic flower structures may be detected, from insignificant outgrowths on awns to flowers with sterile organs. Phenotypically unstable barley double mutants provide a highly promising genetic system for the investigation of gene expression modules and trend orders.
Keywords: Auxin inhibitors, 2, 4-D, barley, Hordeum vulgare, floral organ growth, flower development, flower structure, homeotic mutants, double mutants, phenotypic instability, specific normalization
INTRODUCTION
Significant phenotypic variations in the development of inflorescence–floral organs of cereals may be caused by ecological factors (Bonnett, 1966) or by pathogen infections (Ghareeb et al., 2011). More often, such variations result from mutations in genes that control the development of floral meristems or organ identity, as well as auxin transport and signalling genes. However, in cereals more frequent and drastic disruptions of inflorescence/flower development have been observed in several double (and even triple and quadruple) mutants and hybrids (Ambrose et al., 2000; Ikeda et al., 2007; Thompson et al., 2009; Yoshida and Nagato, 2011; Lee and An, 2012; Gallavotti, 2013). When a broad array of mutants was included in the same study, it was found that drastic phenotypic variations were specific only to definite mutants, double mutants and hybrids (Babb and Muehlbauer, 2003; Dreni et al., 2011; Li et al., 2011).
The same phenomenon has been observed for Hv-Hd/tw2 double mutants that originated from the hybridization of a series of barley (Hordeum vulgare) Hv-Hooded/Kap type mutants with another homeotic mutant, tweaky spike 2. All Hv-Hd mutants develop extra flowers ectopically in place of awns (as in Hooded/Kap in Colsess II; Fig. 1S) or on the awns (as in Lemma hooded; Fig. 1R), which are caused by a 305-bp duplication in intron IV of the BKn3 gene (Müller et al., 1995). In contrast, in typical flowers of Hv-tw mutants the lodicules are transformed into stamens and/or carpels (Fig. 2B–D). Additionally, the Hv-tw mutants have a specific spike (inflorescence) structure. Short naked gaps make spikes appear interrupted, and the upper part of a spike becomes intermedium instead of two-rowed. The allele tw2, used in present study, has a less expressed mutant phenotype (Fig. 1Q).
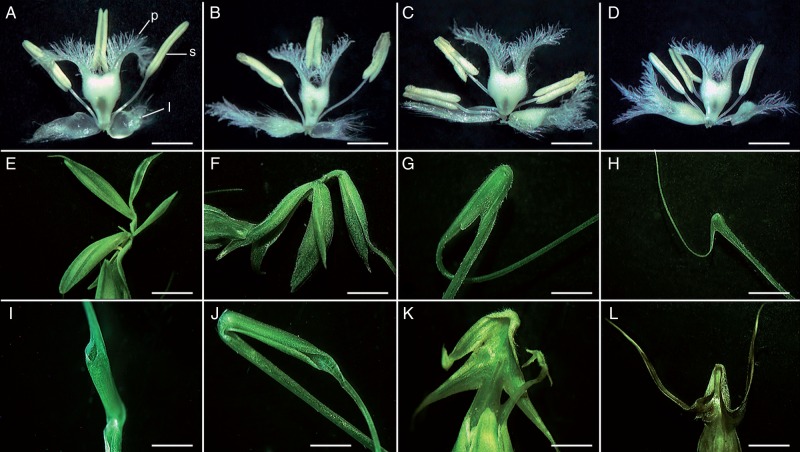
Fig. 1.
Inflorescence variations in barley double mutants. (A–L) Inflorescence variations in Hv-tw2 × Hv-Lh. (A–E) Bract/leaf-like spikes with long naked gaps on rachis. (F) Long gaps with longer bract/lemma. (G) Short gap. (H, I, K, L) Shoot-like spike structures of various sizes (arrows denote insignificant outgrowths on the awn). (M–O) Leaf-like hybrids Hv-tw2 × Hv-H N6 (arrows indicate ectopic spikes). (P–S) Spikes of parents. (P) Hv-WT. (Q) Hv-tweaky spike 2 (Hv-tw2). (R) Hv-Lemma hooded (Hv-Lh). (S) Hv-Hooded (Hv-H). Lm, lemma with awn; Lme, lemma elongation. Scale bars = 20, 40, 8, 8, 20, 20, 20 and 20 mm in L–S, respectively.
Fig. 2.
Flower variations in barley double mutants. (A–D) Variation in basic flower structure. (A) WT (l, lodicule; s, stamen; p, pistil). (E–L) Variation in zone lemma/awn. (E, F) Multiple ectopic outgrowths on the same awn. (G, H) Cap structures in inverted positions and at different developmental stages. (I, J) Tube-like structures: (I) direct position; (J) inverted position. (K, L) Variation in wings: (K) doubled wings; (L) wings with small silky awns. Scale bars (A–D) = 1 mm; (E–G, I–L) = 2 mm, (H) = 4 mm.
Phenotype instability is a well-known phenomenon, but the peculiarities of the phenotypic instability of the Hv-Hd/tw2 double mutants lie in their inherited forms. Apart from stable double mutants in remote (F7–F10) generations, which are of interest for ornamental use as dry bunches (Siuksta et al., 2012), some of these double mutants are phenotypically unstable, and this situation is inherited from the F1 to the F10 generation (Vaitkūnienė et al., 2004). The variations in these phenotypically unstable Hv-Hd/tw2 double mutants represent a cocktail of variations, as observed by Bonnett (1966), and are also found in separate mutants of barley (Babb and Muehlbauer, 2003; Trevaskis et al., 2007; Whipple et al., 2010) and rice (Duan et al., 2003; Ikeda et al., 2007). According to the characteristics of these variations, they resemble several other double mutants of the auxin-signalling pathway (Gallavotti, 2013). The character of the phenotypic variations in the Hv-Hd/tw2 double mutants suggests that their hormone pathway may be unbalanced. Moreover, these variations are, convincingly enough, determined by the interactions of auxin with the Kn1 gene of the KNOX gene family and other homeotic genes involved in apical meristem maintenance and in the differentiation of specialized meristems, by the mutual interaction of the new auxin maxima with the formation of KNOX modules, and by the initiation of lateral plant organs and the boundaries between them (reviewed in McSteen and Leyser, 2005; Hay and Tsiantis, 2010; Müller and Leyser, 2011; Tvorogova et al., 2013). In maize, the Kn1 gene directly regulates genes involved in hormonal pathways and the Kn1 protein binds to nearly half of the AUX-IAA and ARF genes that have been annotated in the maize genome (Bolduc et al., 2012). In turn, auxin negatively regulates the expression of KNOX genes in the primordia of lateral organs (Scanlon et al., 2002; Hay and Tsiantis, 2010; Tabata et al., 2010). However, the expression of KNOX genes is reactivated to promote leaflet initiation, causing the development of compound leaves in Cardamine hirsuta and tomato, but, like KNOX/auxin interactions in the periphery of the shoot apical meristems, the decrease in KNOX expression at the margins of compound leaf primordia allows the auxin maximum to form via the PINFORMED1 (PIN1) auxin efflux transporter, which in turn directs leaflet initiation (Barkoulas et al., 2008; Hay and Tsiantis, 2010; Scarpella et al., 2010). Overexpression of KNOX leads to ectopic auxin maxima, causing the induction of developmental abnormalities (Chow and McCourt, 2004; Scarpella et al., 2010; Tabata et al., 2010; Rast and Simon, 2012), while exogenous auxin application can repress ectopic KNOX expression, and restores a compound leaf phenotype from a simple leaf phenotype caused by pin1 mutation (Barkoulas et al., 2008). However, the role of KNOX genes in the primary and secondary morphogenesis of leaf development is not fully understood (Koenig et al., 2009).
No direct studies of the impact of auxin on the expression of the BKn3 gene in barley have been published, but it has been demonstrated that a 305-bp duplication in intron IV of the BKn3 gene, which causes a Hooded phenotype, has four regulatory cis elements, that are able to bind transcription factors (Santi et al., 2003). To date, this type of regulation in intron IV has only been reported for proteins responsive to the plant hormone ethylene (Osnato et al., 2010). This finding does not exclude the possibility of analogous interactions of cis elements in intron IV of the BKn3 gene with other plant hormones, including auxin. Nevertheless, the BBR (barley B recombinant) protein binds to the (GA)8 repeat located in the fourth element of intron IV and causes morphological alterations of the leaves and flowers when overexpressed in tobacco (Santi et al., 2003). Recently, it has been reported that the barley growth-regulating factor BGRF1 can act as a repressor on an intron sequence in the BKn3 gene and that the Arabidopsis thaliana growth factors AtGRF4, AtGRF7 and AtGRF6 can also repress the Arabidopsis KNOX family gene KNAT2 (Kuijt et al., 2014). These regulatory activities resemble the common regulatory effects of auxin on KNOX family genes. Moreover, the interactions of growth-regulating factors with cis elements in intron sequences may be a common theme for cereals. Orthologues of BKn3 intron IV are found in other cereals, such as maize, wheat and rice (Takumi et al., 2000). Recently, in the third intron of the rice KNOX gene OSH1, which is orthologous to intron IV of the BKn3 gene, the seven cis elements have been found to be involved in the autoregulation and regulation of the other genes (Tsuda et al., 2011), and the OsGRF3 and OsGRF10 proteins bind to the rice KNOX gene OsKn2 (Kuijt et al., 2014). Intron IV of the wild allele of the barley BKn3 gene has 13 conserved non-coding sequences (CNSs) considered to be regulatory cis elements and orthologous to CNSs in the corresponding introns of the maize and rice KNOX genes (Inada et al., 2003). Moreover, 13·9 % of hormone-mediated signalling genes and cellular response to hormone stimulus genes have CNSs (Hettiarachchi et al., 2014).
