Abstract
The largest genetic susceptibility factor for Alzheimer’s disease is the Apolipoprotein E (ApoE) ε4 allele. Cognitive decline and olfactory impairment are greater in those positive for the ε4 allele. This study sought to determine if the olfactory event-related potential (OERP), compared to the visual ERP, would be sensitive to these subtle declines. Participants included 40 individuals from two age groups, half of each group were ε4 allele positive and half were ε4 negative. Visual ERPs did not demonstrate significant differences between ApoE groups. OERPs demonstrated robust age by ApoE interactions. P3 latencies were significantly longer in ε4 young and middle age participants. These findings suggest that very early olfactory and cognitive changes related to ApoE status are detectible via the OERP.
Keywords: Age, Event-related potentials, P3, Visual, Olfaction, Smell, Smell Impairment
1. Introduction
As individuals age, their ability to detect odors declines (for review see Murphy, 1983, 1993, 2008; Schiffman, 1993) and these age-related declines are related to simultaneous declines in cognition (Dulay and Murphy, 2002; Geisler et al., 1999; Larsson et al., 2005). Impairments in odor identification and odor threshold precede noticeable changes in cognitive functioning such as memory (Bacon et al., 1998; Calhoun-Haney and Murphy, 2005; Morgan et al., 1995; Murphy et al., 1998; Olofsson et al., 2009; Serby, 1986). Overall health status decreases as olfactory thresholds increase (Griep et al., 1995) and elderly persons with olfactory dysfunction report significant decreases in quality of life (Miwa et al., 2001). Olfactory thresholds also correlate with degree of dementia (Murphy et al., 1990). Increased olfactory impairment in those with Alzheimer’s disease (AD) and those at risk for AD may be due to physiological changes during early stages of the disease, specifically in areas involved in olfactory processing such as the olfactory bulb, anterior olfactory nucleus, parahippocampal gyrus, hippocampus, amygdala, and orbitalfrontal, insular and temporal neocortex (Averback, 1983; Braak and Braak, 1997, Christen-Zaech, et al., 2003; Esiri and Wilcock, 1984; Getchell et al., 2003; Ohm and Braak, 1987; Price 1993, 1997; Price et al., 1991; Reyes et al., 1985; Struble and Clark, 1992).
The Apolipoprotein E (ApoE) ε4 allele is at present the greatest genetic susceptibility factor for the sporadic and late-onset familial variants of AD (Combarros et al., 2002; Teter et al., 2002). Carriers of the ε4 allele are not only at risk for the familial variant of AD, but also for the sporadic variant (Saunders et al., 1993). Individuals positive for the ε4 allele have an increased rate of cognitive decline compared to those without the ε4 allele (Gilbert and Murphy, 2004; Olofsson et al., 2009; Small, Basun, and Bäckman, 1998; Swan et al., 2005). The ε4 allele in conjunction with measures of olfactory impairment enhance the prediction of cognitive decline. In fact, tasks requiring odor identification have demonstrated improved prediction of cognitive decline over traditional global cognitive functioning tests (Graves et al., 1999; Morgan et al., 1995). Before cognitive functions, such as memory show impairment, participants positive for the ε4 allele experience decreased ability to identify odors compared to persons without the ε4 allele (Handley et al., 2006, Morgan et al., 1995; Murphy et al., 1998).
ERP studies in the auditory and visual modalities have demonstrated that as age increases P3 amplitudes decrease and latencies increase (Fjell et al., 2004; Pfefferbaum et al., 1984; Picton et al., 1984; Polich, 1991, 1997). OERPs have demonstrated similar results and shown robust decreases in peak amplitudes and increased latencies with age (Morgan et al., 1997; Murphy et al., 1994b, 2000; Nordin et al., 1999). OERPs studies in patients with AD have demonstrated larger differences in OERP latency measures than auditory ERP measures (Morgan and Murphy, 2002). OERP latency effect sizes are equal to, if not larger than those of traditional olfactory tests in differentiating those with AD from normal controls (Morgan and Murphy, 2002).
Although individuals with mild cognitive impairment (MCI) show abnormal event-related potentials (Olichney et al., 2002) and recent studies have suggested the potential for ERPs to predict progression from MCI to AD (Chapman et al., 2010, in press; Missionnier et al., 2007; Olichney et al., 2008), little ERP research has been reported on the effects of ApoE status on ERPs. Green and Levey (1999) demonstrated that individuals with multiple risk factors for Alzheimer’s disease, such as family history and the ε4 allele, produce significantly longer auditory N2 and P3 latencies than those with family history of AD but no ε4 allele. Reinvang et al. (2005) demonstrated attenuated auditory peak amplitudes in participants with the ε4 allele than those without the allele. In non-demented older adults, the ε4 allele is associated with significantly longer olfactory peak latencies (Wetter and Murphy, 2001). Furthermore, the same study demonstrated that OERPs are more sensitive than traditional olfactory behavioral measures and have higher specificity than auditory ERPs. Murphy et al. (2009) also described longer P3 latencies in ε4 positive older adults in cross-modal odor-recognition ERPs. ApoE ε4 genetic testing and OERPs combined have the potential to increase risk assessment accuracy at a point significantly earlier than other symptoms of cognitive impairment become apparent.
