Abstract
Background and Aims Nepenthes pitcher plants have evolved modified leaves with slippery surfaces and enzymatic fluids that trap and digest prey, faeces and/or plant detritus. Although the fluid’s contribution to insect capture is recognized, the physico-chemical properties involved remain underexplored and may vary among species, influencing their diet type. This study investigates the contributions of acidity and viscoelasticity in the fluid’s capture efficiency of two ant and two fly species in four Nepenthes species with different nutrition strategies.
Methods Four Nepenthes species were studied, namely N. rafflesiana, N. gracilis, N. hemsleyana and N. ampullaria. Fluid was collected from pitchers of varying ages from plants growing in the field and immediately transferred to glass vials, and individual ants (tribe Campotini, Fomicinae) and flies (Calliphora vomitoria and Drosophila melanogaster) were dropped in and observed for 5 min. Water-filled vials were used as controls. Survival and lifetime data were analysed using models applied to right-censored observations. Additional laboratory experiments were carried out in which C. vomitoria flies were immersed in pH-controlled aqueous solutions and observed for 5 min.
Key Results Pitcher fluid differed among Nepenthes species as regards insect retention capacity and time-to-kill, with differences observed between prey types. Only the fluids of the reputedly insectivorous species were very acidic and/or viscoelastic and retained significantly more insects than the water controls. Viscoelastic fluids were fatal to flies and were able to trap the broadest diversity of insects. Younger viscoelastic fluids showed a better retention ability than older fluids, although with less rapid killing ability, suggesting that a chemical action follows a mechanical one. Insect retention increased exponentially with fluid viscoelasticity, and this happened more abruptly and at a lower threshold for flies compared with ants. Flies were more often retained if they fell into the traps on their backs, thus wetting their wings. Insect retention and death rate increased with fluid acidity, with a lower threshold for ants than for flies, and the time-to-kill decreased with increasing acidity. The laboratory experiments showed that fewer flies escaped from acidic solutions compared with water.
Conclusions In addition to viscoelasticity, the pitcher’s fluid acidity and wetting ability influence the fate of insects and hence the diet of Nepenthes. The plants might select the prey that they retain by manipulating the secretion of H+ ions and polysaccharides in their pitcher fluid. This in turn might participate in possible adaptive radiation of this genus with regard to nutrient sequestration strategy. These plants might even structurally influence insect fall-orientation and capture-probability, inspiring biomimetic designs for pest control.
Keywords: Acidity, ant capture, carnivorous plant, digestive fluid, fly capture, insect capture, Nepenthes, pest control, pitcher plant, viscoelasticity
INTRODUCTION
In habitats naturally poor in nutrients or characterized by a low level of easily assimilated nitrogen, some plants use adaptations that allow them to acquire nutrients through alternative pathways (Chapin, 1980; Brundrett, 2009). Carnivorous plants are conspicuous examples of such alternative strategies of nutrition, as they produce leaves modified into traps which can attract, catch and digest animal prey (mainly arthropods), and assimilate the products of digestion (Lloyd, 1942; Givnish et al., 1984; Juniper et al., 1989; Ellison et al., 2003). These processes involve a variety of mechanisms at the plant–insect interface that play key roles in the plant’s nutrient uptake. Study of their functioning not only helps in understanding how these plants survive in such impoverished environments, but also provides possible clues for bio-mimetically inspired applications (Matusikova et al., 2004; Biteau et al., 2008; Wong et al., 2011).
Among the adaptive characteristics of some carnivorous plants is the capacity of glands to secrete highly acidic fluids. Such fluid, usually called ‘digestive fluid’, is known to play an important role in the digestion of prey by stocking endogenous enzymes (White, 1910; Scala et al., 1969; Hatano and Hamada, 2008; Schulze et al., 2012; Biteau et al., 2013) or by harbouring acidity-specialized bacteria and other aquatic organisms involved in a digestive mutualism with the plant (Bradshaw and Creelman, 1984; Adlassnig et al., 2011; Takeuchi et al., 2011; Chou et al., 2014). In ‘fly-paper’ types of carnivorous plants, such as sundews or butterworts, the fluid also plays a major role in prey capture (Juniper et al., 1989). In these plants, the fluid is produced in the form of droplets secreted by stalked mucilaginous glands and is highly retentive, especially towards flying insects (Darwin, 1875; Juniper et al., 1989). The mucin secreted by leaves of Drosera species (Droseraceae) is composed of a polysaccharide (Rost and Schauer, 1977; Gowda et al., 1982) that might be responsible for viscoelastic properties. In some species of Nepenthes (Nepenthaceae), a sister genus of Drosera, the fluid has similar properties (Gaume and Forterre, 2007; Bonhomme et al., 2011b), probably inherited from common ancestry (Gaume and Forterre, 2007).
In pitcher plants, trapping efficiency relies on their attractiveness, their capture performance and their retention capacity (Juniper et al., 1989). With regard to retention capacity, the fluid plays the last and ultimate role. However, in Nepenthes the involvement of the digestive fluid in the trapping process has long remained unclear, albeit suspected (Gaume et al., 2002), and this might still be the case for several species because the physical properties of the fluid are somehow cryptic, i.e. not conspicuous at first sight (Gaume and Forterre, 2007). Most experiments aimed at clarifying the capture process of the fluid have not been designed to truly distinguish the effect of insect retention of pitcher walls from that of the fluid itself (Gaume et al., 2002; Bohn and Federle, 2004; Di Giusto et al., 2008). Indeed, the focus on the slippery surfaces of the pitcher walls and their importance in the prey capture mechanism (Juniper and Burras, 1962; Bohn and Federle, 2004; Gaume et al., 2004; Gorb et al., 2013) may have consequently undervalued the role of the digestive fluid.
The first experiment to have shown an isolated effect of the fluid on insect retention was carried out on Nepenthes rafflesiana by Gaume and Forterre (2007). In this species, rheological measurements and high-speed video sequences performed under laboratory conditions revealed that the viscoelasticity property of the fluid was crucial to retaining insects, even when fluids were greatly diluted, a situation that can occur in situ during periods of high rainfall (Gaume and Forterre, 2007). However, the contribution of old fluids (i.e. those probably diluted by rain) in the plant’s retention process has been questioned by some field observations (Bauer et al., 2009). It is thus relevant to test the effect of viscoelasticity on fluids of different ages. A field experiment attempted to test the isolated effect of the fluid of two ‘viscoelastic species’ from Borneo, but this was carried out on ants only (Bauer et al., 2011). Yet the Nepenthes species used in these experiments distinguish themselves from other Bornean species in capturing a high proportion of flying prey (Moran, 1996; Moran et al., 1999; Gaume and Di Giusto, 2009). Experimental evidence on the effect of the fluid on flying visitors of Nepenthes plants is lacking. Moreover, although viscoelasticity has been shown to be important for insect retention, it is not a feature shared by all Nepenthes species (Bonhomme et al., 2011b). This raises the question of whether the fluid has a role in insect retention in species having a rheologically ‘water-like’ fluid.
In addition to viscoelasticity, other physico-chemical parameters of the fluid might be involved in prey capture. For instance, insects are known to be susceptible to acidity, which can affect their survival and community structure in freshwater habitats (Tabak and Gibbs, 1991; Brodin and Gransberg, 1993; Harrison, 2001). Despite this, the pH of Nepenthes pitcher fluid is generally disregarded as a potential factor influencing insect capture and survival. It has instead been suspected to have a key role in prey digestion (Adlassnig et al., 2011), especially given that the optimal activity of Nepenthes aspartic proteases occurs at low pH (Athauda et al., 2004; Hatano and Hamada, 2008). Furthermore, acidity has also been shown to assist nutrient uptake by facilitating the functioning of proton pumps, necessary for the active transport of ions through plant tissues (Juniper et al., 1989; Moran et al., 2010). There is therefore a need to clarify the possible multi-functionality of acidic secretions of pitchers, and their possible effect on the whole capture process of the plant. In Drosera capensis, the mucilage composed of an acidic polysaccharide shows maximum viscosity at pH 5, which decreases when pH is raised or lowered (Rost and Schauer, 1977). Therefore, as viscoelasticity and pH might be linked, any attempt to disentangle their possible effects on insect retention will be of value.
