Abstract
This study demonstrates that the polyketide toxin karlotoxin 2 (KmTx 2) produced by Karlodinium veneficum, a dinoflagellate associated with fish kills in temperate estuaries worldwide, alters vertebrate cell membrane permeability. Microfluorimetric and electrophysiological measurements were used to determine that vertebrate cellular toxicity occurs through non-selective permeabilization of plasma membranes, leading to osmotic cell lysis. Previous studies showed that KmTx 2 is lethal to fish at naturally-occurring concentrations measured during fish kills, while sub-lethal doses severely damage gill epithelia. This study provides a mechanistic explanation for the association between K. veneficum blooms and fish kills that has long been observed in temperate estuaries worldwide.
Keywords: pore-forming toxin, non-selective membrane permeability, colloid osmotic lysis, fish kills, karlotoxin
1. Introduction
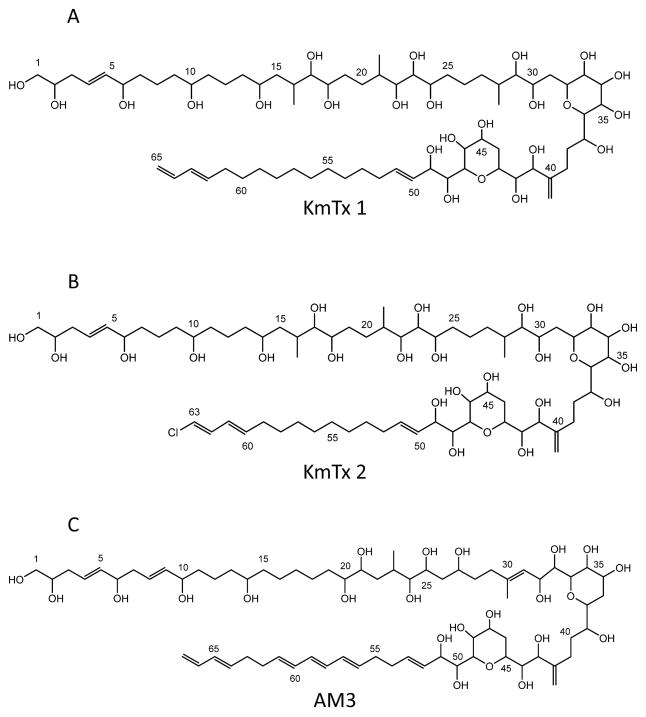
Previous investigations into repeated, dinoflagellate-associated fish kills in Mid and South Atlantic, USA, estuaries have shown that K. veneficum isolates and bloom samples were associated with toxic substances that are ichthyotoxic, hemolytic, and cytotoxic. (Deeds et al., 2002, 2004; Kempton et al. 2002). This small gymnodinioid dinoflagellate has been associated with toxic activity since its discovery in the 1950s and has gone through several taxonomic revisions over the last 60 years (for review see: Place et al. 2012). Although this toxic activity was originally reported by Abbott and Ballantine (1957) from K. veneficum (then Gymnodinium veneficum), assignment of structures to these compounds has become possible only recently (Peng et al., 2010; Van Wagoner et al., 2008, 2010). The karlotoxins (Fig. 1) are similar in structure to the amphidinols produced by dinoflagellates of the genus Amphidinium (Echigoya et al., 2005; Houdai et al., 2001, 2004, 2005; Ishibashi et al., 1994; Morsy et al., 2005; Murata et al., 1999; Paul et al., 1995, 1997; Satake et al., 1991), having a hairpin-like structure (Houdai et al., 2005) with three distinct regions: a polyol arm that exhibits variable hydroxylation and methylation; a hinge region containing two ether rings; and a lipophilic arm that often includes conjugated trienes in amphidinols (Satake et al., 1991) but a terminal diene that can be chlorinated in karlotoxins. Three groups of karlotoxins have now been described differing mainly in the length of the lipophilic arm (KmTx 1: 18 carbons, KmTx 3: 17 carbons, KmTx 2: 16 carbons) (Peng et al., 2010; Van Wagoner et al., 2010). The length of the lipophilic arm appears to modulate the hemolytic activity of these compounds (Van Wagoner et al., 2010). Two families of karlotoxins were initially described, KmTx 1-type (UV maximum ~225 nm) and KmTx 2–type (UV maximum ~235 nm) that were originally believed to have distinct geographic distributions in the U.S. (Deeds et al. 2004). This spectral shift is now known to be due to chlorination of the terminal diene of the lipophilic arm and that some isolates can produce both chlorinated and non-chlorinated, as well as sulfonated and non-sulfonated, versions of the same toxin (Van Wagoner et al., 2010). Bachvaroff et al. (2009) extended these observations to include 16 isolates from U.S. Atlantic waters and, so far, it still holds that U.S. isolates from the Chesapeake Bay and north produce mainly non-chlorinated KmTx-1 type toxins (UV maximum ~225 nm), while isolates from south of the Chesapeake Bay produce mainly chlorinated KmTx-2 type toxins (UV maximum ~235 nm), but these are not mutually exclusive as originally believed (Van Wagoner et al. 2010). Karlotoxins display antifungal and hemolytic activities and these activities depend on interactions with membrane sterols (Deeds and Place, 2006). This is similar to reports for the closely related amphidinols (Houdai et al., 2004; Swasono et al., 2010).