2. Hypotheses
We hypothesized that 1. OERP P3 peak latencies would increase with age. 2. That ApoE ε4 positive participants would show longer OERP peak latencies throughout the lifespan compared to ε4 negative participants. 3. That this difference would increase with age.
We hypothesized that OERP amplitudes would decrease with age, with a large effect for P3 amplitude. It was also expected that the ApoE ε4 positive participants would have significantly smaller peak amplitudes than those negative for the allele.
3. Methods
3.1 Participants
40 participants (20 male, 20 female) participated in the study. They were equally stratified across three different age groups of young (18–28 years old) and middle age (45–56 years old). Half of each participant group were heterozygous for the ε4 allele and experimenters were blinded to ApoE status throughout the experiment. None of the participants were homozygous for the allele. Participants were recruited from the San Diego community and from an ongoing participant pool at the Lifespan Human Senses Laboratory. Exclusionary criteria for this study included butanol thresholds below four (maximum odor sensitivity 9: Cain, et al., 1983, Murphy et al., 1990) and a Dementia Rating Scale (DRS) total score below 129 (Mattis, 1976). The DRS was utilized to ensure that all participants were free of symptoms consistent with early dementia and were within normal limits of cognitive functioning. No participants had DRS scores below 129. Participants were screened for overall nasal health with an in-house questionnaire that included questions regarding overall medical history and specifically history of nasal problems, allergies, loss of smell or taste, etc. Participants were further screened for olfactory dysfunction with the butanol threshold test. Individuals with recent head trauma, upper respiratory infections, severe allergies, or taking antipsychotic medications were screened out of the study (Harris et al., 2006).
3.2 Procedure
In the first session, DNA samples were collected from each participant using a buccal swab to collect genetic material to assay ApoE status. Chemosensory tests included the butanol odor threshold (Cain, et al., 1983; Murphy et al., 1990), and the San Diego Odor Identification Test (SDOIT; Morgan et al., 1995; Murphy et al., 1994a, 2002). Memory and cognitive functioning tests consisted of the Mini-Mental State Exam (MMSE: Folstein et al., 1975) and Dementia Rating Scale (DRS: Mattis, 1976). Olfactory and visual event-related potentials were recorded in separate experiments in the second session. For the visual task, a 76 × 76 mm blue square with a gray background appeared on a computer screen for 200 milliseconds. The olfactory stimulus was amyl acetate presented for 200 milliseconds. While this may appear to be a simple reaction time task, especially for the visual task, a 200 millisecond presentation of a visual stimulus is actually rather difficult, especially given that the subject did not know when in time each stimulus would be presented. Should the subject happen to blink at the same time the stimulus was presented they would miss the stimulus. As such, we did not expect response accuracy to be 100%. Both odor and visual stimuli were presented utilizing a single stimulus paradigm. There are numerous examples in the literature that have implemented the single stimulus paradigm to test ERP hypotheses in auditory, visual, and olfactory research (e.g., Mertens and Polich, 1997, Morgan et al., 1997, 1999; Polich and Heine, 1996; Thesen and Murphy 2001). For OERPs the single stimulus paradigm is especially viable in order to limit the rapid adaptation and habituation of the olfactory system. Olfactory stimuli were delivered by an olfactometer based on previous olfactometer designs (Kobal and Hummel, 1988; Lorig, 2000; Lorig et al., 1999; Morgan et al., 1999; Murphy et al., 1994b). The olfactometer canula remained in the participant’s nostril throughout the OERP experiment. Nostril chosen for stimulation was the nostril identified by the participant as having the best airflow at the time of the experiment. Total airflow from the olfactometer through the canula was 3.75 L/min and the mixture of odor flow to clean air (odor dilution) during stimulation was 80%. Room temperature air was humidified to 80% relative humidity. Stimulus rise time was less than 20 msec. Sensory modality was counterbalanced across participants by alternating the order of experiments between olfaction and visual modalities where some subjects received the visual experiment first and others the olfactory experiment first. Stimuli were presented twenty times with an inter-stimulus interval (ISI) of thirty seconds of clean air or a blank screen presented during the ISI. Participants were instructed to press a response pad button when they detected the visual or olfactory stimulus.
EEG recordings were collected through 64 electrodes following the 10–20 system. All electrodes had an impedance of 10 Ω or less. Ocular artifacts were recorded with two electrodes placed supra- and subocularly at the left eye, and one electrode at each outer canthus. EEG recordings were referenced to linked earlobe electrodes.