In this study we aimed to test, under field conditions, the isolated effect of the fluid in the retention process of Nepenthes pitcher plants, and to explore whether properties other than those inherent to water might be involved. We investigate whether the fluid’s own physico-chemical properties might partly explain inter-specific differences in degrees of insectivory, prey composition and thereby nutrition strategies in these pitcher plants. We used the fluids of four Nepenthes species and different age categories in experiments on four insect species of two types (ants and flies) to address the following questions: (1) Are the fluids of all Nepenthes species generally able to retain and kill insects or are there interspecific differences? (2) Does the age of the fluid influence prey retention and death? (3) Do the different fluids have similar effects on ants and flies, in terms of their retention and killing powers? (4) Which fluid properties (viscoelasticity or acidity) are most likely to explain any observed differences? Another set of experiments was carried out in the laboratory to investigate the specific role of acidity in the fluid trapping process.
MATERIALS AND METHODS
Study site and studied plant species
Experiments were carried out in May–June 2013 in the Tutong District of Brunei (Borneo). Four Nepenthes species, N. rafflesiana, N. gracilis, N. ampullaria and N. hemsleyana Macfarl. (formerly N. rafflesiana var. elongata Hort. or N. baramensis Clarke, Moran and Lee), were selected for their distinct pH and viscoelasticity properties. Ten plants of each species were randomly chosen from two research sites. The two former species were sampled on the open and degraded sandy forests of Tutong (White Sands, 4°44′N, 114°35′E), while the two latter species were sampled from heath forest (kerangas) along the Labi Road (4°35′N; 114°30′E). Among these species, N. ampullaria, which derives an important part of its nitrogen from plant detritus, is the only one that has been proposed to be more detritivore than insectivore (Moran et al., 2003; Pavlovič et al., 2011). The three others are insectivores with N. hemsleyana sometimes completing its diet with bat faeces (Grafe et al., 2011).
Studied insect species
Prior trial experiments revealed that most ant workers [a terrestrial black Crematogaster sp., an arboreal red Crematogaster sp., a Polyrhachis sp. and Oecophylla smaragdina (all 3–5 mm long)] could not escape from a control device (water-filled vials). In the present study, the ant species used belonged to the tribe Campotini, Fomicinae, including a very common visitor of Nepenthes species [Polyrhachis pruinosa (8 mm long)] and a Nepenthes symbiont [Camponotus schmitzi (3 mm long), Clarke and Kitching, 1995], both of which are hardly retained by watery liquids (Bonhomme et al., 2011b). Experimental ants were collected in the field, and held individually in pierced plastic containers for a maximum of 3 h before being used. Two fly species [Calliphora vomitoria (8 mm long) and Drosophila melanogaster (2 mm long)] of approximately the same size as the ants were collected in the field and reared on a nutritive substrate.
Field insect bioassays in Nepenthes fluids and water controls
As pitcher life span varies among species [estimated mean (±s.e.) half lifetime in months from Osunkoya et al. (2008): N. ampullaria, 2·40±0·99; N. gracilis, 2·96±0·99; N. hemsleyana, 0·95±0·31; lifetime of N. rafflesiana from Di Giusto et al. (2008): approx. 2 months], for each Nepenthes species three comparable classes of relative pitcher age were defined as ‘young’, ‘medium’ and ‘old’ – these refer to the estimated first, second and last third fragment of the life span for each species. These age classes were based on the rank of the associated leaf and the pitcher appearance (freshness, stains, colours). Upper pitchers were gently removed from the plants and the experiments were performed immediately. Five replicates were used for each age class of fluid using a different plant for each replicate or five sets of neighbouring plants (see below). Replicates were performed using the fluid of a single pitcher or a mixture of fluids from different pitchers of the same age class from the same plant, except for N. hemsleyana. In this case, an individual plant did not produce enough fluid, so replicates were based on the fluids of several neighbouring plants. Thus, the ‘mixture’ effect tested as a random factor may reflect either a ‘plant’ effect or a ‘locality’ effect. Pyrex vials (height, 80 mm; width, 48 mm) were filled with 30 mL of pitcher fluid for each age class per Nepenthes species. The fluids were previously cleaned of their natural prey items. Five trials per insect species were performed in the same fluid replicate. For each fluid, pH was recorded using pH indicator paper with a precision-scale of 0·5 (Acilit®, Merck KGaA, 64271 Darmstadt, Germany). A proxy of the viscoelasticity was obtained by stretching the fluid between two fingertips and measuring the maximum length reached by the resulting filament before breaking. For each plant species, viscoelasticity was measured in this manner for two fluids of the same age class. A viscoelasticity score was thus noted for each age class of the same species as the mean of the two measures obtained.
Experiments with ants were carried out on different plants from those used for the flies, because freshness of the fluid and of the insects was considered a priority. A total of 1200 bioassays (600 with ants and 600 with flies) were performed on 40 plants (ten plants of each of the four Nepenthes species, with five plants used for the ants and five used for the flies). Fluids of a given age category belonging to the five plants used for the ants were a posteriori checked to have comparable pH and viscoelastic properties to their homologues used for the flies.
The flies were caught passively by covering their container with a funnel, which was connected to a 1·5-cm-diameter plastic pipe. Once a fly moved forwards and reached the extremity of the pipe, the pipe was disconnected from the funnel, which was then quickly closed with a lid, and the fly was gently blown onto the experimental fluid from a height of 2 cm. The way the flies fell onto the fluid was recorded (i.e. right-position fall or back fall). Because humidity in the pipe could have wetted the wings of the flies, the pipe was regularly checked and dried.
The ants were individually held in separate containers. To begin the experiment, the lid of the container was quickly replaced by a funnel coated with Fluon (Whitford, Runcorn, UK). The whole unit was turned upside down 2 cm above the experimental vial containing the fluid, leading the ant to fall onto the fluid without any direct manipulation. Because it was difficult to observe the orientation with which the ant landed in the fluid, this was not scored.
Experimental combinations for each insect category (fly or ant) comprised five individuals of each of the two insect species thrown onto each fluid replicate for the three age categories of each Nepenthes species. Additionally, three control vials were filled with distilled water and insect trials were achieved in water using the same experimental design following each Nepenthes species experiment, for three water replicates per insect species, and a total of 240 bioassays.
A stopwatch was triggered for 5 min, as soon as the insect touched the fluid surface. An insect was scored as ‘escaped’, if it fled away or succeeded in lifting all six legs out of the fluid. Otherwise, if it stopped moving within this timeframe, it was scored as ‘prostrate’. Prostrate individuals were considered to be dead, as none was found to recover (in the fluid) in the following 24-h period. If an insect had not escaped, but remained active in the fluid after 5 min, it was recorded as ‘retained’.
Laboratory fly bioassays in pH-controlled solutions
The isolated effect of fluid acidity on trapping efficiency was tested in the laboratory on the blue fly (Calliphora vomitoria) only because it was the easiest of the two ubiquitous flies to manipulate and because we did not have access to the ants used in the field. An experimental pH 2 solution (mimicking the pH often reached by a number of Nepenthes species) was obtained by diluting 37 % hydrochloric acid into distilled water at 25 °C. The pH of the solution was controlled with a previously calibrated SB70P VWR® sympHony™ pH meter. Hydrochloric acid was chosen to factor out any additional physical effect likely to influence insect trapping, such as the wetting effect observed in acetic acid.
In a preliminary experiment, the blue flies were thrown onto the acidic solution using the same experimental design as in the field but none of the ten flies dropped were retained and all escaped in a few seconds. We concluded from these preliminary observations that acidity alone is not sufficient to catch the flies. We then deliberately used an experimental design that forced the flies to be immersed in the fluid. To this end, the fly was first blocked in a pipe in the same way as in the field experiment. The extremity of the pipe was then plunged into the fluid and the air contained in the pipe aspirated in such a way that the fluid was sucked up into the pipe, wetting the insect for less than 0·5 s. Both fly and liquid were expelled from the pipe, the orientation with which the fly came up to the surface was recorded, and the stopwatch was triggered. Fifty-two fly bioassays were thus carried out, 26 in distilled water and 26 in the acidic solution. When the flies were observed to be caught at the end of the 5-min observation sessions, they were gently removed from the solution and the time before take-off was recorded.
