Abstract
Introduction
Genomic studies of malignant pleural mesothelioma (MPM) have recently identified frequent mutations in the BAP1 gene. In uveal melanoma and clear cell renal cell carcinoma, BAP1 mutations are associated with poor outcomes but their clinical significance in MPM is unknown. We therefore undertook this study to define the characteristics of patients whose MPM tumors harbor somatic BAP1 mutation and to examine the relationship between BAP1 mutation and survival.
Methods
We reviewed the charts of 121 patients with MPM tumors diagnosed between 1990 and 2009 tested for BAP1 mutation and extracted the following information: age at diagnosis, sex, histology, stage, smoking status, asbestos exposure, family or personal history of malignancy, and treatment including surgery, chemotherapy, and radiation as well as survival status.
Results
Twenty-four (20%) of the 121 tumors harbored somatic BAP1 mutations. The percent of current or former smokers among cases with BAP1 mutations was significantly higher than in BAP1 wild-type cases, (75% vs 42%; p=0.006). However, the types of nucleotide substitutions in BAP1 did not suggest that this association was due to a causative role of smoking in BAP1 mutations. No other clinical feature was significantly different among those with and without BAP1 mutations in their MPM. There was also no difference in survival according to somatic BAP1 mutation status.
Conclusions
There is no apparent distinct clinical phenotype for MPM with somatic BAP1 mutation. The significance of the more frequent history of smoking among patients with BAP1-mutated MPM warrants further study.
Keywords: mesothelioma, BAP1, novel therapeutics
Introduction
Despite aggressive multimodality therapy, malignant pleural mesothelioma (MPM) remains almost universally fatal with a median overall survival of 18 months from the time of diagnosis. First-line chemotherapy with cisplatin and pemetrexed improves survival by a few months but the benefit, if any, of additional chemotherapy is modest. To improve these poor outcomes, many novel agents are currently under investigation in clinical trials. Unfortunately, despite the activity of these agents in other malignancies, there has been little efficacy in MPM. In an effort to help develop more effective MPM-specific therapeutics, much effort has focused on identifying the key cancer genes driving MPM oncogenesis.
While it has been known for many years that inactivating mutations in neurofibromatosis 2 (NF2) and deletions of p16 are common in MPM, another commonly mutated gene, BRCA-associated protein 1 (BAP1), was only recently identified.1 In the initial report, BAP1 mutations were identified in 23% of the MPM specimens. A variety of alterations were noted including nonsense mutations, missense mutations, frameshifting indels, and mutations at or near splice sites. Loss of nuclear BAP1 protein expression was confirmed by immunohistochemistry in MPM with BAP1 mutation. No association was identified between BAP1 mutation and other commonly identified genetic alterations including p16 loss and NF2 mutation and/or loss.
BAP1 is a 729 amino acid nuclear ubiquitin hydrolase with multiple functional domains and it has, therefore, been implicated in several cellular processes such as cell proliferation, DNA repair response, and chromatin dynamics.2 Specifically, an interaction between BAP1 and host cell factor 1 (HCF1), which interacts with histone-modifying complexes during cell division, has been described.1 More recently, it has been shown that BAP1, through an interaction with ASXL1 forms the polycomb group repressive deubiquitinase complex, which affects stem cell pluripotency.3
Mutations in BAP1 have also been described in other cancers. In particular, BAP1 mutation is common in uveal melanoma (UM), the most frequent ocular tumor in Caucasian adults, where it is strongly associated with poor outcomes. About 50% of UMs will metastasize at which point no effective treatment exists. A multi-gene expression profiling assay divides UMs into low and high metastatic risk with 84% of metastatic UM harboring BAP1 mutation compared to only 4% of low risk cases.4 Likewise, in clear cell renal cell carcinoma, somatic BAP1 mutations associated with higher grade tumors shorter cancer-specific survival.5 Furthermore, recent reports have described germline BAP1 mutations in families predisposed to MPM and UM6 as well as atypical melanocytic tumors7 and renal cell carcinoma.8 These findings clearly suggest the existence of a new hereditary cancer predisposition syndrome but the phenotype and penetrance of germline BAP1 mutations remains unclear as does the role of gene-environment interactions in the development of these tumors.