During data collection, a filter of DC to 32 Hz was used. A band pass filter was applied to the data with a high of 0.1 and a low of 9. Eyeblink activity was corrected for, using a Neuroscan algorithm calculated from the four ocular electrodes to systematically subtract the eyeblink artifacts from the other electrode channels. Continuous recordings were epoched 500 ms before to 1500 ms after the stimulus. Individual trials that still contained artifactual activity (e.g., motor movement) were rejected and not included in the averages, and only trials for which the participant correctly detected the stimulus were included in the averages. Contingent negative variation was not an issue because the participants received no warning prior to the onset of each stimulus and they were not told the length of the interstimulus interval. All remaining trials were averaged for each participant and condition. Latency windows from previous OERP studies (e.g. Morgan et al., 1997, 1999) were used to manually identify peak component windows for each subject, then the Neuroscan software selected the largest ERP component peaks within those windows, outputting the values into a database for analysis.
4. Results
Table 1 presents mean and standard deviations for behavioral and performance measures. Statistical analyses demonstrated no significant age differences between ApoE positive and negative participants within each age group (p>.05). Education was not significantly different across young and middle age groups (, p>.05). DRS scores, butanol thresholds, and Odor ID scores did not differ between age groups, or ApoE groups, and there were no interaction effects (p>.05). Analysis of ERP performance variables (reaction times, number of trials in averages, and percent correct trials) revealed no significant main effects or interaction effects between age and ApoE groups (p>.05). While there was a 7.5% difference in percent correct trials between middle age ApoE negative and middle age ApoE positive participants, this represents a difference of only one trial, which was not statistically significant. As such, both ApoE negative and positive groups demonstrated no significant difference in vigilance to or performance of the task.
Table 1.
Means and Standard Deviations for demographic, behavioral and performance data for each age and ApoE group.
| ApoE Negative | ApoE Positive | |||
|---|---|---|---|---|
|
| ||||
| Young | Middle | Young | Middle | |
| Age (years) | 23.9 (2.8) | 50.6 (2.4) | 22.2 (2.0) | 49.9 (3.2) |
| Education (years) | 16.6 (2.6) | 14.5 (2.6) | 15.2 (1.8) | 14.9 (2.4) |
| Dementia Rating Scale Score (144 max) | 142.2 (2.0) | 138.5 (5.4) | 140.2 (3.1) | 140.8 (4.1) |
| Odor Threshold (dilution steps, 9 max) | 7.7 (1.2) | 6.0 (1.5) | 7.3 (1.5) | 7.2 (1.6) |
| Odor Identification Test (6 max) | 5.5 (0.7) | 5.4 (0.8) | 5.7 (0.5) | 4.4 (1.4) |
| Visual ERP Reaction Time (msec) | 316 (33) | 413 (177) | 360 (101) | 459 (107) |
| Visual ERP % Correct Trials | 89.0% | 89.5% | 96.0% | 91.0% |
| Visual ERP # Trials in Average (20 max) | 12.1 (4.5) | 10.5 (4.3) | 14.6 (3.6) | 13.4 (3.6) |
| Olfactory ERP Reaction Time (msec) | 1824 (852) | 1752 (862) | 1625 (379) | 2169 (719) |
| Olfactory ERP % Correct Trials | 79.5% | 77.5% | 77.0% | 70.0% |
| Olfactory ERP # Trials in Average (20 max) | 11.0 (3.4) | 10.9 (4.5) | 12.1 (3.1) | 10.3 (2.8) |
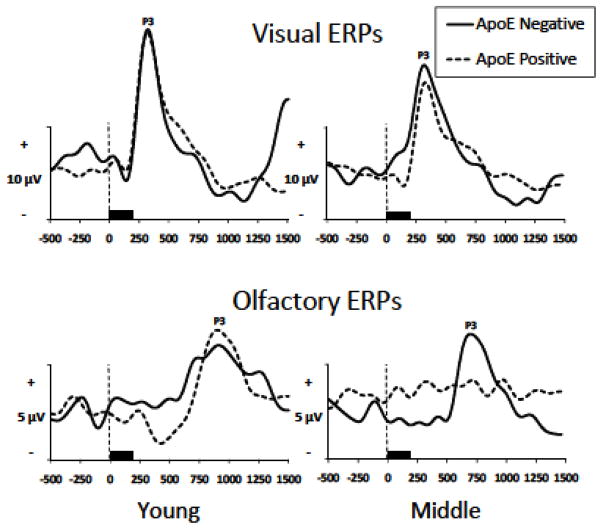
Consistent with previous OERP research, ERP measures included P3 latencies and amplitudes for both visual and olfactory modalities. Repeated measures multivariate analyses of variance (MANOVAs) were performed for each ERP component and each stimulus modality separately by amplitude and latency. The Pz electrode site was analyzed, as this electrode consistently demonstrates reliable OERP responses (Thesen and Murphy, 2002), and in many studies demonstrates larger peak amplitudes than more lateral electrode sites (Morgan et al., 1997, 2010; Murphy et al., 1994b, 2000; Nordin et al., 1999; Olofsson et al., 2006; Thesen and Murphy, 2002). Greenhouse-Geisser corrections were applied to all MANOVAs. Grand averaged visual and olfactory ERPs are displayed in Figure 1.