Statistical analyses
Data were analysed using R version 2.10.1 (www.r-project.org) and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
We first tested whether the retention rates at 5 min (including dead and living insects) differed among (1) the Nepenthes species, (2) the Nepenthes species and a water control, (3) fluid ages, (4) the mixture (equivalent to a ‘plant’ or a ‘locality’ effect), (5) the type of insect (ant/fly) and (6) the insect species. For this we performed logistic regressions using the GLIMMIX procedure for mixed models, with a nested structure, SAS errors of type 3 and backward selection. Selecting variables only when they explained a significant part of the variance when entered in last position in the model (i.e. taking into account errors of type 3) allowed us to overcome any problem of multicollinearity. Type 3 tests thus ensure that each variable used in the model brings its own significant information. The fixed variables were FluidOrigin, FluidAge nested within FluidOrigin, InsectType and InsectSpecies nested within InsectType, and the random variable was Mixture nested within FluidAge and FluidOrigin. In our data set, FluidOrigin matched five modalities (the four Nepenthes species and the ‘water control’) because we aimed to compare the Nepenthes species with this control design. Our aim was then to explore which properties of the plant species or of the fluid age could explain part of the observed effects of these previous variables on the variances of insect retention rates. We thus removed FluidOrigin and FluidAge as explanatory variables in the model and instead tested whether variations in the retention rates could be significantly explained by variations of the fluid pH and fluid viscoelasticity.
Using logistic regressions, we then analysed separately for ants and flies the true survival data [i.e. those for which insect fate (dead/escaped) was certain at the end of the 5-min observation session], and for each insect type, we tested which fluid properties (i.e. age, pH, viscoelasticity) and which insect properties (species, time spent in the fluid, fall orientation) affected their probability of death significantly. The time spent in the fluid was log-transformed as usually done in analyses of survival time. Viscoelasticity was taken both as a quantitative variable (Visco) and as a simplified binary variable (VE). Only the variable out of the two that still explained a significant part of the variance – when the other was taken into account – was kept in the model. Data were corrected for overdispersion when necessary, using the ratio of Pearson’s chi-square to the associated number of degrees of freedom.
Then, for each insect type, we analysed what happened at 5 min (300 s). To this effect, we used the predictions obtained in the previous model dealing with insect fates at times before 5 min. ‘Death’ was ascribed to insect fate at 5 min if the probability of death obtained in the previous model was superior to 0·5, and if not ‘escape’ was otherwise ascribed. We then analysed data for which the predicted fate was different from the observed fate. We observed only three discordances out of 543 known fates for flies and 20 discordances out of 644 known fates for ants. As error rates were below 0·05 in each of these cases, the accuracy of the model was considered satisfactory.
We thus decided to take into account the predictions of the logistic regressions to analyse survival times for each insect category, i.e. using for ‘unknown fates at 5 min’ fates obtained from the predictions of the previous models. To this end, LIFEREG procedures adapted for time analysis of right-censored observations were used with a weight design. For known fates (observed before 5 min), weight = ‘1’ was assigned for death and weight = ‘0’ was assigned for escape. For unknown fates (still alive after 5 min), the weight was equal to the probability of death obtained in the previous model.
For the laboratory experiment, the proportions of flies that were caught in the acidic solution were compared with those caught in water using a χ2 test or Fisher’s exact test when at least one cell had a theoretical number below 5.
RESULTS
Probability of insect retention at 5 min
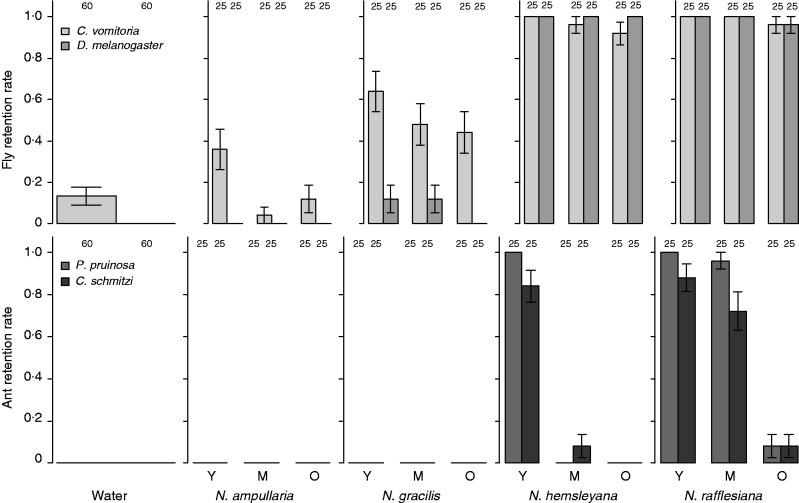
Retention rates of insects in isolated fluids varied greatly according to fluid origin (P < 0·0001) and relative age (P < 0·0001), but there was no random effect of the mixture (Table 1). This absence of a ‘mixture’ effect reflects the absence of any ‘plant’ or ‘locality’ effect. Retention rates also vary significantly with insect type (P < 0·0001) and insect species (P < 0·0001) (Table 1A). The retention probability of flies was higher than that of ants overall (Fig. 1). The small ant C. schmitzi, a symbiont of Nepenthes species (Clarke and Kitching, 1995), had the least chance of being retained by a fluid, while the large fly C. vomitoria was the most likely to be retained (Fig. 1).
Table 1.
Plant and insect parameters affecting fluid retention
| (A) Explanatory variables (Dependent variable = insect retention (0/1)) | ||||
|---|---|---|---|---|
| ndf | ddf | F | P | |
| Mixture: estimated covariance = 4·09×10–18 | n.s. | |||
| FluidOrigin | 4 | 130 | 50·46 | <0·0001 |
| FluidAge(FluidOrigin) | 8 | 130 | 13·26 | <0·0001 |
| InsectType | 1 | 130 | 108·74 | <0·0001 |
| InsectSpecies(InsectType) | 2 | 1294 | 21·69 | <0·0001 |
|
(B) Parameter estimates (least square means) | ||||
|---|---|---|---|---|
| z Value | P | Estimate | s.e. | |
| N. ampullaria vs. Water | 0·11 | 0·9130 | 0·06117 | 0·5600 |
| N. ampullaria vs. N. hemsleyana | 12·25 | <0·0001 | 9·1181 | 0·7443 |
| N. ampullaria vs. N. gracilis | 4·44 | <0·0001 | 2·0674 | 0·4658 |
| N. ampullaria vs. N. rafflesiana | 12·81 | <0·0001 | 11·3268 | 0·8843 |
| Water vs. N. hemsleyana | 12·52 | <0·0001 | 9·0569 | 0·7237 |
| Water vs. N. gracilis | 4·67 | <0·0001 | 2·0062 | 0·4296 |
| Water vs. N. rafflesiana | 12·99 | <0·0001 | 11·2657 | 0·8670 |
| N. hemsleyana vs. N. gracilis | –11·23 | <0·0001 | –7·0507 | 0·6277 |
| N. hemsleyana vs. N. rafflesiana | 4·56 | <0·0001 | 2·2087 | 0·4840 |
| N. gracilis vs. N. rafflesiana | 11·74 | <0·0001 | 9·2595 | 0·7886 |
(A) Results of a mixed nested logistic regression model testing for the random effect of mixture and the fixed effects of fluid origin (four Nepenthes species and water control), fluid age (young, medium, old), type of insect (fly, ant) and insect species on insect retention rates.
(B) Results of pairwise comparisons of the associated least squares means testing differences in the retention rates between fluids of each species or water.
Fig. 1.
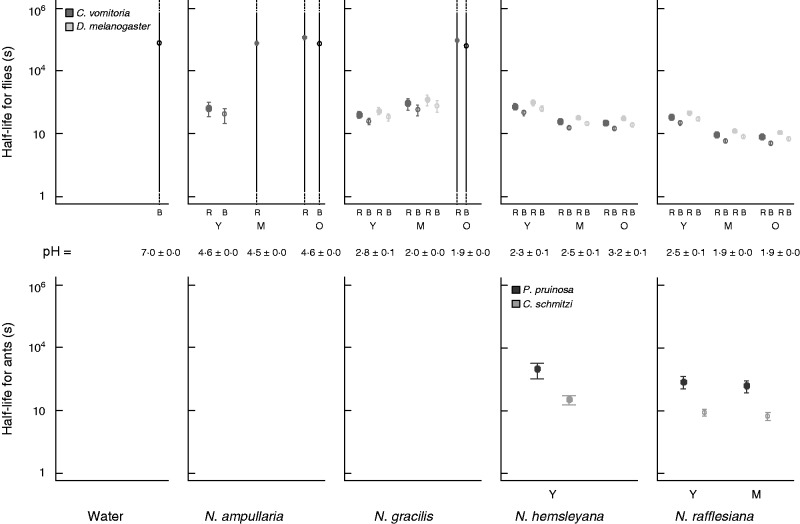
Insect retention probability. Mean (±s.e.) retention rates of two fly species (the blow-fly Calliphora vomitoria and the fruit-fly Drosophila melanogaster) and two ant species (the big ant Polyrhachis pruinosa and the smaller one Camponotus schmitzi) in the fluids of different age categories (Y = young, M= middle age, O = old) of four Nepenthes species and a water control. Numbers above columns indicate the sample size.