Together, these findings suggest that it would be interesting to unravel the interaction of auxin with the Hooded/Kap mutation in the BKn3 gene of barley. Variations in auxin transport and accumulation are usually assessed by means of either chemical inhibitors or specific gene mutations, but there are relatively few functional analyses of the genes involved in the auxin-signalling pathway of monocot species (Gallavotti, 2013).
For these reasons, the effects of auxin inhibitors and the synthetic auxin 2,4-dichloroacetic acid (2,4-D) on variation in flower organs of the basic and ectopic flower-like structures were examined, and the partial rescue of specific phenotypic features by auxin inhibitors and 2,4-D was observed. Two alternative variants of Hv-Hooded mutants were used in our study. In the Hv-Hooded (Colsess II) mutant, the awns are fully transformed into ectopic inflorescence/flower structures. In the Hv-Lemma hooded mutant, ectopic outgrowths develop on a portion of the awns (Fig. 1S and R, respectively).
The phenotypic instability of the double mutants may be caused not only by the ectopic auxin maxima or deficiency or by the expression of organ identity genes, but also by more intensive division of meristematic cells after reprogramming of the epigenetic control of cell division and differentiation via variations in chromatin architecture crosstalk between auxin signal transduction components and chromatin remodelling activities (Murfett et al., 2001; Fukaki et al., 2006; Szemenyei et al., 2008; Anzola et al., 2010; Iwasaki et al., 2013; Nguyen et al., 2013). This presumption was verified by comparing differences in the expression of inflorescence variations with meristem growth intensity in callus cultures of single and double mutants. Rapid changes in DNA methylation were observed in carrot cell cultures in relation to auxin action (LoSchiavo et al., 1989; Arnholdt-Schmitt, 1993). Several inhibitors of epigenetic processes (5-aza-2′-deoxycytidine, sodium butyrate, HC toxin) were tested, but only HC toxin showed an appreciable effect referred in the present study. The HC toxin, produced by the fungus Cochliobolus carbonium in maize (Walton, 2006), was used as a stress factor because the related species Cochliobolus sativus has a strong effect on barley (Ghazvini and Tekauz, 2012). On the other hand, HC toxin is recognized as an inhibitor of histone deacetylases (Walton, 2006) and its effects on callus cultures (Grant-Downton and Dickinson, 2006) may indirectly suggest that the epigenetic processes observed in cultures of meristematic cells may also be involved in causing the differences in meristematic cell growth amongst the single and double mutants tested here (Hv-Hd/tw2).
MATERIALS AND METHODS
A collection of Hv-Hooded barley (Hordeum vulgare) mutants was obtained from the USDA-ARS National Small Grains Collection (GSHO, Aberdeen, ID, USA). The Hv-tw mutants were induced by chemical mutagenesis from the barley ‘Auksiniai II’, which was used as a WT for Hv-tw or wt for Hv-Hd in the present study. To produce Hv-Hd/tw2 double mutants, hybridization of the paternal mutants was performed in 2003. In all cases, a plant with the Hv-tw2 allele was used as the mother plant because all Hv-Hd mutants are dominant and this made it possible to control the success of hybridization; Hv-tw is recessive and the Hv-tw phenotype indicates homozygosity for the Hv-tw allele. Homeotic transformations in Hv-Hd and Hv-tw mutants occur in various flower organs and this feature allows additional discrimination between the effects of Hv-tw and Hv-Hd in double mutants. All tested material was bred and studied in the botanical garden and in the greenhouse of the Department of Botany and Genetics of Vilnius University. Barley Hooded-type mutations differ significantly and, as mentioned in the Introduction, two mutants [Hv-Hooded/Kap in Colsess II (GSHO 67) and Hv-lemma hooded (GSHO 932)] with different expression of the mutant phenotype were used to obtain the Hv-Hd/tw2 double mutants. For exposure to auxin inhibitors or 2,4-D, only double mutant lines (offspring from the initial individual plants) were used. Lines were created using F6 material and were subject to preliminary testing for at least three generations before exposure to auxin inhibitors or 2,4-D. The selection of individual plants was based first on their inflorescence/flower structure according to both Hv-Hd and Hv-tw phenotypes and then on their inflorescence/flower variations. For these reasons, in the Results section and the tables and figures it was necessary to show the history of the tested lines, i.e. the mutants used for hybridization, the numbers of lines and their capacities to form shoot/leaf-like inflorescence structures.
Flowers and spikes were fixed in Carnoy’s solution (ethanol : glacial acetic acid, 3 : 1) and analysed under a stereomicroscope (Motic SMZ-143) connected to a photo camera (Moticam 2000). DNA sequencing of the BKn3 promoter and intron IV regions demonstrated that all of the Hv-Hooded mutants and double mutant lines used in this study had the same allele IIIc (Kap) of the BKn3 gene according to Badr et al. (2000) (results not shown).
Plant treatments with auxin inhibitors and 2,4-D
Under both field and greenhouse conditions, treatment with auxin inhibitors was started at the beginning of the fifth leaf stage because the development of barley floral organs begins very early and this stage is regarded as the most critical stage for inflorescence/flower development (Yagil and Stebbins, 1969; Osnato et al., 2010). Plants were sprayed six times with 100 µm solutions of either the auxin transport inhibitor [9-hydroxyfluorene-9-carboxylic acid (HFCA) or the anti-auxin p-chlorophenoxyisobutyric acid (PCIB)]. Plants were sprayed with 2,4-D sodium salt (2 g L−1) only once to avoid the necrotic injury caused by higher 2,4-D concentrations. Tween 20 (Sigma-Aldrich, USA, final concentration 0·1 %) was added to all spray solutions to facilitate adhesion of the solutions to the leaf surfaces.
Callus cultures and treatment with inhibitors
Seeds for embryo dissection were surface-sterilized with a commercial bleach ACE and water solution (1 : 1) for 18 min and washed five times with sterile water. Water-imbibed embryos were aseptically isolated and cultivated in Petri dishes for 4 weeks at 25 °C in the dark (Incucell, MMM Medcenter, Einrichtungen, Germany). Preliminarily calli were induced and grown on the Murashige–Skoog (MS) medium with 3 mg L−1 2,4-D (Alfa Aesar, Germany), typical for callus induction, or with 6 mg L−1 2,4-D to remove the expected effect of auxin inhibitors on callus growth. Then, the calli were individually divided into portions for treatment with the test compounds and for use as controls (without treatment). All portions were weighed on an analytical balance and placed on MS medium with a particular compound and grown in a thermostat at 25 °C in the dark for 4 weeks. After the 4-week growth period, the calli were once again weighed and the growth index (GI) was calculated according to the formula GI = (m − m0)/m0, where m is the final callus weight and m0 is the initial callus weight. All manipulations, including callus weighing, were conducted in sterile conditions in a laminar box (SAFE 2020, Thermo Scientific). All solutions of thermolabile compounds were filter-sterilized using 0·22 µm pore diameter syringe filters (Roth, Germany).
Two separate experiments were conducted with the callus cultures. In the first experiment the GI was determined for calli from both single and double mutants grown with or without HC toxin (10 µg mL−1 dissolved in DMSO, final concentration 0·1 %). DMSO was also used as a control for the HC toxin. Callus cultures were also tested for the preliminary selection of auxin inhibitors because their effects on various objects can vary significantly (Oono et al., 2003; Krizek, 2011); hence, various auxin inhibitors were tested on two different genotypes, WT and Hv-tw2, and for two different 2,4-D concentrations, 3 mg L−1 (typical) and 6 mg L−1 (increased). All inhibitors (Sigma-Aldrich, USA) were tested at a concentration of 30 µm.
Statistical analysis
Mean values ± s.e. are given in the tables. The significances of differences between means were evaluated by Student’s t-test.
RESULTS
Phenotypic instability of Hv-Hd/tw2 double mutants
Simultaneous variations in the structure of the basic flowers and in the ectopic transformation of the awns to floral structures may be expected naturally in Hv-Hd/tw2 double mutants originating from the hybridization of two different homeotic mutants, Hv-tw2 and Hv-Hd. However, the phenotypic variations exceeded those of the single mutants in basic and ectopic flower organs, and new types of spike (inflorescence) variation were observed (Figs 1 and 2). The wild-type architecture of the two-rowed barley is shown in Fig. 1P. A wild-type flower with the organs discussed in the present study (lodicules, stamens and pistil) is shown in Fig. 2A and the lemma with the awn in Fig. 1P and R. Phenotypic variation was observed not only for the various double mutant lines but also for the same plant and even within the same inflorescence (Fig. 1A–O). This level of variation was especially evident for ectopic outgrowths on awns or instead of awns, which varied from insignificant thicknesses on awns to fully developed but sterile ectopic flowers (Fig. 1A–F, I–K). Statistical data on these variations are presented below, where we discuss the effects of auxin inhibitors (see section Impacts of PCIB and HFCA on the structures of basic and additional ectopic flowers) and 2,4-D (see section Effects of 2,4-D on inflorescence/flower structures) on the structures of basic and ectopic flowers. Moreover, double mutant lines were so widely varied that they covered even the phenotypes of many known mutants of barley and other grasses.