Fig. 1.
Grand averaged olfactory and visual ERPs by age and ApoE groups at the Pz electrode site (40 participants, n=10 per age/ApoE group).
4.1 Amplitude
P3 visual amplitude exhibited a significant main effect of age group (F(1,36)=5.19, p<.05, η2 =.13) with young participants producing significantly larger visual P3 amplitudes than middle age participants. Olfactory P3 amplitude demonstrated a significant main effect of age (F(1,36)=7.96, p<.01, η2 =.18) such that young participants produced significantly larger P3 amplitudes than middle age participants. There were no significant effects of ApoE status on visual or olfactory amplitude (p<.05), and ApoE status did not interact with age (p<.05)
4.2 Latency
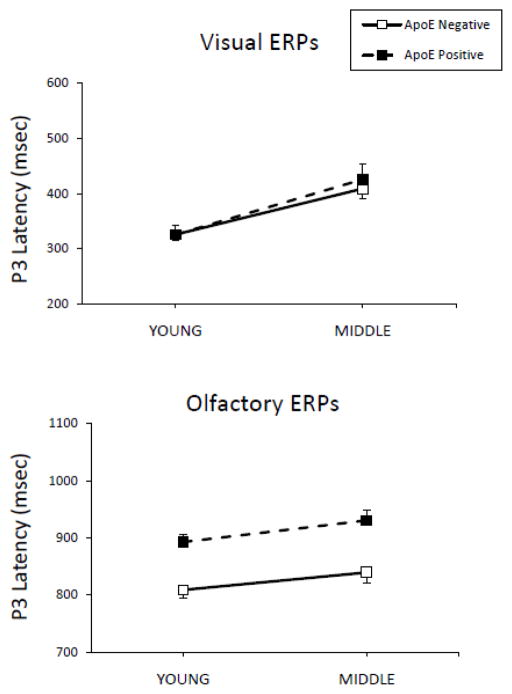
Figure 2 illustrates average visual peak latencies for P3 by age group. Visual latency demonstrated significant effects of age (F(1,36)=21.44, p<.001, η2=.37). Young participants produced significantly shorter peak latencies than middle age subjects for visual P3 (p<.05). OERP latencies in the present study were consistent with latencies found in previous OERP studies. There were significant effects of ApoE status for P3 latency collapsed across age (F(1,36)=21.91, p<.001, η2=.38), with ApoE ε4 negative participants demonstrating shorter latencies than ApoE ε4 positive participants. The age by ApoE status interaction for olfactory latency did not reach significance, however in order to better understand the possible effects of ApoE status in each age group, analyses were performed on young and middle age participant groups separately. Olfactory P3 latency in young subjects was significant with ε4 positive participants producing longer P3 latencies than ε4 negative participants (p<.05, η2=.31). For middle age participants analysis demonstrated longer P3 latencies (p<.01, η2=.47) for ε4 positive participants compared to ε4 negative participants.
Fig. 2.
Visual and olfactory average P3 latencies by age and ApoE group. Error bars represent the standard error of the mean (SEM).
4.5 Logistic Regression Analyses of OERP Variables
In order to better understand the effects of ApoE status on each component of the OERP stepwise logistic regression analyses were performed including P3 amplitudes and latencies. Logistic regressions were performed for each age group separately in order to better understand the ApoE effects at each age. In the young group olfactory P3 latency was the most significant predictor (χ2=7.69, p<.01) resulting in overall classification rate of 75% (Sensitivity=80%, Specificity=70%). In the middle age group olfactory P3 latency was also the most significant predictor (χ2=12.54, p<.001) resulting in overall classification rate of 80% (Sensitivity=80%, Specificity=80%).
5. Discussion
Previous studies have demonstrated significant differences in olfactory threshold and odor identification between age groups and between elderly ApoE ε4 positive and negative individuals (Bacon et al., 1998; Calhoun-Haney and Murphy 2005; Handley et al., 2006; Laakso et al., 2009; Murphy, 1993; Murphy et al., 1998; Olofsson et al., 2010; Schiffman, 1993; Wang et al., 2002). The current study demonstrated no significant differences in odor threshold or odor identification scores in young or middle age groups. The screening out of those without normal olfactory thresholds and odor identification resulted in high functioning adults participating in the current study. As such, significant differences exhibited in the ERPs measures are even more telling.