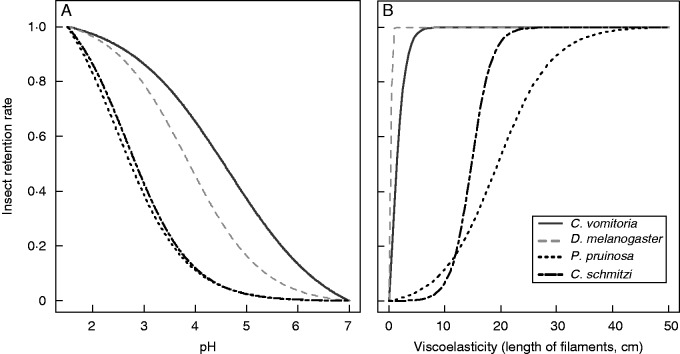
Interestingly, the fluid of N. rafflesiana (P < 0·0001), N. hemsleyana (P < 0·0001) and N. gracilis (P < 0·0001) differed significantly from water in retention rate, but this was not so for N. ampullaria (P = 0·91) (Table 1B). All of the 120 ants and 112 of the 120 flies dropped onto water succeeded in escaping. The fluids associated with the highest rates of retention were those of N. rafflesiana and N. hemsleyana, followed by N. gracilis and N. ampullaria, in order of decreasing importance [Fig. 1, interspecific differences were significant (P < 0·0001)] according to Least Squares Mean tests for the logistic regression). Young fluids were overall better at retaining insects than older ones (Table 1A, Fig. 1). As Nepenthes species and fluid age category are known to vary in pH and fluid viscoelasticity (Clarke, 1997; Bauer et al., 2009), we included the latter two variables in a new model. We also refined the model by testing whether the way the insect (flies) fell onto the fluid affected its retention probability. We showed that both pH and viscoelasticity explained significantly the variances observed for insect retention rate (Table 2). The probability of insect retention decreased with pH more quickly for ants than for flies (significant pH × InsectType interaction, Fig. 2A), whereas retention increased with viscoelasticity more drastically (and at a lower threshold) in flies compared with ants (Fig. 2B). There was also an interspecific effect of insect type, which affected the slope for retention rate of the fly species against pH (that of the blow-fly decreased more slowly than that of the fruit-fly), and the slope for retention rate of the ant species against viscoelasticity (the retention of C. schmitzi being slower than that of P. pruinosa) (Fig. 2B, significant interactions, Table 2). The way the flies fell onto the fluid strongly influenced their probability of being retained (Table 2), with retention rates being significantly higher when they landed on their backs (dorsal surfaces).
Table 2.
Fluid and insect parameters affecting retention rates
| Explanatory variable |
ndf | χ2 | P |
|---|---|---|---|
| Visco | 1 | 688·77 | <0·0001 |
| pH | 1 | 44·20 | <0·0001 |
| InsectType | 1 | 4·78 | 0·0312 |
| InsectSpecies(InsectType) | 2 | 7·39 | 0·0367 |
| FallOrientation(InsectType) | 1 | 14·31 | 0·0002 |
| Visco × InsectType | 1 | 44·00 | <0·0001 |
| pH × InsectType | 1 | 23·28 | <0·0001 |
| Visco × InsectSpecies(InsectType) | 2 | 58·86 | <0·0001 |
| pH × InsectSpecies(InsectType) | 2 | 5·85 | 0·0537 |
Results of a nested logistic regression model testing for the effects of the fluid’s viscoelasticity (considered as a quantitative variable), the fluid’s pH, the type of insect and the insect species on insect retention rates. Dependent variable = insect retention (0/1).
Fig. 2.
Insect retention probability as functions of the physico-chemical properties of pitcher fluid. Proportions of retained insects during a 5-min exposure, as a function of pH (A) and viscoelasticity (B). The curves represent the estimated probabilities of insect retention (360 repetitions by species), as predicted by the logistic regressions.
Insect survival probability or killing ability of the plant
Results of the model that best explained the variance for fly death and that minimized discordance between observed and predicted fly fates are given in Table 3A. The variables that best explained the probability of death were time spent in the fluid and fluid viscoelasticity (when encoded as a binary variable). This means that as long as the fluid is slightly viscoelastic, the flies are very likely to be retained and killed, in relation to duration in the fluid. Only flies that had remained in the fluid for a few seconds, not long enough to wet their wings, successfully escaped. pH and fall orientation alone did not significantly affect survival probability (Table 3A). Their effects were probably concealed by the strong effect of time in fluid, a variable highly correlated with both pH and fall orientation (replacing time in the fluid with these variables revealed their significance: χ2 = 5·09, P = 0·02; χ2 = 6·04, P = 0·01, respectively, using a logistic regression). Note, however, that the interaction of these variables was significant, which means that the survival probability of a fly decreases with fluid acidity more quickly when it falls on its back.
Table 3.
Parameters affecting insects’ death
| Explanatory variable | ndf | Wald χ2 | P |
|---|---|---|---|
| (A) Flies, Dependent variable = death (0/1) | |||
| VE | 1 | 6·0786 | 0·0137 |
| pH | 1 | 2·1514 | 0·1424 |
| FallOrientation | 1 | 2·8056 | 0·0939 |
| LogTime | 1 | 15·2531 | <0·0001 |
| pH × FallOrientation | 1 | 3·8491 | 0·0498 |
| (B) Ants, Dependent variable = death (0/1) | |||
| FluidOrigin | 4 | 6·3020 | 0·1777 |
| Visco | 1 | 31·6832 | <0·0001 |
| FluidAge(FluidOrigin) | 7 | 25·8307 | 0·0005 |
Results of a logistic regression model testing for the effects of fluid origin, fluid age, fluid viscoelasticity (‘Visco’ as a quantitative variable and ‘VE’ as a qualitative variable), fluid pH, insect species and their interactions on death rates of (A) flies and (B) ants. Only significant variables were kept in the final models according to the backward selection procedure.
As for the ants, results of the model that best explained the variances for insect death and that minimized the discordance number between observed and predicted insect fates are displayed in Table 3B. The time spent in the fluid was not a good predictor of survival for ants (χ2 = 1·43, P = 0·23). The degree of viscoelasticity was a better predictor than the viscoelasticity taken as a binary variable, as the latter was no longer significant when considering the former (χ2 = 0·001, P = 0·99). Relative age of the fluid significantly affected the survival probability of ants, while this variable was not retained by the model performed on the flies (χ2 = 3·26, P = 0·92). To sum up, the probability of death of an ant increases with degree of viscoelasticity of the fluid and fluid relative age. Here again, the effect of pH (not significant: χ2 = 0·64, P = 0·42) was probably masked by effects of the Nepenthes species and fluid relative age. Nonetheless, pH became significant in the model when tested independently of these two variables (χ2 = 17·30, P < 0·0001).
Influence of fluid and insect-fall orientation on prey composition
Figures 3 and 4 summarize the known insect fates and the assigned insect fates obtained from predictions of the logistic models (see the procedure in the Methods for insects not killed at the end of the 5-min session).
Fig. 3.