While BAP1 mutation is considered a crucial event in the progression of UM,4 the clinical impact of BAP1 mutation in MPM remains unknown. Therefore, the purpose of this study was to characterize the clinical features of MPM patients whose tumors harbor BAP1 mutations. Additionally, we examined the relationship between BAP1 mutation and survival.
Materials and Methods
With the approval of the Memorial Sloan-Kettering Cancer Center Institutional Review Board, the clinical records of 121 patients whose MPM tumors had been tested for BAP1 mutation by conventional Sanger sequencing were reviewed. These 121 tumor specimens were collected over 18 years from 1991 to 2009. Clinical features were extracted including age at diagnosis, sex, histology, stage, smoking status, asbestos exposure, family or personal history of malignancy, and treatment including surgery, chemotherapy, and radiation, as well as survival status.
The relationship between BAP1 mutation status and age, sex, histology, stage, smoking status, asbestos exposure, and family or personal history of malignancy was assessed using Fisher’s exact tests. To ensure that outcomes stratified by BAP1 mutation status were not confounded by differences in therapy, the relationship between BAP1 mutation status and surgery, chemotherapy, and radiation was also assessed using Fisher’s exact tests. Overall survival (OS) was estimated using Kaplan-Meier method, with patients followed from the time of diagnosis to death. Patients alive at the end of the study were censored at the time of the last available follow-up. The BAP1 mutant and BAP1 wild-type groups were compared with respect to OS using the log-rank test.
Results
Twenty-four of 121 MPM tumors harbored a somatic BAP1 mutation, giving a frequency of 20% (95% CI: 13–27%). Baseline characteristics of all patients included in this analysis are displayed in Table 1. The median age was 64 years (range 33–81). Seventy percent were men. Histology was distributed with 76% epithelioid, 15% mixed, and 9% sarcomatoid. Stage at diagnosis was 7% stage I, 26% stage II, 42% stage III, and 25% stage IV. Forty-nine percent of patients were former or current smokers while 40% reported known asbestos exposure. Treatment included extrapleural pneumonectomy in 54%, pleurectomy/decortication in 36%, and no surgery in 10%. Fifty-six percent received chemotherapy and 60% received radiation. A family history of mesothelioma was reported in 2% of patients and a family history of any cancer in 55%. Ten percent of patients had a personal history of cancer prior to the diagnosis of MPM. In addition, 8% of patients had a personal or family history of skin cancer.
Table 1.
Patient characteristics
| % (N=121) | |
|---|---|
| Age, median (range) | 64 (33–81) |
| Sex | |
| • Male | 70 (85) |
| • Female | 30 (36) |
| Histology | |
| • Epithelioid | 76 (92) |
| • Mixed | 15 (18) |
| • Sarcomatoid | 9 (11) |
| Stage | |
| • I | 7 (8) |
| • II | 26 (32) |
| • III | 42 (51) |
| • IV | 25 (30) |
| Former and current smoking | 49 (59) |
| Known asbestos exposure | 40 (48) |
| Surgery | |
| • EPP | 54 (66) |
| • P/D | 36 (43) |
| • None | 10 (12) |
| Chemotherapy | 56 (68) |
| Radiation | 60 (73) |
| Family history of mesothelioma | 2 (3) |
| Family history of cancer | 55 (67) |
| Personal history of cancer | 10 (12) |
| Personal or family history of skin cancer | 8 (10) |
EPP = extrapleural pneumonectomy; P/D = pleurectomy/decortication
As shown in Table 2, there was no significant difference in age, sex, asbestos exposure, surgery, chemotherapy, radiation, family history of mesothelioma, family history of cancer, and personal history of cancer among patients with BAP1 mutant and BAP1 wild-type MPM. However, a smoking history, either former or current, was more common among those with BAP1 mutant tumors, 75% versus 42% (p=0.006). Based on this association, we reviewed the spectrum of nucleotide substitutions in BAP1-mutated cases for evidence of a causal link to smoking. Fourteen of the 24 mutations identified in BAP1 were point mutations. Among those 14 point mutations, only 2 represented classic smoking-associated nucleotide transversions (Supplementary Table 1).
Table 2.