Fig. 1. Structures for select karlotoxins and amphidinols.
A. karlotoxin 1 (KmTx 1), B. karlotoxin 2 (KmTx 2) [used in this study], C. amphidinol 3 (AM3). Structures recreated from Van Wagoner et al. (2008) using ChemBioDraw Ultra (ver. 13.0) (PerkinElmer, Waltham, MA, USA).
This report extends previous observations on the biological activities of the karlotoxins by providing a detailed description of the cellular mode of toxicity for one of these toxins, KmTx 2, using osmotic protection assays combined with microfluorimetric and electrophysiological measurements.
2. Materials and Methods
2.1. KmTx 2 isolation
KmTx 2 used in this study was isolated directly from water collected during a fish kill in a South Carolina brackish pond described in Kempton et al. (2002). This is the primary compound produced by CCMP isolates 2282, 2283, and 2388 (isolated from South Carolina), 2064 (isolated from Georgia), and 2778 (isolated from Florida) (Provasoli-Guillard National Center for Marine Algae and Microbiota, East Boothbay, ME, USA) (Bachvaroff et al. 2009; Kempton et al. 2002; Wang et al. 2005). Previously frozen and thawed water samples (1.6 liter total) were first passed through type GF/F filters (Whatman International Ltd., Maidstone, England), then lipophilic materials were isolated from filtrates using several small (3 ml) disposable C18 cartridges according to manufacturer’s instructions (Sep-Pak Plus tC18, Waters Corporation, Milford, MA). After 40% and 60% MeOH washing steps (15 ml each), hemolytic materials were eluted from the cartridges with 80% MeOH (15 ml). The 80% MeOH fraction was dried under N2, re-suspended in a small volume of MeOH and fractionated further using HPLC. Aliquots were injected onto a LiChroDART 125-4/RP8 (5 μm) reversed-phase column (Waters Corporation, Milford, MA) and eluted at 30°C with a linear MeOH/H2O gradient (30–95% MeOH over 20 min), at a flow rate of 1 ml/min (Hewlett Packard Series 1100 HPLC System, Agilent Technologies, Inc. Wilmington, DE). Fractions were collected every 0.5 min and assayed for hemolytic activity using rainbow trout (Oncorhynchus mykis) erythrocytes as described below. This particular sample contained only one HPLC peak with hemolytic activity (Kempton et al. 2002). The molecular weight of the material in this HPLC fraction was determined by mass spectrometry (MSn) analysis, according to Bachvaroff et al. (2008), and found to be identical (m/z 1367.67 [M + Na]+) to KmTx 2 as described by Peng et al. (2010).
2.2 Hemolysis assays
Whole blood (3–5 ml) was extracted from the caudal vein of adult rainbow trout (Oncorhynchus mykis), maintained by the University of Maryland Center for Environmental Science Aquaculture Research Facility, according to approved animal care and use protocols. Heparinized syringes were used to prevent clotting. Whole blood was transferred to 15 ml disposable centrifuge tubes containing a small amount of heparin solution and centrifuged (2000 g for 5 min.); thereafter, plasma and buffy coat, containing white blood cells, were removed. Erythrocyte (RBC) suspensions were prepared by washing cells three times (2000 g for 5 min.) with ice-cold RBC wash buffer [150 mM NaCl, 3.2 mM KCl, 1.25 mM MgSO4, and 12.2 mM Tris base]. Buffer pH was adjusted to 7.4 at 10 °C with 1N HCl, then filter sterilized (0.22 μm). After the third wash, cells were resuspended in RBC storage buffer (RBC wash buffer + 3.75 mM CaCl2), at 50% of the original hemotocrit. Erythrocyte suspensions were stored at 4 °C for no longer than 10 days.