Consistent with previous studies (Fjell et al., 2004; Pfefferbaum et al., 1984; Picton et al., 1984; Polich, 1991, 1997), the current study demonstrated increased P3 visual peak latencies with age. No published studies have reported ApoE effects on the visual ERP, so it is not surprising that most ApoE visual effects also did not reach significance in the present study. Overall the minimal amount of significant visual findings would suggest that ApoE status has minimal effect on the processing of visual stimuli in the brain, at least as measured by ERPs.
In contrast, the present study demonstrated very robust findings for olfactory ERPs, not only for age, but also for ApoE status and interactions between age and ApoE status. This may suggest that presence of the ApoE ε4 allele affects the olfactory processing areas in the brain more so than visual processing areas. The most salient new findings in the current study are differences in the olfactory ERP in the middle age and young groups dependent on ApoE status. Additionally, significant differences in P3 latency were demonstrated in young ApoE positive participants suggesting very mild changes in speed of the cognitive processing of olfactory stimuli are present even in persons in their early 20s. This suggests that subtle cognitive declines associated with the ApoE ε4 allele and expressed in the brain activity captured in the OERP may occur much earlier than previously thought. Overall the present study suggests that the presence of the ApoE ε4 allele has differing effects in different age groups. In young participants similar amplitudes in ApoE positive and negative participants would suggest similar ability to attend to and process a stimulus (Donchin et al., 1984; Polich 1998), while slower latencies in young ApoE positive participants would suggest slightly slower cognitive processing speed (Magliero et al., 1984). In middle age, ApoE positive participants not only process olfactory stimuli slower than ApoE negative individuals (latency), but also demonstrate decreased ability to attend to and process the stimulus (amplitude).
One limitation of the current study is the number of ERP trials obtained for each participant. While this was consistent across groups it does affect the signal to noise ratio, leading to less visually clean waveforms, especially when displaying grand averages. Given the nature of the olfactory system, compared to other sensory systems, including slower stimulus processing times and quicker adaptation/habituation of the neurons, OERPs necessitate longer ISIs than other modalities. OERP stimuli can only be presented every 30–45 seconds compared to every 2–3 seconds with other modalities. Longer ISIs increase the length of testing sessions and the longer the testing session, the more increase in participant fatigue. Participant fatigue can affect vigilance to and correct performance of the task. In order to balance these effects and to increase the difficulty and demands on the olfactory processing system, in the present study we utilized a shorter ISI (30 seconds) than in previous studies. We correctly hypothesized that by increasing the demands on the olfactory system, especially in participants susceptible to Alzheimer’s disease due to the ApoE ε4 allele, that we might be able to demonstrate previously unseen olfactory processing deficits. As a result of the increased demands and difficulty of the task, the number of usable trials was less than would be obtained for another modality. Even so, significant and robust results were obtained demonstrating previously unseen deficits in middle age ApoE+ participants.
Another limitation is the difficulty of seeking to display the current group differences via a grand averaged OERP graph. Data used in statistical analyses are taken from each participant’s own averaged waveforms (not from the grand average). When individual EPRs are averaged together to create a grand average, some details are lost. Given latency differences between each participant within the ApoE groups, especially the ApoE positive group, a grand average does not well capture actual differences that exist between groups.
In conclusion, the OERP appears to be sensitive to very subtle changes in the brain associated with the ApoE ε4 allele, even at much younger ages than previously demonstrated. Additionally, the OERP appears to be more sensitive to these changes than traditional tests of olfactory functioning. Future research should investigate ApoE ε4 positive individuals in young and middle adulthood in longitudinal studies to fully characterize the nature of neuropsychological deficits, to investigate cerebrovascular lesion load, and to better determine at what age and at what speed these subtle olfactory and cognitive declines take place.
Highlights.
Olfactory event-related potential (OERP) response discriminated ε4+ from ε4− adults
OERPs demonstrated robust effects of the ApoE ε4 allele on P3
Subtle cognitive declines associated with the ε4 allele and captured in the OERP may occur very early
Acknowledgments
Supported by NIH Grants DC02064-14 and AG04085-24 to Claire Murphy. The authors would like to thank the late Dr. Leon Thal and the UCSD ADRC (P50AG005131-28) for genotyping, Dr. John Polich, Joel Kowalewski, Jessica Bartholow, Dr. Barbara Cerf-Ducastel, Roberto Zamora, Richard Vail and Lori Haase for research assistance.