Clinical features of patients with BAP1 mutant versus BAP1 wild-type MPM
| BAP1 mutant % (N=24) |
BAP1 wild-type % (N=97) |
p-value | |
|---|---|---|---|
| Sex (M/F) | 79/21 | 68/32 | 0.33 |
| Median age | 65 | 63 | 0.31 |
| Asbestos exposure | 54 | 46 | 1 |
| Former and current smoking | 75 | 42 | 0.006 |
| Surgery | |||
| • EPP | 54 | 55 | 1 |
| • P/D | 38 | 35 | |
| • None | 8 | 10 | |
| Chemotherapy | 54 | 57 | 0.82 |
| Radiation | 71 | 58 | 0.35 |
| Family history of mesothelioma | 4 | 2 | 1 |
| Family history of cancer | 38 | 47 | 0.49 |
| Personal history of cancer | 8 | 8 | 0.46 |
EPP = extrapleural pneumonectomy; P/D = pleurectomy/decortication
There was no significant difference (p=0.43) in the stage at presentation for BAP1 mutant versus wild-type disease (Figure 1). Similarly, there was no difference (p=0.28) in the distribution of histologies among BAP1 mutant versus wild-type disease (Figure 2). No difference in overall survival was associated with BAP1 mutation status (Figure 3). Those with BAP1 mutant tumors had a median overall survival of 14.3 months (95% CI: 12.4–19.4) while those with BAP1 wild-type tumors had a median overall survival of 14.8 months (95% CI: 10.6–37.3), p=0.81. Adjusted for stage, the p-value remains non-significant (p=0.77).
Figure 1.
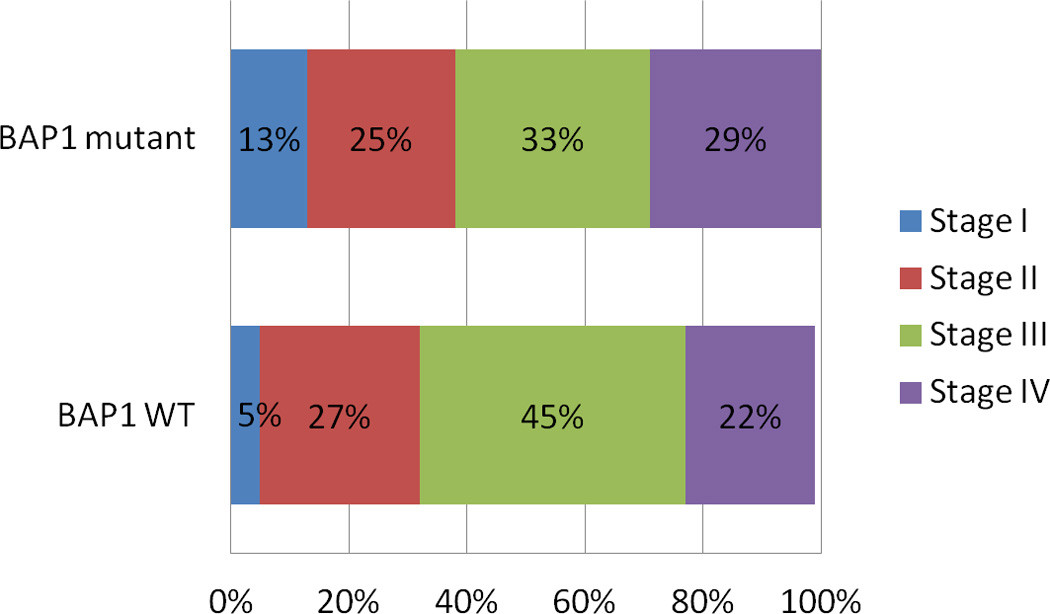
The distribution of stage by plotted by BAP1 mutation status. There is no observed difference (p=0.43).
Figure 2.

The distribution of MPM histology is plotted by BAP1 mutation status. There is no observed difference (p=0.28).
Figure 3.

These are the Kaplan-Meier survival curves for patients based on BAP1 mutation status. There is no difference in survival (p=0.81).