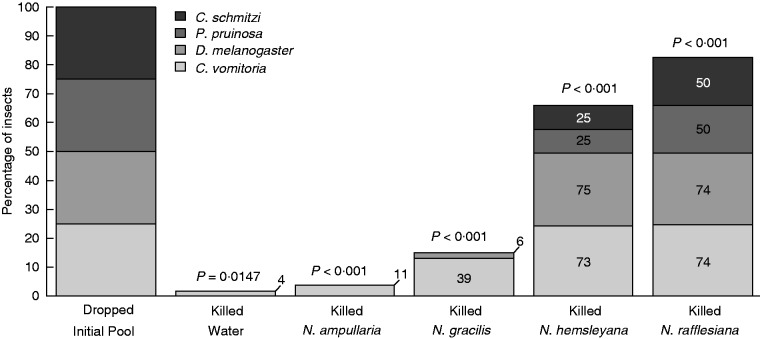
Influence of the fluid’s trapping ability on prey quantity and prey spectrum. The numbers refer to the numbers of insects that were killed. The fluid does not kill all the insect species initially dropped. The difference between the compositions of prey and initially dropped insects was significant in each case. The only two insect species that were systematically killed were the two fly species in the two viscoelastic Nepenthes species, N. rafflesiana and N. hemsleyana. These species also had the richest and the most diverse prey spectra. The number of insects initially dropped in the fluid was 75 by insect species for each Nepenthes species and 60 by insect species for water.
Fig. 4.
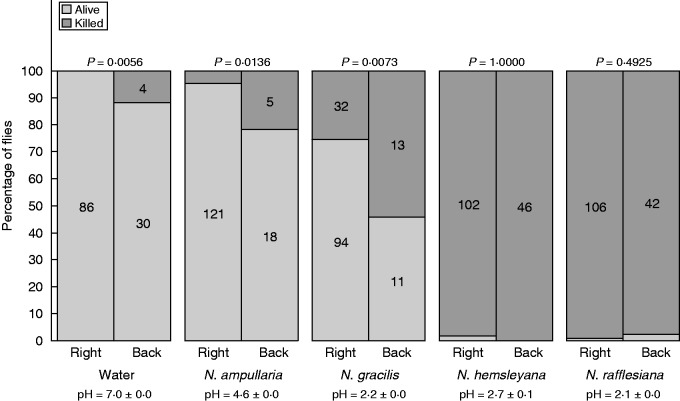
Influence of the fly’s impact side onto the fluid on its death probability. Flies were predicted to be killed in greater proportions when they impacted the fluid on their back (dorsal surface) than on the right position (ventral surface). Sample numbers are indicated. According to the Fisher’s exact tests, this effect was only significant for non-viscoelastic fluids for which it increased with fluid acidity, illustrating the results of the statistical tests that were performed in the logistic regression (interaction FallOrientation × pH).
As shown in Fig. 3, the fluid greatly influences prey composition (i.e. the relative abundances of insect species in the prey spectrum), which was, for each Nepenthes species, very different from the composition of insects initially dropped (Fisher exact test, P < 10–6 for each Nepenthes species). Interestingly, it is the difference between the two types of insects (flies/ants) that essentially explained the interspecific differences in death rates. This is particularly clear for the two viscoelastic Nepenthes species (i.e. N. rafflesiana and N. hemsleyana) for which the death rates within a type of insect were equivalent. These Nepenthes species were able to kill almost all the flies initially dropped, but were significantly less efficient (albeit far more than their congeners) against ants. Note also that these species have the most diversified prey spectra.
Figure 4 illustrates how the way the flies initially touch the fluid influences their fate and thus the composition of the prey spectrum. This effect of fall orientation was significant for water and for the two Nepenthes species having a non-viscoelastic fluid (Fisher exact tests, Pwater = 0·0056, Pampullaria = 0·0136, Pgracilis = 0·0073). The strongest effect of viscoelasticity masked this effect, which thus was non-significant for N. hemsleyana (P = 1) and N. rafflesiana (P = 0·49). Confirming results previously statistically treated, for non-viscoelastic species, this difference seems to increase when pH decreases, being stronger for N. gracilis, which has the most acidic fluid.
Survival times
The estimated half-life of the prey (time to 50 % mortality, mean±s.e.) in Nepenthes fluids was variable among the insect species (Fig. 5). For example, in viscoelastic fluids, P. pruinosa had the longest estimated half-life (1206·91±563·78 s, n = 75), followed by the two flies (C. vomitoria: 258·5±45·5 s, n = 147; D. melanogaster: 355·8±64·0 s, n = 149) and then C. schmitzi (127·45± 37·89 s, n = 75).
Fig. 5.
Predicted times to death (log-scale) for (A) flies and (B) ants according to the results of the censored models. The error bars refer to the standard deviations of predictions. The estimated insect half-lives (i.e. time to 50 % mortality) are given by the models for the two fly species (the blow-fly Calliphora vomitoria and the fruit-fly Drosophila melanogaster) and the two ant species (the big ant Polyrhachis pruinosa and the smaller one Camponotus schmitzi) in the fluids of different age categories (Y = young, M= middle age, O = old) of four Nepenthes species and a water control. R and B refer to the way the dropped fly initially touched the fluid (R = right/ventral, B = back/dorsal).
For the flies, according to the right-censored model applied to lifetime data, time to death in any fluids (including water) was significantly affected by fluid age, fluid origin, pH, insect species and fall orientation (Table 4A, Fig. 5). Time to death was shorter for the blow-fly than for the fruit-fly. It decreased significantly when acidity increased and it was significantly shorter when the insect fell on the back side (dorsal surface) rather than on the right side (ventral surface) (Table 4A, parameter estimates). Time to death also varied with fluid age, but differently according to fluid origin. It tended to decrease with fluid age for species with a viscoelastic fluid (N. rafflesiana and N. hemsleyana), i.e. those for which the estimations based on higher numbers were also the most accurate.
Table 4.
Parameters affecting insects’ time to death
| Explanatory variable |
ndf | Wald χ2 | P |
|---|---|---|---|
| (A) Flies, Dependent variable = DeathTime (right-censored at 300 s) | |||
| pH | 1 | 9·8366 | 0·0017 |
| FluidOrigin | 4 | 50·9673 | <0·0001 |
| FluidAge(FluidOrigin) | 8 | 81·6622 | <0·0001 |
| InsectSpecies | 1 | 7·5618 | 0·0060 |
| FallOrientation | 1 | 18·9018 | <0·0001 |
| (B) Ants, Dependent variable = DeathTime (right-censored at 300 s) | |||
| FluidOrigin | 1 | 3·1756 | 0·0747 |
| FluidAge(FluidOrigin) | 3 | 13·1007 | 0·0044 |
| InsectSpecies | 1 | 25·7284 | <0·0001 |
Results of a right-censored survival time model (censured at 300 s) testing for the effects of fluid origin, fluid age, fluid viscoelasticity (‘VE’ as a binary variable and ‘Visco’ as a quantitative variable), insect species, fall orientation, pH and their interactions on time to death of (A) flies and (B) ants. Only significant variables were kept in the final models according to the backward selection procedure.
As for ants, which died only in species having a viscoelastic fluid, i.e. N. hemsleyana and N. rafflesiana, time to death was affected by fluid age, being shorter for older fluids (Table 4B, Fig. 5). It varied considerably between the insect species, being shorter for C. schmitzi than for P. pruinosa according to the model applied to right-censored observations (Fig. 5).
Isolated effect of acidity on flies
Eleven of the 26 flies promptly immersed in the acidic solution were observed to be caught at the end of the 5-min observation sessions versus only four of the 26 flies promptly immersed in water. The differences were significant according to a χ2 test (χ2 = 4·6, P = 0·03). Interestingly, none of the six flies observed in the back side position succeeded in escaping from the acidic solution whereas eight out of the ten flies observed in the back side position managed to escape from water, the difference being highly significant (P = 0·007, Fisher’s exact test). All the flies observed to be caught in the 5-min sessions were alive. They all spent time cleaning themselves after removal from the fluid at the end of the experiment. However, the time elapsed before the flies that were immersed flew away was on average increased threefold in acidic solution compared with water solution (acid, 38±9·2 s; water, 12·8±3·3 s).
DISCUSSION
This study shows that the fluid of carnivorous species of the genus Nepenthes has retentive properties that differ from those inherent to water. Both the viscoelasticity and the pH of the pitcher fluids are strongly correlated to insect capture. The effect of viscoelasticity is shown to be ‘all or nothing’ for flies and more progressive for water-resistant ants but with a delayed threshold. Insect retention increased with fluid acidity earlier but more gradually in flies than in ants. The way the flies initially touched the fluid influenced their fate, all the more so as the fluid was acidic. Additional experiments in prepared acidic solutions showed an effect of acidity only in situations of insect wetting. According to their properties, the fluids of Nepenthes species do not trap the same quantity and quality of insect species. These findings raise some important questions. What are the fine mechanisms at the insect–fluid interface that can explain insect trapping and the differences observed between insect categories? What are the possible implications for pest control? To what extent are these results relevant for the evolutionary ecology of Nepenthes carnivorous plants?