Footnotes
Conflict of interest statement
The authors have no actual or potential conflicts of interest associated with this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Averback P. 2 New Lesions in Alzheimers-Disease. Lancet. 1983;2(8360):1203. doi: 10.1016/s0140-6736(83)91256-4. [DOI] [PubMed] [Google Scholar]
- Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–31. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Cain WS, Gent J, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. Am J Otolaryngol. 1983;4(4):252–6. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Calhoun-Haney R, Murphy C. Apolipoprotein epsilon 4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cognition. 2005;58(2):178–82. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Chapman RM, McCrary JW, Gardner MN, Sandoval TC, Guillily MD, Reilly LA, DeGrush E. Brain ERP components predict which individuals progress to Alzheimer’s disease and which do not. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.010. doi:10:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, Miklossy J. Early olfactory involvement in Alzheimer’s disease. Can J Neurol Sci. 2003;30(1):20–25. doi: 10.1017/s0317167100002389. [DOI] [PubMed] [Google Scholar]
- Combarros O, Alvarez-Arcaya A, Sanchez-Guerra M, Infante J, Berciano J. Candidate gene association studies in sporadic Alzheimer’s disease. Dement Geriatr Cogn. 2002;14(1):41–54. doi: 10.1159/000058332. [DOI] [PubMed] [Google Scholar]
- Donchin E, Heffley E, Hillyard SA, Loveless N, Maltzman I, Ohman A, Rosler F, Ruchkin D, Siddle D. Cognition and event-related potentials. II. The orienting reflex and P300. Ann N Y Acad Sci. 1984;425:39–57. doi: 10.1111/j.1749-6632.1984.tb23522.x. [DOI] [PubMed] [Google Scholar]
- Dulay MF, Murphy C. Olfactory acuity and cognitive function converge in older adulthood: Support for the common cause hypothesis. Psychology and Aging. 2002;17(3):392–404. [PubMed] [Google Scholar]
- Esiri MM, Wilcock GK. The Olfactory Bulbs in Alzheimers-Disease. J Neurol Neurosur Ps. 1984;47(1):56–60. doi: 10.1136/jnnp.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Life-span changes in P3a. Psychophysiology. 2004;41(4):575–83. doi: 10.1111/j.1469-8986.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geisler MW, Morgan CD, Covington JW, Murphy C. Neuropsychological performance and cognitive olfactory event-related brain potentials in young and elderly adults. J Clin Exp Neuropsyc. 1999;21(1):108–26. doi: 10.1076/jcen.21.1.108.935. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Peng XJ, Stromberg AJ, Chen KC, Green CP, Subhedar NK, Shah DS, Mattson MP, Getchell ML. Age-related trends in gene expression in the chemosensory-nasal mucosae of senescence-accelerated mice. Ageing Res Rev. 2003;2(2):211–43. doi: 10.1016/s1568-1637(02)00066-1. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheimer’s disease. Exp Gerontol. 2004;39(3):433–41. doi: 10.1016/j.exger.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline - Interaction with apolipoprotein E epsilon 4 status. Neurology. 1999;53(7):1480–7. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Green J, Levey AI. Event-related potential changes in groups at increased risk for Alzheimer disease. Arch Neurol-Chicago. 1999;56(11):1398–403. doi: 10.1001/archneur.56.11.1398. [DOI] [PubMed] [Google Scholar]
- Griep MI, Mets TF, Vercruysse A, Cromphout I, Ponjaert I, Toft J, Massart DL. Food Odor Thresholds in Relation to Age, Nutritional, and Health-Status. J Gerontol a-Biol. 1995;50(6):B407–B14. doi: 10.1093/gerona/50a.6.b407. [DOI] [PubMed] [Google Scholar]
- Handley OJ, Morrison CM, Miles C, Bayer AJ. ApoE gene and familial risk of Alzheimer’s disease as predictors of odour identification in older adults. Neurobiology of Aging. 2006;27(10):1425–30. doi: 10.1016/j.neurobiolaging.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Harris R, Davidson TM, Murphy C, Gilbert PE, Chen M. Clinical evaluation and symptoms of chemosensory impairment: 1000 consecutive cases from the Nasal Dysfunction Clinic in San Diego. Am J Rhinology. 2006;20:101–108. [PubMed] [Google Scholar]
- Kobal G, Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol. 1988;71(4):241–50. doi: 10.1016/0168-5597(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Tervo S, Hanninen T, Vanhanen M, Hallikainen M, Soininen H. Olfactory identification in non-demented elderly population and in mild cognitive impairment: a comparison of performance in clinical odor identification versus Boston Naming Test. J Neural Transm. 2009;116(7):891–5. doi: 10.1007/s00702-009-0235-8. [DOI] [PubMed] [Google Scholar]
- Larsson M, Oberg CN, Backman L. Odor identification in old age: demographic, sensory and cognitive correlates. Aging, Neuropsychology, and Cognition. 2005;12:231–44. doi: 10.1080/138255890968385. [DOI] [PubMed] [Google Scholar]