The main features that attracted attention and were absent in the parental mutants were bract/leaf-like and shoot-like structures inside a spike and long, naked gaps on the rachis (spike axis), varying in the degree of expression (Fig. 1M–O). In barley and related cereals, bract development is usually suppressed, but it has been observed in several mutants and transgenic plants (Pozzi et al., 2000; Forster et al., 2007; Whipple et al., 2010; Houston et al., 2012; Lee and An, 2012; Gallavotti, 2013). Within the commonly observed phenotypic groups, plants simultaneously having two features – bract/leaf-like structures and long, naked gaps without florets on the rachis (Fig. 1A–F, Supplementary Data Table S1) – were assigned to separate groups. Rare spike phenotypes (‘bulbs’) consisted of multiple lemma–bract structures (Fig. 1O), resembling mutants called ‘supernumerary bracts’ (Lee and An, 2012). The other phenotypic group consisted of plants forming shoot-like structures within the spike (Fig. 1H, I, K, L). Rarely, ectopic shoots also developed extra spikes (Fig. 1M). The ectopic leafy/shoot-like phenotype is also a feature of several other barley mutants and hybrids (Babb and Muehlbauer, 2003; Forster et al., 2007; Trevaskis et al., 2007; Curaba et al., 2013), but the plants with long, naked rachis gaps were the most intriguing in our study. This latter phenotype, or even fully naked pin-formed structures, is specific to mutations in cereal genes that are involved in auxin synthesis, transport or response (McSteen et al., 2007; Morita and Kyozuka, 2007; Gallavotti, 2013). The naked part of a spike was very vigorous (Fig. 1C, N, O) or had a zigzag structure (Fig. 1K, N) similar to that in the rice mutants leafy head (Duan et al., 2003) and aberrant panicle organisation 1 (Ikeda et al., 2007) or in the spikes of barley line OxBM10, which overexpresses the BM10 gene (MADS superfamily), called ‘concertina’ (Trevaskis et al., 2007) or Hvu pri-miR171a (Curaba et al., 2013). These results demonstrate the various origins of naked gaps in a spike as a result of internode elongation (Fig. 1F), as well as the introduction of numerous spike nodes into the naked spike region and simultaneous internode elongation, forming a zigzag structure (Fig. 1K, N). The latter phenomenon also confirms the involvement of factors that control the inflorescence length and prevent the development of lengthy spikes.
Despite the variation in their frequency amongst different generations, which can also be caused by ecological factors, phenotypic instability of the most notable features, ectopic leaf/shoot-like structures and naked rachis gaps, was observed in all the tested generations up to F10 (e.g. F7 and F8 in Supplementary Data Table S1).
As mentioned above, a high degree of variation was also observed in the degree of ectopic outgrowths in the lemma/awn transition zone and more distantly along the awn (Figs 1 and 2), including such awn malformations as the presence of more than one ectopic flower on the same awn (Fig. 2E, F), the presence of ‘cap’ structures in inverted positions and of different developmental levels (Fig. 2G, H), and occurrences of tube-like structures; notably, the latter were observed in both direct and inverted positions (Fig. 2I, J). The inverted position of ectopic flowers is a common peculiarity of Hv-Hooded mutants and this phenomenon deserves special consideration (Williams-Carrier et al., 1997; Zanotti et al., 2010). The existence of two possibilities for initial awn transformation into flower structures, such as a tube, is of special interest. Noticeable variations were observed for the so-called wings on the lemma and for the short awn-like structures on ectopic flowers. In some cases, the wings were doubled or even converted into awn-like structures (Fig. 2K, L). Awn-like structures also varied widely. In several cases, the ectopic awn-like structures resembled the Zm-silky1 phenotype in maize (Ambrose et al., 2000). This suggests the existence of special factors or modules that control wing and awn development. Lemma elongation was also observed. This feature may be considered a slightly intermediate degree of transformation of the lemma into a leaf-like structure.
In conclusion, the high variation amongst ectopic flower-like structures suggests a definite developmental trend in flower development, varying from insignificant outgrowths on the awn to a tube (in non-inverted/inverted positions) and finally to flowers with sterile sexual organs. In parallel, variations in lemma wings may represent another developmental trend. Contrary to Bonnett (1966), ectopic variations were observed in both field and greenhouse conditions but were more pronounced under field conditions.
Comparison of meristem growth intensity in callus cultures
The data presented in the previous section suggest that the phenotypic instability of Hv-Hd/tw2 double mutants may be caused not only by the ectopic expression of organ identity genes but also by more intensive division of meristematic cells. This presumption was verified by examining callus cultures from the initial mutants and double mutant lines that had varying expressions of leaf/shoot-like inflorescence phenotypes (Table 1).
Table 1.
Intensity of callus meristem growth expressed as growth index (GI; see text) in barley single and double mutants with and without DMSO or HC toxin
| Mutant/hybrid | Control (H2O) | DMSO | DMSO + HC | Height of intact plant (cm) |
|---|---|---|---|---|
| WT (‘Auksiniai II’) (−) | 4·18 ± 0·57 | 3·18 ± 0·50 | 4·26 ± 0·66 | 75·2 ± 1·2 |
| Hv-tw (−) | 0·35 ± 0·08c3 | 0·70 ± 0·16c3 | 1·30 ± 0·24c3 | 71·7 ± 0·9 |
| Hv-tw2 (common mother) (−) | 3·90 ± 0·58 | 3·97 ± 0·54 | 2·82 ± 0·30a | 66·4 ± 1·33 |
| Hv-Lemma hooded (Lh) (−) | 1·46 ± 0·24c3 | 1·91 ± 0·35a2 | 1·61 ± 0·30c2 | 50·3 ± 1·03 |
| Hv-tw2 × Hv-Lh N13 (−) | 2·00 ± 0·33c2 | 1·84 ± 0·32a3 | 1·30 ± 0·22c3 | 49·3 ± 0·8 |
| Hv-tw2 × Hv-Lh N17 (+−) | 2·61 ± 0·52a | 2·08 ± 0·342 | 1·48 ± 0·19c3 | 57·9 ± 1·03 |
| Hv-tw2 × Hv-Lh N11-18 (+) | 2·17 ± 0·40b1 | 2·41 ± 0·222 | 2·86 ± 0·35 | 67·4 ± 1·33 |
| Hv-tw2 × Hv-Lh N19 (+) | 1·11 ± 0·18c3 | 1·65 ± 0·27b3 | 2·39 ± 0·48a | 70·6 ± 1·33 |
| Hv-Hooded (H) (−) | 2·03 ± 0·28c2 | 2·17 ± 0·252 | 2·00 ± 0·31b | 71·4 ± 1·2 |
| Hv-tw2 × Hv-H N8-20 (−) | 2·14 ± 0·25b2 | 1·51 ± 0·18b3 | 1·63 ± 0·19c3 | 75·2 ± 0·8 |
| Hv-Dense wing hood (Hv-Dwh) (−)* | 2·08 ± 0·42b1 | 2·35 ± 0·262 | 3·85 ± 0·391 | 30·4 ± 0·83 |
| Hv-tw2 × Hv-Dwh N25 (+) | 2·38 ± 0·30b1 | 2·55 ± 0·331 | 3·15 ± 0·50 | 80·8 ± 1·03 |
| Hv-Hoods on center spikelet (Hv-Hcs) (−)* | 1·59 ± 0·28c3 | 0·68 ± 0·11c3 | 2·82 ± 0·29a | 91·8 ± 1·13 |
| Hv-H × Hv-Hcs N9 (+) | 2·10 ± 0·20c2 | 2·07 ± 0·252 | 3·57 ± 0·45 | 89·8 ± 1·1 |
| Hv-Multiflorous (Hv-Mf) (−)* | 2·01 ± 0·51b1 | 2·23 ± 0·77 | 1·41 ± 0·38c2 | 44·2 ± 0·93 |
| Hv-tw2 × Hv-Mf N14 (+) | 2·45 ± 0·25b1 | 2·13 ± 0·252 | 2·77 ± 0·79 | 66·6 ± 0·93 |
*Accession numbers in USDA-ARS National Small Grains Collection are GSHO 928, GSHO 666 and GSHO 79, respectively.
(+,−) formation of leafy/shoot-like structures: (+), present, (−), absent; (+−), present under field conditions, absent in greenhouse.
a,b,cIn comparison with WT (wt) (‘Auksiniai II’): aP < 0·05; bP < 0·01; cP < 0·001.