Discussion
Twenty percent of MPM tumors harbor mutations in BAP1. We find that a smoking history is significantly more common in MPM patients whose tumors harbor a BAP1 mutation. The biological significance of this observation is presently unclear. Perhaps, BAP1 mutation is a sequela of toxic exposure to smoking but the wide variety of mutations is not typical of tobacco smoke mutagenesis (only 2 of 24 mutations were G to T transversions consistent with classic smoking associated changes). Alternatively, BAP1 somatic mutation could be an early event that might impair the response to carcinogenic damage inflicted by smoking. Other clinical characteristics were similar among those with mutant and wild-type BAP1 MPM. While another group reported that BAP1 mutated cases were more likely to be partly or entirely epithelioid, this difference was not statistically significant in our larger cohort.9 No difference in survival was observed. Similarly, while the initial report of BAP1 mutations in MPM found an association between older age and BAP1 mutation,1 this is not confirmed in this larger series.
This analysis has some limitations. The retrospective nature of this project precluded thorough investigation of smoking status, asbestos exposure, personal history of malignancy, and family history of mesothelioma and other malignancies. Finally, as these specimens were obtained over an 18 year period, changes in therapy could have obscured differences in survival by BAP1 mutation status.
Since the initial reports of germline BAP1 mutations, numerous other neoplasms including meningiomas, renal cell carcinoma, lung cancer, breast cancer, ovarian cancer, pancreas cancer, and leukemia have been associated with BAP1 mutation.10–15 While there is no apparent distinct phenotype for MPM with somatic BAP1 mutation, further study is needed to identify and characterize patients with germline BAP1 mutations. To help describe the spectrum of disease associated with germline BAP1 mutation, we have initiated a prospective clinical protocol for patients with MPM, UM, and choroidal nevus (a premalignant eye tumor). First, patients will provide samples to participate in an anonymous estimate of the prevalence of germline BAP1 mutations. Additionally, patients whose tumors harbor BAP1 mutation or meet pre-specified criteria will be offered identified germline BAP1 testing. Those patients identified as carrying germline BAP1 mutations will be asked to invite both potentially affected and unaffected family members for testing through our Clinical Genetics Service. We will also continue to explore the interaction of BAP1 mutation with other somatic mutations, environmental exposures, and single nucleotide polymorphisms. As with other malignancies, insights into the biology of MPM may help identify new, disease-specific therapeutic targets.
Acknowledgments
Supported in part by the Mesothelioma Applied Research Foundation
References
- 1.Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladanyi M, Zauderer MG, Krug LM, et al. New Strategies in Pleural Mesothelioma: BAP1 and NF2 as Novel Targets for Therapeutic Development and Risk Assessment. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheuermann JC, de Ayala Alonso AG, Oktaba K, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakimi AA, Chen YB, Wren J, et al. Clinical and Pathologic Impact of Select Chromatinmodulating Tumor Suppressors in Clear Cell Renal Cell Carcinoma. European urology. 2012 doi: 10.1016/j.eururo.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011 doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiesner T, Obenauf AC, Murali R, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011 doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshikawa Y, Sato A, Tsujimura T, et al. Frequent inactivation of the BAP1 gene in epithelioidtype malignant mesothelioma. Cancer science. 2012 doi: 10.1111/j.1349-7006.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Rahman MH, Pilarski R, Cebulla CM, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. Journal of medical genetics. 2011;48:856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone M, Korb Ferris L, Baumann F, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. Journal of translational medicine. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey A, Seshasayee D, Noubade R, et al. Loss of the Tumor Suppressor BAP1 Causes Myeloid Transformation. Science. 2012 doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan LH, Tang LN, Yue L, et al. BAP1 is a good prognostic factor in advanced non-small cell lung cancer. Clinical and investigative medicine Medecine clinique et experimentale. 2012;35:E182. doi: 10.25011/cim.v35i4.17146. [DOI] [PubMed] [Google Scholar]
- 14.Njauw CN, Kim I, Piris A, et al. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PloS one. 2012;7:e35295. doi: 10.1371/journal.pone.0035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadt K, Choi J, Chung JY, et al. A cryptic BAP1 splice mutation in a family with uveal and cutaneous melanoma, and paraganglioma. Pigment cell & melanoma research. 2012 doi: 10.1111/pcmr.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]