Mechanisms at the insect–fluid interface in Nepenthes pitcher plants: research avenues for bio-inspired traps?
First, viscoelasticity is shown to be the fluid’s most powerful property regarding insect retention in Nepenthes pitcher plants. The role of viscoelasticity in the trapping system of Nepenthes species has been previously demonstrated, but only in the laboratory using greenhouse plants and non-native insects (Gaume and Forterre, 2007; Bonhomme et al., 2011b). Here the bioassays are shown to be conclusive in the field, using insects found on Nepenthes pitcher plants. Our results further showed that viscoelasticity is lethal for the great majority of insects tested and that it allows the plants to kill insects, such as water-resistant ants and flying insects that may not have been killed by rheologically ‘water-like’ fluids. It seems clear that C. schmitzi, which is able to swim in the Nepenthes fluid (Clarke and Kitching, 1995; Bohn et al., 2012), has behavioural adaptations that prevent it from sinking. The ant P. pruinosa has a thick waxy layer on its cuticle, the bloom or ‘pruine’ from which it takes its name, and which has, like any lipid surface, hydrophobic and anti-wetting properties (Holdgate, 1955). This may explain why no specimen of these two species has died in water or ‘water-like’ fluids while all of the ant specimens of two Crematogaster species and Oecophylla smaragdina were found to sink in water. Moreover, flies bear on their body numerous hairs which, among other functions, may protect them against wetting (Perez-Goodwyn, 2009). This is at least so for Calliphora flies, which exhibit wide contact angles of water droplets on their hairy cuticle (Holdgate, 1955). In Nepenthes viscoelastic fluids, which have water-comparable surface tension, trapping does not occur because of surface properties but rather because of the fluid’s extensional viscosity, which offers resistance to insect stretching (Gaume and Forterre, 2007). Trapping occurs as soon as the elastic forces created by insect movements have no time to relax. Thus, an abrupt transition of capture rate with fluid viscoelasticity is expected. This is precisely what is observed, as attested by the sigmoid functions obtained for ants and flies (Fig. 2). The earlier threshold of capture for flies may largely be explained by the presence of wings, because their large surface/weight ratios increase the fluid’s resistance to their movement. Interestingly, the capture rate of the ant C. schmitzi is slower than that of P. pruinosa. As discussed by Gaume and Forterre (2007), escape of an insect from a viscoelastic fluid depends on how slowly it moves. This is probably the strategy adopted by the N. bicalcarata symbiont C. schmitzi (Clarke and Kitching, 1995; Bazile et al., 2012). Indeed, C. schmitzi showed less sign of agitation than the other insects when dropped into the fluid and has been measured to have low stepping frequencies and a large phase delay within a given pair of legs (Bohn et al., 2012). This slow period of swimming stroke might be the result of coevolution and denotes its adaptation to pitcher life. While insect definitive capture is a positive function of viscoelasticity, there is no significant correlation between time to death and viscoelasticity for the two insect types of this study (flies and ants). Unexpectedly, in species having a viscoelastic fluid, the estimated time to death was found to decrease with fluid age, i.e. to decrease while viscoelasticity decreases. This means that something else should explain rapid death of prey in old, weakly viscoelastic fluids. A conspicuous singularity of old fluids compared with younger fluids is that they are in contact with numerous prey items. It has been reported that secretion of naphtoquinones, known to have cytotoxic effects, is induced by prey in the pitcher of N. khasiana (Eilenberg et al., 2010; Raj et al., 2011) and N. alata (Buch et al., 2013). It is thus tempting to suggest that in older plants, alkaloids, triggered by the insects themselves, in addition to having potential antimicrobial activity (Buch et al., 2013), play a chemical and ultimate role in the death of insects in Nepenthes pitcher plants. Supporting this idea is our observation that no blue-fly died in the acidic solution during the 5-min experiments carried out in the laboratory.
Without being lethal, acidity is revealed to affect insect performance. This result is new and worthy of attention as acidity was not previously suspected to take part in the trapping system of Nepenthes pitcher plants. Although the observed correlation between fluid acidity and insect life span might only be the by-product of other pH-dependent factors such as enzyme cytolytic activity, which may be the main cause of an insect’s slowdown, there are several arguments that support a direct effect of pH instead. First, it seems more credible that a cytolytic effect, such as that caused by enzymes, would be measurable over a longer time scale. Acidity is more likely to trigger a rapid insect response, as observed in this study. Second, acidity is known to negatively influence insect behaviour and survival in freshwater habitats (Tabak and Gibbs, 1991; Brodin and Gransberg, 1993; Harrison, 2001). Last but not least, we experimentally showed the isolated effect of acidity in laboratory experiments on fly retention in wetting situations. Acidity alone may not prevent the escape of flies but it may greatly impede flies’ take-off or recovery provided that they have been immersed. This highlights a synergetic effect of acidity and wetting properties of the fluid. In viscoelastic fluids, wetting occurs as soon as the insect begins to move (wetting occurs in dynamic not in static situations, Gaume and Forterre 2007). To explain the flies’ capture in the acidic fluid of N. gracilis, one might expect the presence of either a wetting agent or, at very weak concentration, a polysaccharide responsible for the viscoelastic properties. According to our results, C. schmitzi seems to die more quickly than P. pruinosa in the acidic and viscoelastic fluids of N. hemsleyana and N. rafflesiana. This might be explained by the better anti-wetting properties of P. pruinosa, a frequent visitor of Nepenthes (Di Giusto et al., 2008; Bonhomme et al., 2011a). Indeed, C. schmitzi is easily wetted by fluids (Bohn et al., 2012) and its cuticle, not covered by a thick layer of ‘pruine’ such as in P. pruinosa, is probably more permeable and thereby more sensitive to homeostatic and pH changes of the liquid (Harrison, 2001). This is therefore not a hazard if the ant is associated with N. bicalcarata, one of the species having the less acidic fluid (Moran et al., 2010; Bonhomme et al., 2011a). It is even argued that N. bicalcarata actively manipulates its fluid pH to maintain low acidity (Moran et al., 2010), which thus appears to be of high adaptive significance for the symbiotic but pH-sensitive ant C. schmitzi. In the genus Nepenthes, fluid acidity might have evolved primarily in response to the plant’s digestion requirements with regard to enzyme activity and constitutes only a secondary adaptation to insect retention. However, some genera of carnivorous or proto-carnivorous pitcher plants, although believed to lack enzymes in their fluid, display species with low-pH (<4) fluids, such as a few Sarracenia species or Brocchinia tatei (Juniper et al., 1989). It is thus tempting to consider that for those species, particularly those forming substantial tanks, the primary role of acidity is insect retention.
Finally, the way the fly initially touches the fluid influences its fate. This orientation effect appears to be significant when it is not concealed by the stronger effects of viscoelasticity. The magnitude of the fall orientation effect on death rate increases with the pH of the fluid. This is observed from our experiments using Nepenthes fluids and secondarily confirmed by our experiments in acidic solutions. Falling dorsally on its wings reduces the survival chances of a fly, probably because (1) the wings offer a larger contact area and retention points compared with tarsi, and (2) the wings are not as rough as the rest of the insect body, and hence are more easily wetted (Holdgate, 1955; Perez-Goodwyn, 2009). This effect may explain the higher capture and death rate of flies than of ants in all of the fluids tested. As nectar glands of Nepenthes pitcher plants are often sunk between the curved teeth of the pitcher rim (Owen and Lennon, 1999), tentatively this may lead insects to bend in a reversed position before losing their foothold (Juniper et al., 1989) and land on the fluid ventral surface upwards.