1,2,3In comparison with the mother plant Hv-tw2: 1P < 0·05; 2P < 0·01; 3P < 0·001; similarly for plant height in the comparison of the corresponding mutant with WT (wt) or of father mutant with the corresponding hybrid.
A very weak callus growth intensity of plants with the Hv-tw allele was noted, while plants with the Hv-tw2 allele, used as mother plants, did not differ significantly from the WT (Table 1). In general, lower callus growth intensity was a common feature of all Hv-Hd mutants involved in the hybridization, as well as Hv/Hd-tw2 double mutants. Callus growth was slightly more intensive in several double mutants than in the respective father mutants. However, the growth intensities of the intact plants were not as uniform under field conditions. The height of the Hv-Hcs mutant and the double mutant lines Hv-H × Hv-Hcs N9 and Hv-tw2 × Hv-Dwh N25 significantly exceeded that of Hv-wt (‘Auksiniai II’) despite their low callus growth under baseline conditions (without DMSO and HC toxin; Table 1). Despite the fact that DMSO was only used for dissolving HC toxin in water, it unexpectedly caused a stimulatory effect on callus growth in plants with the strongest Hv-tw allele, which was 2.0-fold greater than without DMSO. However, the stimulatory effect of HC toxin on the growth of Hv-tw callus was still stronger, ∼2.0-fold greater than that of DMSO. In addition to Hv-tw, DMSO only affected one of the Hv-Hd type mutants, Hv-Hcs; however, in contrast to Hv-tw, the effect of DMSO was inhibitory in the case of Hv-Hcs. The effect of the HC toxin clearly depended on the plant genotype. The HC toxin increased callus growth in two Hv-Hd type mutants, Hv-Dwh and Hv-Hcs, and in several double mutants of different origin: Hv-tw2 × Hv-Lh N11–18, N19; Hv-tw2 × Hv-Dwh N25 and Hv-H × Hv-Hcs N9. Notably, all these double mutants had ‘leaf/shoot’-forming phenotypes (Table 1).
Impacts of PCIB and HFCA on the structures of basic and additional ectopic flowers
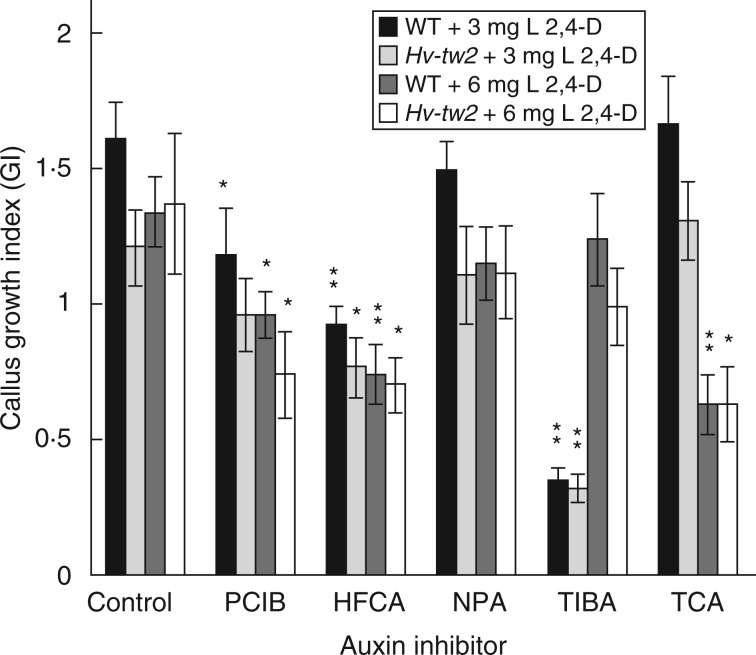
Callus cultures were also used for the preliminary selection of various auxin inhibitors. The majority of the preliminary test compounds, namely [HFCA, N-(1-naphthyl)phthalamic acid (NPA) and 2,3,5-triiodobenzoic acid (TIBA)] are inhibitors of auxin polar transport; PCIB is an anti-auxin; only the action of trans-cinnamic acid (TCA) as an auxin inhibitor is not fully recognized (Oono et al., 2003). In instances of inhibitory effects of these compounds on callus growth, it is expected that an increased (double) concentration of the synthetic auxin 2,4-D in the medium could act as a compensatory factor. However, this effect was only observed for TIBA, which showed a very strong inhibitory effect on both WT and Hv-tw2 callus growth; in medium with 6 mg L−1 of 2,4-D the inhibitory effect of TIBA either fully disappeared or was insignificant (Fig. 3). The opposite effect was observed for TCA. In MS medium with 3 mg L−1 of 2,4-D (typical for callus culture), no effect of TCA was observed on callus growth, but when the concentration of 2,4-D was doubled the inhibitory effect of TCA was obvious. For PCIB and especially for HFCA, the inhibitory effect did not depend on 2,4-D concentration. Assuming that plants with different genotypes may have different auxin levels, PCIB and HFCA were selected for further experiments with intact barley plants.
Fig. 3.
Comparison of WT and Hv-tw2 barley responses to auxin inhibitors and 2,4-D expressed as the callus growth index (GI). Control, without inhibitors; PCIB, p-chlorophenoxyisobutyric acid; HFCA, 9-hydroxyfluorene-9-carboxylic acid; NPA, N-(1-naphthyl)phthalamic acid; TIBA, 2,3,5-triiodobenzoic acid; TCA, trans-cinnamic acid. Differences between inhibitor and corresponding control: *P < 0·05; **P < 0·01.
The effect of auxin inhibitors on WT and Hv-tw2 callus growth intensities may also be important. The absence of significant differences in the response of both WT and Hv-tw2 calli to the auxin transport inhibitors (HFCA, NPA and TIBA) and the anti-auxin (PCIB) may suggest that Hv-tw2 itself is not a mutation of an auxin pathway gene.
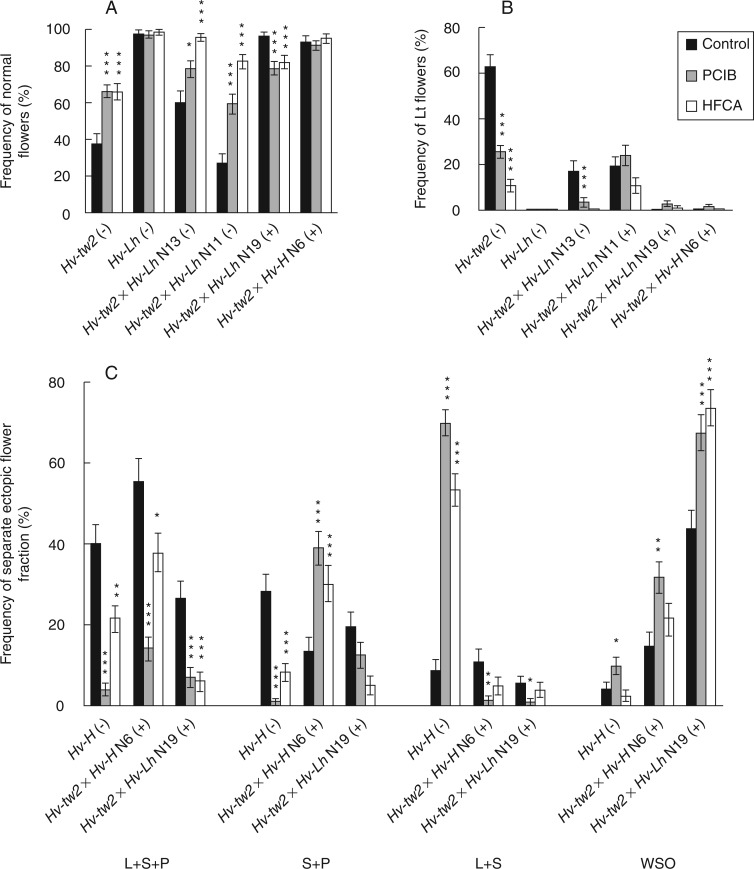
However, this conclusion conflicts with the results of the basic flower structure analysis of the Hv-tw2 mutant after plant treatment with PCIB or HFCA because partial normalization of the basic flower structure occurred. The effect was expressed as an increase in the fraction of normal basic flowers with two lodicules, three stamens and one pistil (2L + 3S + 1P) by ∼2.0-fold (Fig. 4A, B) and by a decrease in flowers with the typical to Hv-tw-type mutant lodicule transformation (Lt) to stamens and/or pistils, in which the total of L + S + P was not altered. Similarly, both auxin inhibitors, but particularly HFCA, increased the proportion of flowers in which only the number of sexual organs (SO) or Lt + SO was changed (Supplementary Data Table S2).
Fig. 4.
Effects of PCIB and HFCA on fractions of normal flowers (A), flowers with lodicule transformations (Lt) to stamens and/or pistils (B) in basic flowers and on several fractions of ectopic flowers (C) cultivated under greenhouse conditions. In (C), only genotypes with a wide spectrum of ectopic outgrowths are shown. L, lodicule; S, stamen; P, pistil; WSO, without sexual organs. Differences between inhibitor and corresponding control: *P < 0·05; **P < 0·01; ***P < 0·001.