- Lorig TS. The application of electroencephalographic techniques to the study of human olfaction: a review and tutorial. Int J Psychophysiol. 2000;36(2):91–104. doi: 10.1016/s0167-8760(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Lorig TS, Elmes DG, Zald DH, Pardo JV. A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behav Res Methods Instrum Comput. 1999;31(2):370–5. doi: 10.3758/bf03207734. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore TR, Coles MG, Donchin E. On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology. 1984;21(2):171–86. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Katasu TB, editors. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. Grune and Statton; New York: 1976. pp. 77–121. [Google Scholar]
- Mertens R, Polich J. P300 from a single-stimulus paradigm: passive versus active tasks and stimulus modality. Electroencephalogr Clin Neurophysiol. 1997;104:488–497. doi: 10.1016/s0168-5597(97)00041-5. [DOI] [PubMed] [Google Scholar]
- Missionnier P, Deiber MP, Gold G, Herrmann FR, Millet P, Michon A, Fazio-Costa L, Ibanez V, Giannakopouos P. Working memory load-related electroencephalographic parameters can differential progressive from stable mild cognitive impairment. Neuroscience. 2007;150:346–356. doi: 10.1016/j.neuroscience.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Miwa T, Furukawa M, Tsukatani T, Costanzo RM, DiNardo LJ, Reiter ER. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol. 2001;127(5):497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Covington JW, Geisler MW, Polich J, Murphy C. Olfactory event-related potentials: older males demonstrate the greatest deficits. Electroencephalogr Clin Neurophysiol. 1997;104(4):351–8. doi: 10.1016/s0168-5597(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Geisler MW, Covington JW, Polich J, Murphy C. Olfactory P3 in young and older adults. Psychophysiology. 1999;36(3):281–7. doi: 10.1017/s0048577299980265. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Murphy C. Olfactory event-related potentials in Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8(6):753–63. doi: 10.1017/s1355617702860039. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Murphy C. Differential effects of active attention and age on event-related potentials to visual and olfactory stimuli. Int J Psychophysiol. 2010;78(2):190–199. doi: 10.1016/j.ijpsycho.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Nordin S, Murphy C. Odor identification as an early marker for Alzheimer’s disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol. 1995;17(5):793–803. doi: 10.1080/01688639508405168. [DOI] [PubMed] [Google Scholar]
- Murphy C. Age-Related Effects on the Threshold, Psychophysical Function, and Pleasantness of Menthol. Journals of Gerontology. 1983;38(2):217–22. doi: 10.1093/geronj/38.2.217. [DOI] [PubMed] [Google Scholar]
- Murphy C. Senescence and clinical changes in the olfactory system: Psychological considerations [NIDCD Monograph] Development, Growth and Senescence in the Chemical Senses. 1993;3:153–60. [Google Scholar]
- Murphy C. The chemical senses and nutrition in the elderly. J Nutri for the Elderly. 2008;27:247–65. doi: 10.1080/01639360802261862. [DOI] [PubMed] [Google Scholar]
- Murphy C, Anderson JA, Markison S. Psychophysical assessement of chemosensory disorders in clinical populations. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and Taste XI. Springer-Verlag; Tokyo: 1994a. pp. 609–13. [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–50. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol Aging. 1990;11(4):465–9. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Morgan CD, Geisler MW, Wetter S, Covington JW, Madowitz MD, Nordin S, Polich JM. Olfactory event-related potentials and aging: normative data. Int J Psychophysiol. 2000;36(2):133–45. doi: 10.1016/s0167-8760(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, de Wijk RA, Cain WS, Polich J. Olfactory-evoked potentials: assessment of young and elderly, and comparison to psychophysical threshold. Chem Senses. 1994b;19(1):47–56. doi: 10.1093/chemse/19.1.47. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal Amer Med Assoc (JAMA) 2002;288(18):2307–12. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Murphy C, Solomon ES, Haase L, Wang MR, Morgan CD. Olfaction in Aging and Alzheimer’s Disease Event-related Potentials to a Cross-modal Odor-Recognition Memory Task Discriminate ApoE epsilon 4(+) and ApoE epsilon 4(−) Individuals. International Symposium on Olfaction and Taste. 2009;1170:647–57. doi: 10.1111/j.1749-6632.2009.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin S, Quinonez C, Morgan CD, Geisler MW, Polich J, Murphy C. Olfactory event-related potentials in young and elderly adults: Evaluation of tracking task versus eyes open/closed recording. Chem Senses. 1999;24(4):459–64. doi: 10.1093/chemse/24.4.459. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Braak H. Olfactory-Bulb Changes in Alzheimers-Disease. Acta Neuropathol. 1987;73(4):365–9. doi: 10.1007/BF00688261. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iraqui VJ. Abnormal verbal event-related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iraqui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Broman DA, Gilbert PE, Dean P, Nordin S, Murphy C. Laterality of the olfactory event-related potential response. Chem Senses. 2006;31:699–704. doi: 10.1093/chemse/bjl011. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-epsilon 4 is independent of clinical dementia. Neurobiology of Aging. 2010;31(4):567–77. doi: 10.1016/j.neurobiolaging.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical-Application of the P3 Component of Event-Related Potentials .1. Normal Aging. Electroen Clin Neuro. 1984;59(2):85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Champagne SC, Nelson RF. The Effects of Age on Human Event-Related Potentials. Psychophysiology. 1984;21(3):312–25. doi: 10.1111/j.1469-8986.1984.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 in the evaluation of aging and dementia. In: Brunia CHM, GM, Verbaten MN, editors. Event-related brain potential research (EEG Suppl) Elsevier; Amsterdam: 1991. pp. 304–23. [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Evoked Potential. 1997;104(3):244–56. doi: 10.1016/s0168-5597(97)96139-6. [DOI] [PubMed] [Google Scholar]
- Polich J, Heine M. P300 topography and modality effects from a single-stimulus paradigm. Psychophysiology. 1996;33:747–752. doi: 10.1111/j.1469-8986.1996.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Hoffman LD. P300 and handedness: on the possible contribution of corpus callosal size to ERPs. Psychophysiology. 1998;35(5):497–507. doi: 10.1017/s0048577298970792. [DOI] [PubMed] [Google Scholar]
- Price JL. The Relationship between Tangle and Plaque-Formation during Healthy Aging and Mild Dementia. Neurobiology of Aging. 1993;14(6):661–3. doi: 10.1016/0197-4580(93)90062-g. [DOI] [PubMed] [Google Scholar]
- Price JL. Diagnostic criteria for Alzheimer’s disease. Neurobiology of Aging. 1997;18(4):S67–S70. doi: 10.1016/s0197-4580(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The Distribution of Tangles, Plaques and Related Immunohistochemical Markers in Healthy Aging and Alzheimers-Disease. Neurobiology of Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Gjerstad L. Cognitive ERPs are related to ApoE allelic variation in mildly cognitively impaired patients. Neuroscience Letters. 2005;382(3):346–51. doi: 10.1016/j.neulet.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Reyes PF, Golden GT, Fariello RG, Fagel L, Zalewska M. Olfactory pathways in Alzheimer’s disease (AD): Neuropathological studies [Abstract] Society for Neurosciences. 1985;1(168) [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, Georgehyslop PHS, Pericakvance MA, Joo SH, Rosi BL, Gusella JF, Crappermaclachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of Apolipoprotein-E Allele Epsilon-4 with Late-Onset Familial and Sporadic Alzheimers-Disease. Neurology. 1993;43(8):1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Perception of taste and smell in elderly persons. Crit Rev Food Sci Nutr. 1993;33(1):17–26. doi: 10.1080/10408399309527608. [DOI] [PubMed] [Google Scholar]
- Serby M. Olfaction and Alzheimers-Disease. Prog Neuro-Psychoph. 1986;10(3–5):579–86. doi: 10.1016/0278-5846(86)90027-8. [DOI] [PubMed] [Google Scholar]
- Small BJ, Basun H, Backman L. Three-year changes in cognitive performance as a function of apolipoprotein E genotype: Evidence from very old adults without dementia. Psychology and Aging. 1998;13(1):80–7. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- Struble RG, Clark HB. Olfactory bulb lesions in Alzheimer’s disease. Neurobiol Aging. 1992;13(4):469–473. doi: 10.1016/0197-4580(92)90074-8. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon 4 and change in cognitive functioning in community-dwelling older adults. J Geriatr Psych Neur. 2005;18(4):196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- Teter B, Raber J, Nathan B, Crutcher KA. The presence of apoE4, not the absence of apoE3, contributes to AD pathology. Journal of Alzheimer’s Disease. 2002;4:155–63. doi: 10.3233/jad-2002-4305. [DOI] [PubMed] [Google Scholar]
- Thesen T, Murphy C. Age-related changes in olfactory processing detected with olfactory event-related brain potentials using velopharyngeal closure and natural breathing. Int J Psychophysiol. 2001;40:119–127. doi: 10.1016/s0167-8760(00)00157-4. [DOI] [PubMed] [Google Scholar]
- Thesen T, Murphy C. Reliability analysis of event-related brain potentials to olfactory stimuli. Psychophysiology. 2002;39:733–738. doi: 10.1111/1469-8986.3960733. [DOI] [PubMed] [Google Scholar]
- Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN. Olfactory identification and apolipoprotein E epsilon 4 allele in mild cognitive impairment. Brain Research. 2002;951(1):77–81. doi: 10.1016/s0006-8993(02)03137-2. [DOI] [PubMed] [Google Scholar]
- Wetter S, Murphy C. Apolipoprotein E epsilon 4 positive individuals demonstrate delayed olfactory event-related potentials. Neurobiology of Aging. 2001;22(3):439–47. doi: 10.1016/s0197-4580(01)00215-9. [DOI] [PubMed] [Google Scholar]