This observation on the importance of the insect landing position and the results pertaining to pH, wetting and viscoelasticity may help the design of biomimetic traps. Synthetic pitfalls devoted to the trapping of flying insects could be greatly and easily improved if they also included an acidic substrate and a landing platform. Both the inclination angle and the height of the platform should be optimized and adapted to the insect weight, to make an insect systematically fall upside down on its wings and with a kinetic energy sufficient to cause body immersion. Such physical parameters have been shown to influence the fall orientation of buttered toast, which does not merely follow a binomial law with equiprobability (random phenomenon: 0·5/0·5) or ‘Murphy’s law’ (‘If it can go wrong, it will’) as previously thought (Matthews, 1995). The viscoelastic properties of the Nepenthes fluid may also serve as a model for the development of insect glues. Indeed, biopolymers could be added to a water-based fluid (Bergeron et al., 2000) to obtain the necessary viscoelastic properties, i.e. its propensity to make long-lived filaments that resist stretching and prevent insect escape. Finally, at a larger scale, the development of pesticide or fertilizer sprays that avoid the problem of drop bouncing on plants may be economically and ecologically valuable, as it would prevent the release of large amounts of chemicals that otherwise would have spread with the well-known consequences for soil pollution.
Fluid characteristics and their influence on the plant’s diet
The fluid is thus an important part of the complex mechanism of capture in Nepenthes carnivorous pitcher plants, which have long being considered to rely on passive gravity-dependent pitfall traps. It is clear that the fluid, by its own physical properties, is able to influence the pitcher’s prey composition and thereby the plant’s diet. Differences in both acidity and rheology partly explain inter-specific differences in capture strategies. First, it is noteworthy that fluids with trapping physico-chemical properties such as acidity and viscoelasticity characterize only insectivorous species. Species such as N. ampullaria, which is known to be more detritivorous than carnivorous (Moran et al., 2003; Pavlovič et al., 2011), do not need to have an insect-retentive fluid. This may explain why its fluid is neither very acidic nor viscoelastic. The absence of strong acidity in this species may reflect only the absence of functional proteolytic enzymes such as nepenthesins, which optimally function at low pH (Athauda et al., 2004). Indeed, the plant relies on microorganisms (Takeuchi et al., 2011) and a complex food web to decompose organic matter (Mogi and Yong, 1992) and our results pertaining to the effect of pH on insect fitness also support the hypothesis that N. ampullaria also actively maintains low acidity to protect such a mutualistic infauna (Alcala and Dominguez, 2003; Moran et al., 2010). The ‘all or nothing’ effect on flying prey of fluid viscoelasticity, which is observed here at a low level of viscoelasticity, confirming previous laboratory results (Bonhomme et al., 2011b), clearly demonstrates that fluid viscoelasticity gives no chance to flying prey. Species such as N. rafflesiana have a trapping feature that successfully targets flying insects, such as the production of sweet odours (Di Giusto et al., 2010), and lack the waxy layer particularly efficient for ant capture (Gaume and Di Giusto, 2009). But why do the young fluids of such Nepenthes species have high degrees of viscoelasticity if a weak viscoelasticity is sufficient to catch flies? First, species with viscoelastic fluids grow in perhumid regions (Moran et al., 2013) and are thus more likely to have their fluid often diluted by rainfall, as probably attested to by the observed decrease of viscoelasticity with fluid age. Therefore, fluids initially having high concentrations of polysaccharides have a greater chance of circumventing the water-dilution problem. Secondly, the effect of fluid viscoelasticity is more progressive on at least water-resistant ants, which require a greater degree of viscoelasticity than flies to be retained. As ants are abundant in most tropical forests (Davidson and Patrell-Kim, 1996), it is important for the plant to have a weapon that is also widely efficient on this more ubiquitous type of prey.
Species such as N. gracilis, which has a crawling and terrestrial habit, and which encounters and traps mostly terrestrial ants (Moran et al., 1999), do not need to have a viscoelastic and retentive fluid, but rather rely on the waxy layer to efficiently retain their prey (Bonhomme et al., 2011b) and on the acidity to kill them (this study). The question remains as to why N. hemsleyana, which is reputed to be coprophagous (Grafe et al., 2011), maintains a viscoelastic fluid with high retentive ability and that is potentially harmful for the so-called mutualistic bat reported to defecate in its pitcher? It is possible that this viscoelastic fluid is a remnant character from a common ancestry with the sister species, N. rafflesiana. A second hypothesis is that insects are a more reliable food resource than bat faeces to N. hemsleyana. On the whole, bats visit only relatively few individual plants (Grafe et al., 2011), while insects are always found in the plants’ traps in significant diversity (Di Giusto et al., 2009), suggesting that bats could be engaged more opportunistically than mutualistically in their relationship with N. hemsleyana.
In conclusion, Nepenthes pitcher plants might select the prey they retain and thereby their type of diet by manipulating the secretion of H+ ions and polysaccharides in their pitcher fluid. This finding is of high adaptive significance and might partly explain the possible adaptive radiation of this genus with regard to the nutrient sequestration strategies of different species in various habitats (Pavlovič, 2012). Moreover, our unique observation that the orientation with which a fly lands on acidic fluids determines its fate leads to several novel questions as to how the structural associations of nectar glands, curved teeth and pitcher rim may manipulate the feeding position of an insect and its probability of being trapped. This provides a basis for the design of bio-inspired traps.
ACKNOWLEDGMENTS
We would like to dedicate this work to Michaël Guéroult, our friend and exceptional co-worker, who died last year after a long struggle against cancer. We are deeply grateful to Hadzid, Ieney, Fina and the whole Hadzid family for their kind hospitality at Telamba homestay in Brunei. The Brunei Forestry Department allowed us to carry out research in the forest. Zulhilmi Ab is acknowledged for his field assistance. Two anonymous reviewers are thanked for their helpful comments. The work was supported by the Centre National de la Recherche Scientifique (PEPS/INEE-CNRS-CarniBiop to L.G.).
LITERATURE CITED
- Adlassnig W, Peroutka M, Lendl T. 2011. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Annals of Botany 107: 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala RE, Dominguez CA. 2003. Patterns of prey capture and prey availability among populations of the carnivorous plant Pinguicula moranensis (Lentibulariaceae) along an environmental gradient. American Journal of Botany 90: 1341–1348. [DOI] [PubMed] [Google Scholar]
- Athauda SBP, Matsumoto K, Rajapakshe S, et al. 2004. Enzymic and structural characterization of nepenthesin, a unique member of a novel subfamily of aspartic proteinases. Biochemical Journal 381: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Willmes C, Federle W. 2009. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Annals of Botany 103: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Grafe TU, Federle W. 2011. Evidence for alternative trapping strategies in two forms of the pitcher plant, Nepenthes rafflesiana. Journal of Experimental Botany 62: 3683–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile V, Moran JA, Le Moguédec G, Marshall DJ, Gaume L. 2012. A carnivorous plant fed by its ant symbiont: a unique multi-faceted nutritional mutualism PloS ONE 7: e36179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron V, Bonn D, Martin JY, Vovelle L. 2000. Controlling droplet deposition with polymer additives. Nature 405: 772–775. [DOI] [PubMed] [Google Scholar]
- Biteau F, Bourgaud F, Gontier E, Fèvre J-P. 2008. Process for the production of recombinant proteins using carnivorous plants, WO/2008/040599. Nancy: Plant Advanced Technologies. [Google Scholar]
- Biteau F, Nisse E, Miguel S, et al. 2013. A simple SDS–PAGE protein pattern from pitcher secretions as a new tool to distinguish Nepenthes species (Nepenthaceae). American Journal of Botany 100: 2478–2484. [DOI] [PubMed] [Google Scholar]
- Bohn HF, Federle W. 2004. Insect aquaplanning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences USA 101: 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn H, Thornham D, Federle W. 2012. Ants swimming in pitcher plants: kinematics of aquatic and terrestrial locomotion in Camponotus schmitzi. Journal of Comparative Physiology A 198: 465–476. [DOI] [PubMed] [Google Scholar]