Notably, the transformation of L to S or P in basic flowers denotes the phenotype and homozygosity of the recessive Hv-tw mutant allele, while ectopic outgrowths in place of the awn or on the awn are indicative of the dominant Hv-Hooded mutation, and only double mutant lines expressing both mutant phenotypes in the spike were used for HFCA, PCIB and 2,4-D experiments.
For the Hv-Hd/tw2 double mutants, the effects of HFCA and PCIB on basic flower structure differed even for leaf/shoot-forming double mutant lines, e.g. Hv-tw2 × Hv-Lh N11 (+) and N19 (+) (Fig. 4A and B). As we found for Hv-tw2, a partial normalization of flower structure occurred for the Hv-tw2 × Hv-Lh N11 (+) and N13 (−) lines and was expressed as an increased fraction of flowers with normal structure (Fig. 4A), but a statistically significant decrease in Lt was observed only for line N13 (Fig. 4B). In line N11, the proportion of both the SO and Lt + SO fractions also clearly decreased, while in line N13 this effect was observed only after exposure to HFCA (Supplementary Data Table S2).
In contrast, an opposite effect of auxin inhibitors was noted for line N19 (+), but this line was characterized by a very high frequency of normal flowers without any treatment, and both inhibitors decreased the proportion of normal flowers in the spectrum (Fig. 4A) and increased the proportion of SO flowers (Supplementary Data Table S2). No effects of HFCA or PCIB on the structure of the basic flowers were evident for genotypes WT, Hv-Lh and Hv-tw2 × Hv-H N6 (+), where Lt was not observed at all.
In regard to ectopic flower structures, Hv-Hd type mutants differed significantly amongst themselves and, as mentioned in the Introduction, representatives of the two alternative Hv-Hd mutants, Hv-Hooded (Hv-H) and Hv-Lemma hooded (Hv-Lh), were tested. For the mutant Hv-H, ectopic transformation of awns into flower structures occurred, and a significant portion of the ectopic flowers developed sterile sexual organs. In contrast, the rudimentary flower-like structures constituted a significant portion of the ectopic outgrowths on the awns of the mutant Hv-Lh (Fig. 1R, S).
A significant amount of diversity was observed amongst the double mutant lines according to the presence of the ectopic awn transformations, even within the same cross-combination, as noted for the Hv-tw2 × Hv-Lh hybrids (Supplementary Data Table S3). Within the Hv-tw2 × Hv-Lh lines, two alternatives were also tested for their responses to the auxin inhibitors PCIB and HFCA. The first alternative was represented by the father mutant Hv-Lh and the second consisted of its hybrids with Hv-tw2, namely N13 (−) and N11 (+), in which only rare rudimentary flower-like structures developed on the awns. A wider spectrum of ectopic flower structures could be expected after PCIB and HFCA treatment; however, for the first group of double mutants there were no appreciable effects of inhibitors on ectopic outgrowths (Supplementary Data Table S3).
At the same time, the effects of auxin inhibitors were clearly expressed on representatives of the other group of genotypes, namely Hv-H and its hybrid lines Hv-tw2 × Hv-H N6 (+) and Hv-tw2 × Hv-Lh N19 (+), which were characterized by a wide spectrum of ectopic outgrowths (Fig. 4C). A significant portion of the flower spectrum of the latter group comprised ectopic flowers with both sexual organs, L + S + P and S + P. Both flower types may be considered as highly advanced towards the final development of flowers because the organ identity genes specifying the fourth floral whorl (representatives of class C according to the ABC model of flower development) must be expressed for these phenotypes.
The effects of HFCA and PCIB were most evident on these fractions of ectopic flowers (Fig. 4C). However, while the fraction of L + S + P flowers decreased for all representatives of this group, the effect of auxin inhibitors on the S + P fraction was more genotype-specific. For the mutant Hv-H and hybrid line Hv-tw2 × Hv-Lh N19 (+), both auxin inhibitors decreased the S + P portion of the spectrum, while the opposite effect of auxin inhibitors was observed for Hv-tw2 × Hv-H N6 (+) (Fig. 4C).
A decrease in one portion of the spectrum must be accompanied by an increase in another portion(s). In this respect, significant variation was observed amongst the tested mutants and lines of double mutants. For mutant Hv-H, both auxin inhibitors, PCIB and HFCA, very clearly increased the L + S fraction (by 8.0- and 6.1-fold, respectively). As mentioned above, in the line Hv-tw2 × Hv-H N6 (+), the fraction of S + P flowers was increased by both inhibitors. We propose that such varying effects of inhibitors on the ectopic flower fractions with P may be explained by the fact that, in contrast to dicots, grasses have more than one class C gene (Yoshida and Nagato, 2011). An increased frequency of outgrowths without sexual organs was also observed in double mutant lines (Fig. 4C), but in mutant Hv-H and in line N6 (+) this effect was clearly expressed only after treatment with PCIB. In the hybrid Hv-tw2 × Hv-Lh N19 (+), the fraction of outgrowths without sexual organs comprised a significant proportion of the spectrum without any treatment, but both inhibitors significantly increased the proportion of this fraction in the spectrum (Fig. 4C). Hv-WT and the mutant Hv-tw2 did not develop any ectopic flower structures instead of awns or on awns, and PCIB and HFCA did not cause any effects on awn development.
Effects of 2,4-D on inflorescence/flower structures
If several of the phenotypic instability features in Hv-Hd/tw2 double mutants associated with auxin inhibitors are really caused by auxin concentration deficiencies, an additional treatment with an exogenous auxin should induce the opposite effects. This presumption was tested on two different features of the phenotypic instability of double mutant lines: on leaf/shoot-like structures (Table 2) and on variations in the frequencies of basic and ectopic flower fractions (Fig. 5 and Supplementary Data Table S4).
Table 2.
Effects of 2,4-D on spike structure of selected double mutant lines (F10) cultivated under field conditions
| Mutant/hybrid | Treatment | n | Phenotypic group |
|||
|---|---|---|---|---|---|---|
| I | II | III | Others | |||
| Hv-tweaky spike 2 (Hv-tw2) (−) | 0 | 282 | 0 | 17 ·7 ± 2 ·3 | 0 | 0 |
| 2,4-D | 288 | 0 | 0c | 0 | 0 | |
| Hv-tw2 × Hv-Lh N1 (−) | 0 | 420 | 13 ·3 ± 1 ·7 | 0 ·7 ± 0 ·4 | 0 | 0 |
| 2,4-D | 215 | 1 ·4 ± 0 ·8c | 0 | 0 ·5 ± 0 ·5 | 0 | |
| Hv-tw2 × Hv-Lh N2 (+) | 0 | 326 | 3 ·1 ± 1 ·0 | 2 ·8 ± 0 ·9 | 12 ·0 ± 1 ·8 | 1 ·5 ± 0 ·7 |
| 2,4-D | 322 | 0 ·6 ± 0 ·4a | 1 ·6 ± 0 ·7 | 11 ·2 ± 1 ·8 | 1 ·2 ± 0 ·6 | |
| Hv-tw2 × Hv-Lh N11 (+) | 0 | 262 | 20 ·2 ± 2 ·5 | 10 ·3 ± 1 ·9 | 15 ·3 ± 2 ·2 | 0 |
| 2,4-D | 210 | 16 ·7 ± 2 ·6 | 7 ·1 ± 1 ·8 | 5 ·2 ± 1 ·5c | 0 | |
| Hv-tw2 × Hv-Lh N17 (+−) | 0 | 324 | 9 ·3 ± 1 ·6 | 9 ·9 ± 1 ·7 | 18 ·5 ± 2 ·2 | 4 ·0 ± 1 ·1 |
| 2,4-D | 200 | 6 ·5 ± 1 ·7 | 15 ·5 ± 2 ·6 | 15 ·0 ± 2 ·5 | 9 ·0 ± 2 ·0a | |
| Hv-tw2 × Hv-Lh N19 (+) | 0 | 533 | 9 ·4 ± 1 ·3 | 12 ·4 ± 1 ·4 | 11 ·6 ± 1 ·4 | 0 ·4 ± 0 ·3 |
| 2,4-D | 435 | 3 ·4 ± 0 ·9c | 17 ·2 ± 1 ·8a | 2 ·5 ± 0 ·7c | 0 | |
| Hv-tw2 × Hv-H N21 (+) | 0 | 437 | 3 ·7 ± 0 ·9 | 14 ·9 ± 1 ·7 | 5 ·9 ± 1 ·1 | 0 |
| 2,4-D | 352 | 2 ·3 ± 0 ·8 | 2 ·0 ± 0 ·7c | 9 ·7 ± 1 ·6 | 0 | |
| Hv-tw2 × Hv-H N6 (+) | 0 | 147 | 3 ·4 ± 1 ·5 | 10 ·2 ± 2 ·5 | 2 ·7 ± 1 ·3 | 0 ·7 ± 0 ·7 |
| 2,4-D | 75 | 0a | 2 ·7 ± 1 ·9a | 1 ·3 ± 1 ·3 | 0 | |
Phenotypic groups: I, with long naked gaps on rachis (Fig. 1A, F); II, with short gaps (Fig. 1G); III, leafy/shoot-like (Fig. 1H, I, K, L); for Hv-wt, Hv-Lh and Hv-H the above variations of spike structure are absent.