Hemolysis assays were performed by diluting test material in RBC storage buffer (100 μl) and adding this to 100 μl of a 5% erythrocyte suspension for a total assay volume of 200 μl. Assays were run in 96 well, V-bottom, polystyrene plates (Corning Inc., Corning NY) sealed with Falcon 3073 pressure sensitive film (Becton Dickinson Labware, Lincoln Park NJ). Plates were incubated on an orbital shaker (80–100 rpm) at 20 °C for 1 hour. Plates were then centrifuged at 2000 g for 5 min. and the supernatant (100 μl) was transferred to a new flat-bottom 96 well polystyrene plate, where the absorbance of released hemoglobin was measured at 540 nm. Saponin (from Quillaja bark; Sigma Chemical Co., St. Louis MO) was used as a positive hemolysin control. Percent hemolysis (0–100%) was calculated from comparison to untreated controls and samples treated with 10 μg saponin. All treatments were run in quadruplicate.
2.3. Osmotic protection assays
The hypothesis that KmTx 2 induces lysis through osmotic imbalance was tested by incubating rainbow trout erythrocytes with various molecular weight osmolytes. The following osmolytes were prepared as 30 mM solutions using the hemolytic assay RBC storage buffer described above: sucrose (MW 342.3), poly(ethylene glycol)s (PEG MW 400, 600, and 8,000), maltohexaose (MW 990.0), and dextran (MW 10,000). Osmolarity of each solution was measured using a vapor pressure osmometer (Vapro 5520, Wescor Inc., Logan, Utah). Osmolarity of all solutions of osmolytes with MW ≤ 8,000 did not differ from that of the RBC buffer (300–320 mOsm). The osmolarities of PEG 8,000 and 10,000 MW dextran were ~640 mOsm. Inhibition of hemolysis was assayed by preparing erythrocyte suspensions and toxin dilutions (0, 0.25 0.5, and 1 μg/ml) in the appropriate osmolyte solution and performing hemolysis assays as described above.
2.4. Ion permeation measurements
Cellular responses to KmTx 2 challenge were studied by single-cell microfluorimetry or digital fluorescence imaging microscopy using commercially available intracellular indicators for the cations Ca2+ and Na+. The following cell types were tested: rat embryonic fibroblasts (REF52), rat intestinal epithelial cells (IEC-6), human T-lymphocytes (Jurkat), rabbit vagal sensory neurons acutely isolated from the nodose ganglion, and acutely dissociated rat ventricular cardiac myocytes (cell isolation and culturing described in section 2.6 below). Cells on No. 1 glass coverslips (Fisher Scientific, Newark, DE) were loaded with fluorescent indicator by incubation with the acetoxymethyl (AM) ester of the appropriate fluorescent indicator (fura-2 for Ca2+ or Mn2+; SBFI for Na+; Molecular Probes, Inc., Eugene OR) for ~60 min. at room temperature. Arg8-vasopressin (Sigma Chemical Co., St. Louis, MO), which activates phosphoinositide signaling, was used as a positive control for eliciting Ca2+ signals in REF52 fibroblasts.
Toxin-induced membrane permeability to Mn2+ was measured in REF52 cells essentially as previously described (Raimondi et al., 2000). Briefly, fluorescene from REF52 cells pre-loaded with fura-2 were quantified continuously by microfluorimetry. Prior to KmTx2 addition, 50 μM Mn2+ was added to the external medium, and the basal rate of decline of fluorescence was monitored. Upon KmTx 2 addition, a sharp decline in fluorescence would reflect increased influx of Mn2+ ions, which bind to fura-2 indicator molecules with higher affinity than Ca2+ and quenches their fluorescence (Grynkiewicz et al., 1985).
For single-cell microfluorimetry, cells were examined on an inverted epifluorescence microscope (model Diaphot; 40X CF Fluor objective, N.A. 1.30; Nikon Corp. Melville, NY) coupled to a spectrofluorimeter (model CM1T10I, SPEX Industries, Edison, NJ) operating in the microfluorimetry mode. Fura-2 and SBFI were alternately excited at 340 and 380 nm. Fluorescence emission was passed through a 510-nm bandpass filter before photometric quantitation. DATAMAX software (SPEX Industries) was used for data acquisition and instrument control. For fluorescence imaging, cells were examined on an inverted epifluorescence microscope (Eclipse TE200, 40X SuperFluor objective, N.A. 1.3, Nikon Corp.). A PolyChrome II monochromator (TILL Photonics, Gräfelfing, Germany) was used for excitation. Fluorescence images were captured by a cooled CCD camera (CoolSnap HQ, Roper Scientific, Tucson, AZ). MetaFluor software (Universal Imaging Corp., Downingtown, PA) was used for image acquisition and instrument control. Cells were bathed in 4 ml of medium buffered with HEPES (pH 7.4). KmTx 2 (1 μg/ml stock solution in DMSO) was directly bath-applied with gentle mixing. Data reduction and analysis, including the calibration of microfluorimetric measurments to obtain [Ca2+]i or [Na+]i, were performed as previously described (for Ca2+: Kao, 1994; Kao et al. 2010; for Na+: Harootunian et al. 1989). Origin software (OriginLab Corp., Northampton, MA) was used for data reduction and analysis.