- Bonhomme V, Gounand I, Alaux C, Jousselin E, Barthélémy D, Gaume L. 2011a. The plant-ant Camponotus schmitzi helps its carnivorous host-plant Nepenthes bicalcarata to catch its prey. Journal of Tropical Ecology 27: 15–24. [Google Scholar]
- Bonhomme V, Pelloux-Prayer H, Jousselin E, Forterre Y, Labat J-J, Gaume L. 2011b. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytologist 191: 545–554. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Creelman RA. 1984. Mutualism between the carnivorous purple pitcher plant and its inhabitants. American Midland Naturalist 112: 294–304. [Google Scholar]
- Brodin YW, Gransberg M. 1993. Responses of insects, especially Chironomidae (Diptera), and mites to 130 years of acidification in a Scottish lake. Hydrobiologia 250: 201–212. [Google Scholar]
- Brundrett M. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320: 37–77. [Google Scholar]
- Buch F, Rott M, Rottloff S, et al. 2013. Secreted pitfall-trap fluid of carnivorous Nepenthes plants is unsuitable for microbial growth. Annals of Botany 111: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS. 1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233–260. [Google Scholar]
- Chou L, Clarke CM, Dykes G. 2014. Bacterial communities associated with the pitcher fluids of three Nepenthes (Nepenthaceae) pitcher plant species growing in the wild. Archives of Microbiology 196: 709–717. [DOI] [PubMed] [Google Scholar]
- Clarke CM. 1997. Nepenthes of Borneo . Kota Kinabalu: Natural History Publications. [Google Scholar]
- Clarke CM, Kitching RL. 1995. Swimming ants and pitcher plants: a unique ant-plant interaction from Borneo. Journal of Tropical Ecology 11: 589–602. [Google Scholar]
- Darwin C. 1875. Insectivorous plants . London: John Murray. [Google Scholar]
- Davidson DW, Patrell-Kim L. 1996. Tropical arboreal ants: why so abundant? In: Gibson AC, ed. Neotropical biodiversity and conservation. Los Angeles: University of California Press, 127–140. [Google Scholar]
- Di Giusto B, Grosbois V, Fargeas E, Marshall D, Gaume L. 2008. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. Journal of Biosciences 33: 121–136. [DOI] [PubMed] [Google Scholar]
- Di Giusto B, Guéroult M, Rowe N, Gaume L. 2009. The waxy surface in Nepenthes pitcher plants: variability, adaptive significance and developmental evolution. In: Gorb SN, ed. Functional surfaces in biology. Berlin: Springer, 183–203. [Google Scholar]
- Di Giusto B, Bessière J-M, Guéroult M, et al. 2010. Flower-scent mimicry masks a deadly trap in the carnivorous plant Nepenthes rafflesiana. Journal of Ecology 98: 845–856. [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Rahamim Y, et al. 2010. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany 61: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ, Brewer JS, et al. 2003. The evolutionary ecology of carnivorous plants. Advances in Ecological Research 33: 1–74. [Google Scholar]
- Gaume L, Di Giusto B. 2009. Adaptive significance and ontogenetic variability of the waxy zone in Nepenthes rafflesiana. Annals of Botany 104: 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Forterre Y. 2007. A viscoelastic deadly trap in Nepenthes pitcher plants. PloS ONE 2: e1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb SN, Rowe N. 2002. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist 156: 479–489. [DOI] [PubMed] [Google Scholar]
- Gaume L, Perret P, Gorb EV, Gorb SN, Labat J-J, Rowe N. 2004. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Structure and Development 33: 103–111. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. 1984. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. The American Naturalist 124: 479–497. [Google Scholar]
- Gorb EV, Baum MJ, Gorb SN. 2013. Development and regeneration ability of the wax coverage in Nepenthes alata pitchers: a cryo-SEM approach. Scientific Reports 3: 3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda DC, Reuter G, Schauer R. 1982. Structural features of an acidic polysaccharide from the mucin of Drosera binata. Phytochemistry 21: 2297–2300. [Google Scholar]
- Grafe TU, Schöner CR, Kerth G, Junaidi A, Schöner MG. 2011. A novel resource-service between bats and pitcher plants. Biology Letters 7: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JF. 2001. Insect acid-base physiology. Annual Review of Entomology 46: 221–250. [DOI] [PubMed] [Google Scholar]
- Hatano N, Hamada T. 2008. Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. Journal of Proteome Research 7: 809–816. [DOI] [PubMed] [Google Scholar]
- Holdgate MW. 1955. The wetting of insect cuticles by water. Journal of Experimental Biology 32: 591–617. [Google Scholar]
- Juniper BE, Burras J. 1962. How pitcher plants trap insects. New Scientist 13: 75–77. [Google Scholar]
- Juniper BE, Robins RJ, Joel D. 1989. The carnivorous plants . London: Academic Press. [Google Scholar]
- Lloyd FE. 1942. The carnivorous plants . Waltham: Chronica Botanica Co. [Google Scholar]
- Matthews RAJ. 1995. Tumbling toast, Murphy’s Law and the fundamental constants. European Journal of Physics 16: 172–176. [Google Scholar]
- Matusikova I, Libantova J, Moravcikova J, Mlynarova L, Nap JPH. 2004. The insectivorous sundew (Drosera rotundifolia, L.) might be a novel source of PR genes for biotechnology. Biologia Bratislava 56: 719–725. [Google Scholar]
- Mogi M, Yong HS. 1992. Aquatic arthropod communities in Nepenthes pitchers: the role of niche differentiation, aggregation, predation and competition in community organization. Oecologia 90: 172–184. [DOI] [PubMed] [Google Scholar]
- Moran JA. 1996. Pitcher dimorphism, prey composition and the mechanism of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology 84: 515–525. [Google Scholar]
- Moran JA, Booth WE, Charles JK. 1999. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implications for prey capture. Annals of Botany 83: 521–528. [Google Scholar]
- Moran JA, Clarke CM, Hawkins BJ. 2003. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. International Journal of Plant Science 164: 635–639. [Google Scholar]
- Moran JA, Hawkins BJ, Gowen BE, Robbins SL. 2010. Ion fluxes across the pitcher walls of three Bornean Nepenthes pitcher plant species: flux rates and gland distribution patterns reflect nitrogen sequestration strategies. Journal of Experimental Botany 61: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JA, Gray LK, Clarke CM, Chin L. 2013. Capture mechanism in palaeotropical pitcher plants (Nepenthaceae) is constrained by climate. Annals of Botany 112: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. 2008. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany 102: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen TP, Lennon KA. 1999. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). American Journal of Botany 86: 1382–1390. [PubMed] [Google Scholar]
- Pavlovič A. 2012. Adaptive radiation with regard to nutrient sequestration strategies in the carnivorous plants of the genus Nepenthes. Plant Signaling & Behavior 7: 295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Slováková Lu, Šantrůček J. 2011. Nutritional benefit from leaf litter utilization in the pitcher plant Nepenthes ampullaria. Plant, Cell & Environment 34: 1865–1873. [DOI] [PubMed] [Google Scholar]
- Perez-Goodwyn P. 2009. Anti-wetting surfaces in heteroptera (Insecta): hairy solutions to any problem. In: Gorb SN, ed. Functional surfaces in biology: little structures with big effects . Berlin: Springer, 55–76. [Google Scholar]
- Raj G, Kurup R, Hussain AA, Baby S. 2011. Distribution of naphthoquinones, plumbagin, droserone, and 5-O-methyl droserone in chitin-induced and uninduced Nepenthes khasiana: molecular events in prey capture. Journal of Experimental Botany 62: 5429–5436. [DOI] [PubMed] [Google Scholar]
- Rost K, Schauer R. 1977. Physical and chemical properties of the mucin secreted by Drosera capensis. Phytochemistry 16: 1365–1368. [Google Scholar]
- Scala J, Iott K, Schwab DW, Semersky FE. 1969. Digestive secretion of Dionaea muscipula (Venus’s-flytrap). Plant Physiology 44: 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze WX, Sanggaard KW, Kreuzer I, et al. 2012. The protein composition of the digestive fluid from the venus flytrap sheds light on prey digestion mechanisms. Molecular & Cellular Proteomics 11: 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak L, Gibbs KE. 1991. Effects of aluminum, calcium and low pH on egg hatching and nymphal survival of Cloeon triangulifer McDunnough (Ephemeroptera: Baetidae). Hydrobiologia 218: 157–166. [Google Scholar]
- Takeuchi Y, Salcher MM, Ushio M, et al. 2011. In situ enzyme activity in the dissolved and particulate fraction of the fluid from four pitcher plant species of the genus Nepenthes. PLoS ONE 6: e25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. 1910. The proteolytic enzyme of Drosera. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character83: 134–139. [Google Scholar]
- Wong T-S, Kang SH, Tang SKY, et al. 2011. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477: 443–447. [DOI] [PubMed] [Google Scholar]