(+), (−) as in Table 1.
n, number of tested spikes; n for Hv-wt: 0–201; 2,4-D, 215; for Hv-Lemma hooded (Hv-Lh): 0–155; 2,4-D, 147; for Hv-Hooded (Hv-H): 0–193; 2,4-D, 173.
a,b,cIn comparison with plants not treated with 2,4-D: aP < 0 ·05; bP < 0 ·01; cP < 0 ·001.
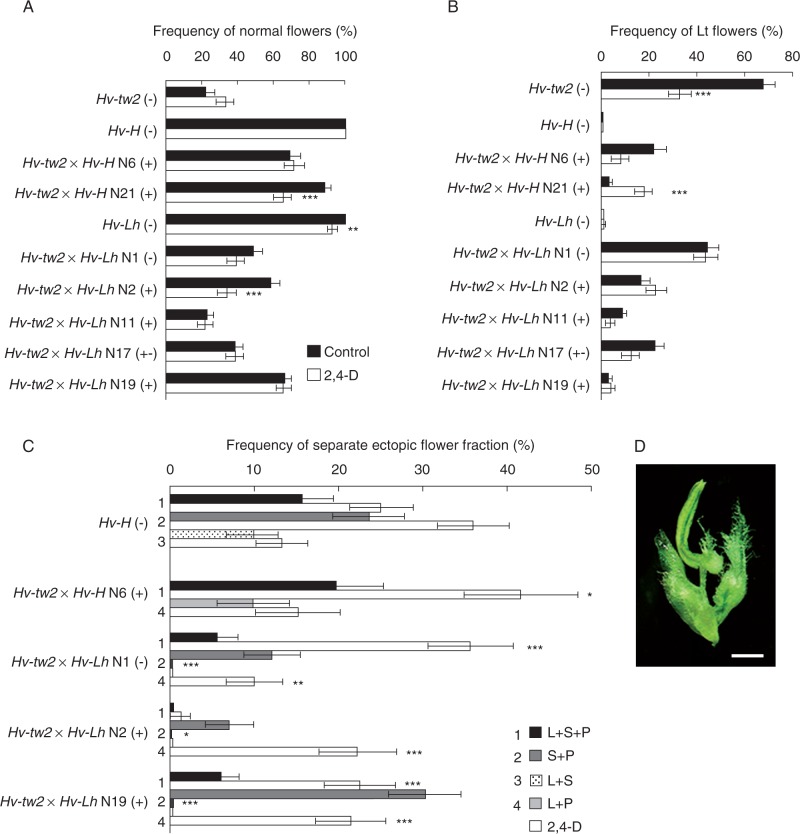
Fig. 5.
Effects of 2,4-D on the frequencies of (A) basic normal flowers and (B) flowers with lodicule transformations to stamens and/or pistils (Lt) and (C) on the spectrum of ectopic flower structures in place of awn or on awns, cultivated under field conditions. (D) Specific chimeric structures observed only after 2,4-D exposure. L, lodicule; S, stamen; P, pistil. Scale bar (D) = 1 mm. Differences compared with 2,4-D treatment: *P < 0·05; **P < 0·01; ***P < 0·001.
The effect of 2,4-D was rather specific to ectopic malformations. Exposure to 2,4-D lowered the frequency of spikes with long naked gaps (Table 2, group I). However, statistically significant effects were observed in only four of the seven double mutant lines tested. As mentioned above in the section Phenotypic instability of Hv-Hd/tw2 double mutants, this feature is rather characteristic of an auxin deficiency because it is similar to phenotypes of auxin pathway mutants or to phenocopies induced by auxin inhibitors. Polymorphism of double mutant lines was also clearly expressed for other tested features, such as short gaps (group II) and leaf/shoot-like structures (III). After exposure of the mutant Hv-tw2 to 2,4-D, short gaps in spikes completely disappeared. Polymorphism of this feature was clearly expressed amongst most of the tested double mutant lines. In lines Hv-tw2 × Hv-H N21 (+) and N6 (+), the frequencies of spikes with short gaps decreased significantly, while the opposite (statistically significant) effect was observed in line Hv-tw2 × Hv-Lh N19 (+). In other lines, the effects were either statistically insignificant or the frequency of that spike type was low.
A significant amount of polymorphism in the response to 2,4-D was also evident for the leaf/shoot-like spike structures. In two double mutant lines, Hv-tw2 × Hv-Lh N11 (+) and N19 (+), the frequency of such variations observed after exposure to 2,4-D decreased, and in other tested lines the effect of 2,4-D was absent (Table 2, group III).
The effects of 2,4-D on the spectra of basic and ectopic flowers were not uniform (Fig. 5). For the mutant Hv-tw2, 2,4-D decreased the frequency of basic flowers with Lt (Fig. 5), but at the same time it significantly increased the frequency of Lt + SO flowers (Supplementary Data Table S4). The frequency of Lt flowers was also decreased by 2,4-D in the two double mutant lines Hv-tw2 × Hv-H N6 (+) and Hv-tw2 × Hv-Lh N17 (+−), but the opposite effect was observed in line Hv-tw2 × Hv-H N21 (+) (Fig. 5A). However, for four genotypes, including the Hv-Lh (−) mutant, the frequency of normal basic flowers decreased to various extents. This effect may be considered as opposite to the action of auxin inhibitors (cf. Fig. 4A and B). It is paradoxical that, in the Hv-tw2 mutant, the frequency of normal flowers increased after treatment with 2,4-D as well as after treatment with the auxin inhibitors PCIB and HFCA.
A specific type of stamen malformation was also induced by 2,4-D: a chimeric stamen (Sc) with an additional rudimentary carpel (Fig. 5D). However, this effect was observed in only a portion of the double mutant lines (Supplementary Data Table S4).
A more uniform effect of 2,4-D was noted on ectopic flower structures. First, exposure to 2,4-D increased the frequency of L + S + P flowers in all genotypes in which such flowers were developed (Fig. 5C and Supplementary Data Table S4). This effect may be considered to some extent as opposite to the effect of auxin inhibitors on the same fraction of ectopic flowers (cf. Fig. 5C and Table S4 with Fig. 4C). Additionally, a significant modification of the proportion of ectopic S + P flowers occurred. Their proportion decreased in cases where Hv-Lh was used as the father plant. In contrast, the fraction of L + P ectopic flowers increased in this scenario.
Nevertheless, the responses of the double mutant lines to 2,4-D varied across a wide range for both the basic and ectopic flower structures and specifically concerned the development of sexual organs in ectopic flowers and of long naked gaps. A specific effect of 2,4-D was also expressed on the short gaps in the spikes of the Hv-tw2 mutant. The effects of auxin inhibitors on this spike feature were not investigated.
DISCUSSION
The modifications of phenotypic instability features caused by the auxin inhibitors PCIB and HFCA and the synthetic auxin 2,4-D are of considerable significance because, to date, auxin imbalance has not been described as a cause of phenotypic instability. The inherited forms of phenotypic instability across all of the tested generations of barley Hv-Hd/tw2 double mutants, which originated from hybridization between the homeotic mutant Hv-tweaky spike (Hv-tw2 allele) and various Hv-Hooded mutants, showed varying effects on inflorescence/flower development that were an intriguing and unexpected phenomenon. DNA sequencing of two regions of the barley BKn3 gene that serve as specific markers (20- and 33-bp insertions) differentiating the BKn3 gene into alleles I, II, IIIa, IIIb and IIIc (Badr et al., 2000) demonstrated that all of the tested Hooded-type mutants and hybrids used in the present study have the molecular markers of the same allele IIIc (Kap), while WT and the Hv-tw2 mutant contain the European BKn3 allele I (unpublished data).
Our presumption that the phenotypic instability of the double mutant lines may be caused by an imbalance in auxin distribution, based on the similarity of the long naked inflorescence gaps to the pin-like phenotype of auxin transport mutants (McSteen et al., 2007; Morita and Kyozuka, 2007; Gallavotti, 2013) and on the fact that the LEAFY gene, mutations of which are expressed as a phenotype similar to that of the leafy/shoot spike structures of double mutants Hv-Hd/tw2, has auxin-response elements in its promoter and may be recognized as an auxin response factor (ARF) (Bai and DeMason, 2008), was confirmed by the significant decrease in this type of spike variation after exposure to 2,4-D, the partial normalization of the structure of basic flowers and the effects on ectopic flowers of the auxin inhibitors HFCA and PCIB. Moreover, the effects of 2,4-D, HFCA and PCIB on the flower spectra were considerably greater for the flower fractions in which both sexual organs (stamens and pistils) were developed.
The gene BKn3, mutation of which results in the Hv-Hd phenotype (Müller et al., 1995), is a representative of the Kn1 class of genes, which exhibit mutual interactions with auxin (Woodward and Bartel, 2005; Tabata et al., 2010; Rast and Simon, 2012) and form complex developmental modules with auxin pathway genes in the flower meristem (Hay and Tsiantis, 2010). We have hypothesized that the disturbance of such modules, as well as of the ectopic auxin maxima (Krizek, 2011) or of the auxin gradients (Benková et al., 2003; Tanaka et al., 2006), may lead to phenotypic instability in the inflorescences and flowers of double mutant lines. This supposition was also strengthened by the described pleiotropic effects in transgenic barley plants ectopically expressing the maize gene Zm-Kn1 (Williams-Carrier et al., 1997) and in the Zm-semaphore1 mutant with disturbed auxin/Kn1 gene interaction (Scanlon et al., 2002). The pleiotropic effects of awn transformation into flower structures may also contribute to phenotypic instability because the awn is the main photosynthetic organ in a barley spike (Abebe et al., 2009). Photosynthesis is also one of the main regulators of numerous plant processes and genes.
However, the various phenotype normalization effects caused by the auxin inhibitors PCIB and HFCA indicate that ectopic auxin hyperaccumulation has occurred or that ectopic auxin maxima have arisen. This conclusion is sustained by the contrasting effect of 2,4-D on the frequency of the normal basic flowers. Frequently, auxin inhibitors cause phenocopies of auxin pathway mutations (Morita and Kyozuka, 2007; Krizek, 2011; Gallavotti, 2013). The opposite effect – the rescue of the mutant phenotype by auxin inhibitors – is a rare event (Morita and Kyozuka, 2007; Staldal et al., 2008) and the inherited phenotypic instability in double homeotic mutants (i.e. Hv-Hd/tw2) is a suitable model system for the further investigation of ectopic auxin maxima formation, its consequences and the possible mechanisms of modifications. According to Krizek (2011), it is difficult to determine the auxin concentration in developing flower organs, and the phenomenon of phenotypic instability in the Hv-Hd/tw2 barley double mutants may be a useful system for investigating the integrative processes that occur at the organ initiation sites of a flower.
The specific effects of 2,4-D on spikes (short gaps in Hv-tw2 and long naked gaps in double mutants) and of the auxin inhibitors HFCA and PCIB on flower fractions with both sexual organs may indicate that various regulatory modules are involved, with varying relationships to auxin distributions in particular cells of the spike/flower primordium. The results also suggest that certain features characterized by the phenotypic instability of double mutants may differ.
This variation in the nature of phenotypic instability was especially clear for ectopic flowers. For the development of sexual organs, the expression of organ identity genes (classes B and C) in the third and fourth whorls is necessary, which may indirectly suggest that unbalanced overexpression of B/C genes may have occurred in barley hybrids, and this unbalanced expression may be caused by occasional auxin accumulations. In turn, this situation may induce phenotypic variations in organ initiation. In the present work, the involvement of different regulatory modules in relation to auxin responses also indicates significant differences between the effects of auxin inhibitors on callus meristematic cells and on the flower structure of the Hv-WT and the Hv-tw2 mutant.
The high polymorphism of the response to 2,4-D and auxin inhibitors in the tested double mutant lines is noteworthy. This polymorphism of the response to auxin, as well as the unexpected effect of 2,4-D on short spike gaps in the Hv-tw2 mutant and several other double mutant lines, deserves further study. Our presumption is that differences among double mutant lines, especially those with the same origin, may be explained mainly by the different genetic background that resulted from segregation, resembling observations in inbred lines of maize. The penetrance of the maize Kn1 gene was genetic background-dependent (Vollbrecht et al., 2000). In turn, the pleiotropic action of auxin was involved in an extraordinary variety of plant growth and developmental processes (Woodward and Bartel, 2005; Vanneste and Friml, 2009; Sauer et al., 2013). It may have variable effects on different genetic backgrounds, creating variations in auxin concentrations, deficiency or ectopic maxima in different single and double mutants. Furthermore, natural variations in auxin response were observed (Delker et al., 2010; Laskowski, 2013). The increase in callus growth in several mutants after exposure to HC toxin, an inhibitor of histone deacetylases, and the diverse response of different single and double mutants to the HC toxin suggest that variations in mutants may be caused also by epigenetic factors.
Ectopic outgrowths in the lemma/awn transition zone, especially on the awns of double mutants, allowed us to hypothesize (1) that a redistribution of auxin occurs in spikes and flower meristems of single mutants and Hv-Hd/tw2 double mutants and that an ectopic auxin maximum in one founder cell induces a deficiency in another founder cell, and (2) that the various levels of gene products necessary for flower organ development are achieved in different cells, resulting in a wide range of expression of ectopic outgrowths. Based on the variation in the extent of ectopic outgrowths, definite trends in flower structure development may be outlined: insignificant outgrowths on awns → tubes of two kinds (non-inverted → inverted) → variations in flower-like structures → flowers with sterile sexual organs. Perhaps in parallel to this, other organizational centres arise at sites of the so-called wings and cause other malformations: lengthening of wings → their transformation to awn-like structures. The occurrences of various malformations of spike structure, including leaf/bract-like structures, also demonstrate the existence of other developmental trends. Consequently, phenotypically unstable barley double mutants are a highly promising genetic system for the investigation of gene expression modules and trend orders.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: frequency of leafy/shoot-like structures and naked gaps in spikes of various barley double mutant lines cultivated under field conditions. Table S2: PCIB and HFCA effects on basic flower structures of barley single and double mutants cultivated under greenhouse conditions. Table S3: effects of PCIB and HFCA on the development of ectopic flower structures in barley single and double mutants cultivated under greenhouse conditions. Table S4: effects of 2,4-D on the basic and ectopic flower structures of selected barley single and double mutant lines (F10) cultivated under field conditions
ACKNOWLEDGEMENTS
We would like to thank two anonymous reviewers for their useful critical comments on the manuscript.
LITERATURE CITED
- Abebe T, Wise RP, Skadsen SW. 2009. Comparative transcriptional profiling established the awn as the major photosynthetic organ of the barley spike while the lemma and the palea primarily protect the seed. Plant Genome 2: 247–259. [Google Scholar]
- Ambrose BA, Lerner DR, Ciceri P, Padilla CM, Yanofsky MF, Schmidt RJ. 2000. Molecular and genetic analyses of the Silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Molecular Cell 5: 569–579. [DOI] [PubMed] [Google Scholar]
- Anzola JM, Sieberer T, Ortbauer M, et al. 2010. Putative Arabidopsis transcriptional adaptor protein (PROPORZ1) is required to modulate histone acetylation in response to auxin. Proceedings of the National Academy of Sciences of the USA 107: 10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnholdt-Schmitt B. 1993. Rapid changes in amplification and methylation pattern of genomic DNA in cultured carrot root explants (Daucus carota L.). Theoretical and Applied Genetics 85: 793–800. [DOI] [PubMed] [Google Scholar]
- Babb S, Muehlbauer GJ. 2003. Genetic and morphological characterization of the barley uniculm2 (cul2) mutant. Theoretical and Applied Genetics 106: 846–857. [DOI] [PubMed] [Google Scholar]
- Badr A, Müller K, Schäfer-Pregl R, et al. 2000. On the origin and domestication history of barley (Hordeum vulgare). Molecular Biology and Evolution 17: 499–510. [DOI] [PubMed] [Google Scholar]
- Bai F, DeMason DA. 2008. Hormone interactions and regulation of PsPK2::GUS compared with DR5::GUS and PID::GUS in Arabidopsis thaliana. American Journal of Botany 95: 133–145. [DOI] [PubMed] [Google Scholar]
- Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. 2008. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nature Genetics 40: 1136–1141. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, et al. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- Bolduc N, Yilmaz A, Mejia-Guerra MK, et al. 2012. Unravelling the KNOTTED1 regulatory network in maize meristems. Genes & Development 26: 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnett OT. 1966. Hood and supernumerary spike development in barley. Illinois Agricultural Experiment Station Bulletin 721: 78–91. [Google Scholar]
- Chow B, McCourt P. 2004. Hormone signalling from a developmental context. Journal of Experimental Botany 55: 247–251. [DOI] [PubMed] [Google Scholar]
- Curaba J, Talbot M, Li Z, Helliwell C. 2013. Over-expression of microRNA171 affects phase transitions and floral meristem determinancy in barley. BMC Plant Biology 13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delker C, Pöschl Y, Raschke A, et al. 2010. Natural variation of transcriptional auxin response networks in Arabidopsis thaliana. Plant Cell 22: 2184–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreni L, Pilatone A, Yun D, et al. 2011. Functional analysis of all AGAMOUS subfamily members in rice reveals their roles in reproductive organ identity determination and meristem determinacy. Plant Cell 23: 2850–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Wu W, Liu H, et al. 2003. Genetic analysis and gene mapping of leafy head (lhd), a mutant blocking the differentiation of rachis branches in rice (Oryza sativa L.). Chinese Science Bulletin 48: 2201–2205. [Google Scholar]
- Forster BP, Franckowiak JD, Lundqvist U, Lyon J, Pitkethly I, Thomas WTB. 2007. The barley phytomer. Annals of Botany 100: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Taniguchi N, Tasaka M. 2006. PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant Journal 48: 380–389. [DOI] [PubMed] [Google Scholar]
- Gallavotti A. 2013. The role of auxin in shaping shoot architecture. Journal of Experimental Botany 64: 2593–2608. [DOI] [PubMed] [Google Scholar]
- Ghareeb H, Becker A, Iven T, Feussner I, Schirawski J. 2011. Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiology 156: 2037–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini H, Tekauz A. 2012. Molecular diversity in the barley pathogen Bipolaris sorokiniana (Cochliobolus sativus). Australasian Plant Pathology 41: 283–293. [Google Scholar]
- Grant-Downton RT, Dickinson HG. 2006. Epigenetics and its implications for plant biology 2. The ‘epigenetic epiphany’: epigenetics, evolution and beyond. Annals of Botany 97: 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A, Tsiantis M. 2010. KNOX genes: versatile regulators of plant development and diversity. Development 137: 3153–3165. [DOI] [PubMed] [Google Scholar]
- Hettiarachchi N, Kryukov K, Sumiyama K, Saitou N. 2014. Lineage-specific conserved noncoding sequences of plant genomes: their possible role in nucleosome positioning. Genome Biology and Evolution 6: 2527–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston K, Druka A, Bonar N, et al. 2012. Analysis of the barley bract suppression gene Trd1. Theoretical and Applied Genetics 125: 33–45. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y. 2007. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant Journal 51: 1030–1040. [DOI] [PubMed] [Google Scholar]
- Inada DC, Bashir A, Lee C, et al. 2003. Conserved noncoding sequences in the grasses. Genome Research 13: 2030–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Takahashi H, Iwakawa H, et al. 2013. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis . Development 140: 1958–1969. [DOI] [PubMed] [Google Scholar]
- Koenig D, Bayer E, Kang J, Kuhlemeier C, Sinha N. 2009. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development 136: 2997–3006. [DOI] [PubMed] [Google Scholar]
- Krizek A. 2011. Auxin regulation of arabidopsis flower development involves members of the AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) family. Journal of Experimental Botany 62: 3311–3319. [DOI] [PubMed] [Google Scholar]
- Kuijt SJ, Greco R, Agalou A, et al. 2014. Interaction between the GROWTH-REGULATING FACTOR and KNOTTED1-LIKE HOMEOBOX families of transcription factors. Plant Physiology 164: 1952–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M. 2013. Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response. Journal of Experimental Botany 64: 2609–2617. [DOI] [PubMed] [Google Scholar]
- Lee DY, An G. 2012. Two AP2 family genes, SUPERNUMERARY BRACT (SNB) and OsINDETERMINATE SPIKELET 1 (OsIDS1), synergistically control inflorescence architecture and floral meristem establishment in rice. Plant Journal 69: 445–461. [DOI] [PubMed] [Google Scholar]
- Li H, Liang W, Yin C, Zhu L, Zhang D. 2011. Genetic interaction of OsMADS3, DROOPING LEAF and OsMADS13 in specifying rice floral organ identities and meristem determinacy. Plant Physiology 156: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoSchiavo F, Pitto L, Giuliano G, et al. 1989. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theoretical and Applied Genetics 77: 325–331. [DOI] [PubMed] [Google Scholar]
- McSteen P, Leyser O. 2005. Shoot branching. Annual Review of Plant Biology 56: 353–374. [DOI] [PubMed] [Google Scholar]
- McSteen P, Malcomber S, Skirpan A, et al. 2007. barren inflorescence2 encodes a co-ortholog of the PINOID serine/threonine kinase and is required for organogenesis during inflorescence and vegetative development in maize. Plant Physiology 144: 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Kyozuka J. 2007. Characterisation of OsPID, the rice ortholog of PINOID, and its possible involvement in the control of polar auxin transport. Plant and Cell Physiology 48: 540–549. [DOI] [PubMed] [Google Scholar]
- Murfett J, Wang XJ, Hagen G, Guilfoyle T J. 2001. Identification of Arabidopsis histone deacetylase HDA6 mutants that affect transgene expression. Plant Cell 13: 1047–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Leyser O. 2011. Auxin, cytokinin and the control of shoot branching. Annals of Botany 107: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KJ, Romano N, Gerstner O, et al. 1995. The barley Hooded mutation caused by a duplication in a homeobox gene intron. Nature 374: 727–730. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Kim JH, Jeong CY, Hong SW, Lee H. 2013. Inhibition of histone deacetylation alters Arabidopsis root growth in response to auxin via PIN1 degradation. Plant Cell Reports 32: 1625–1636. [DOI] [PubMed] [Google Scholar]
- Oono Y, Ooura C, Rahman A, et al. 2003. p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiology 133: 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osnato M, Stile MR, Wang Y, et al. 2010. Cross talk between the KNOX and ethylene pathways is mediated by intron-binding transcription factors in barley. Plant Physiology 154: 1616–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi P, Faccioli V, Terzi AM, et al. 2000. Genetics of mutations affecting the development of a barley floral bract. Genetics 154: 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rast MI, Simon R. 2012. Arabidopsis JAGGED LATERAL ORGANS acts with ASYMMETRIC LEAVES2 to coordinate KNOX and PIN expression in shoot and root meristems. Plant Cell 24: 2917–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi L, Wang Y, Stile MR, et al. 2003. The GA octodinucleotide repeat binding factor BBR participates in the transcriptional regulation of the homeobox gene Bkn3. Plant Journal 34: 813–826. [DOI] [PubMed] [Google Scholar]
- Sauer M, Robert S, Kleine-Vehn J. 2013. Auxin: simply complicated. Journal of Experimental Botany 64: 2565–2577. [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Henderson DC, Bernstein B. 2002. SEMAPHORE1 functions during the regulation of ancestrally duplicated knox genes and polar auxin transport in maize. Development 129: 2663–2673. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Barkoulas M, Tsiantis M. 2010. Control of leaf and vein development by auxin. Cold Spring Harbor Perspectives in Biology 2: a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuksta R, Vaitkuniene V, Rancelis V, et al. 2012. Barley homeotic mutants and their hybrids for ornamental purposes. Acta Horticulturae 953: 337–343. [Google Scholar]
- Staldal V, Sohlberg JJ, Eklund DM, Ljung K, Sundberg E. 2008. Auxin can act independently of CRC, LUG, SEU, SPT and STY1 in style development but not apical-basal patterning of the Arabidopsis gynoecium. New Phytologist 180: 798–808. [DOI] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. 2008. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386. [DOI] [PubMed] [Google Scholar]
- Tabata R, Ikezaki M, Fujibe T, et al. 2010. Arabidopsis AUXIN RESPONSE FACTOR6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class1 KNOX genes. Plant and Cell Physiology 51: 164–175. [DOI] [PubMed] [Google Scholar]
- Takumi S, Kosugi T, Murai K, Mori N, Nakamura C. 2000. Molecular cloning of three homoeologous cDNAs encoding orthologs of the maize KNOTTED1 homeobox protein from young spikes of hexaploid wheat. Gene 249: 171–181. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J. 2006. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cellular and Molecular Life Sciences 63: 2738–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BE, Bartling L, Whipple C, et al. 2009. bearded-ear encodes a MADS box transcription factor critical for maize floral development. Plant Cell 21: 2578–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Tadege M, Hemming MN, Peacock WJ, Dennis ES, Sheldon C. 2007. Short vegetative phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiology 143: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Ito Y, Sato Y, Kurata N. 2011. Positive autoregulation of a KNOX gene is essential for shoot apical meristem maintenance in rice. Plant Cell 23: 4368–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvorogova VY, Osipova MA, Doduyeva IY, Lutova LA. 2013. Interactions between transcription factors and phytohormones in the regulation of plant meristem activity. Russian Journal of Genetics: Applied Research 3: 325–337. [Google Scholar]
- Vaitkūnienė V, Varnaitė A, Rančelis V. 2004. Interaction of barley mutants Hooded and tweaky spike in F1 hybrids. Biologija 3: 13–20. [Google Scholar]
- Vanneste S, Friml J. 2009. Auxin: a trigger for change in plant development. Cell 136: 1005–1016. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S. 2000. Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 12: 3161–3172. [DOI] [PubMed] [Google Scholar]
- Walton JD. 2006. HC-toxin. Phytochemistry 67: 1406–1413. [DOI] [PubMed] [Google Scholar]
- Whipple CJ, Hall DH, DeBlasio S, Taguchi-Shiobara F, Schmidt RJ, Jackson DP. 2010. A conserved mechanism of bract suppression in the grass family. Plant Cell 22: 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Carrier RE, Lie YS, Hake S, Lemaux PG. 1997. Ectopic expression of the maize kn1 gene phenocopies the Hooded mutant of barley. Development 124: 3737–3745. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E, Stebbins GL. 1969. The morphogenetic effects of the Hooded gene in barley. II. Cytological and environmental factors affecting gene expression. Genetics 62: 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Nagato Y. 2011. Flower development in rice. Journal of Experimental Botany 62: 4719–4730. [DOI] [PubMed] [Google Scholar]
- Zanotti CA, Pozner R, Morrone O. 2010. Understanding spikelet orientation in Paniceae (Poaceae). American Journal of Botany 97: 717–729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.