2.5. Electrophysiological measurements
The whole-cell patch-clamp technique was used to measure membrane currents in individual vagal sensory neurons from male New Zealand White rabbits upon exposure to KmTx 2 (cell isolation and culturing described in section 2.6 below). Neurons were superfused with physiological saline solution (20–24°C) that contained (mM): 120 NaCl, 3.0 KCl, 1.0 NaH2PO4, 25.0 NaHCO3, 1.5 MgCl2, 2.2 CaCl2, and 10.0 dextrose, equilibrated with 95%O2-5%CO2; pH adjusted to 7.4. A recording chamber with a narrow rectangular flow path allowed superfusion of neurons cultured on a glass coverslip at 7 ml/min via a gravity flow system. The chamber was mounted on an inverted microscope (Diaphot, Nikon, Melville, NY) equipped with a ×40 phase-contrast oil-immersion objective (Fluor, N.A. 1.3, Nikon) to allow microfluorimetry and direct visualization of neurons for positioning patch pipettes.
Patch pipettes were filled with intracellular solutions containing (mM): 152 KCH3SO3, 10.0 HEPES, 2.0 MgCl2, 1.0 Na3ATP, 1.0 Na3GTP, and 1.0 KCl; pH adjusted with KOH to 7.2. K5Fura-2 was added to the pipette solution to a final concentration of 50 μM; sufficient CaCl2 was added to set free [Ca2+] = 100 nM (taking the Ca2+ dissociation constant of fura-2 under physiological conditions to be 224 nM) (Grynkiewicz et al., 1985).
The whole-cell configuration of the patch clamp technique (Hamill et al., 1981) was used to measure membrane currents. Patch pipettes (2 – 3 MΩ resistance), fabricated from borosilicate glass stock (O.D. 1.5 mm, I.D. 1.12 mm World Precision Instruments, Sarasota, FL) on a Flaming-Brown P97 micropipette puller (Sutter Instruments, Novato, CA) were connected to an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Data acquisition through the Digidata 1200 interface was controlled with pClamp 8 software (Axon Instruments). Rabbit nodose ganglion neurons were first loaded with fura-2/AM (as previously described) to allow fluorimetric measurement of [Ca2+]i in parallel with electrophysiological recording. After a gigaohm seal (>1.0 GΩ) was formed, the whole-cell configuration was established, with neurons voltage-clamped to −50 mV. Membrane input resistance and capacitance were determined from current transients elicited by 5-mV depolarizing voltage steps from the holding potential. Cells were considered suitable for study if membrane input resistance was >150 MΩ and holding current was <200 pA.
To determine current-voltage (I-V) relations, the following three-phase voltage command protocol was applied to voltage-clamped neurons: 1) From a holding potential of −50 mV, the membrane potential was stepped to +50 mV for 100 msec to inactivate voltage-gated Na+ channels; 2) An I-V relation was then generated by a voltage ramp that decreased from +70 mV to −110 mV at 1.125 mV/msec; and 3) At the end of the ramp, the cell was returned to a holding potential of −50 mV. This protocol was applied twice to each cell tested: first immediately before toxin application, and again 90 sec after a 60 sec toxin application by superfusion, when the toxin-induced current had developed substantially. Taking the difference between these two I-V relations yielded the I-V relation for the toxin-induced current.
2.6. Cell isolation and culturing
Rat embryo fibroblasts (REF52 line, gift of Dr. James Feramisco, Univ. of California, San Diego) and rat intestinal epithelial cells (IEC-6 line, ATCC No. CRL-1592) were trypsinized and plated onto coverslips at ~20% coverage, cultured at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% v/v fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin, in humidified 5% CO2/95% air for at least 24 hours before experimentation. Human T lymphocytes (Jurkat line, gift of Dr. Alfredo Garzino-Demo, Institute of Human Virology, University of Maryland School of Medicine) were grown in suspension in RPMI 1640 medium supplemented with 10% v/v FBS, 100 units/ml penicillin and 100 μg/ml streptomycin, in humidified 5% CO2/95% air at 37°C. All cell culture reagents were purchased from Gibco-BRL (Life Technologies, Grand Island, NY).
For isolating vagal sensory neurons, male New Zealand White rabbits, weighing 3 – 4 kg (RSI Biotechnology, Clemmons, NC) were killed by sodium pentobarbital over-dose (100 mg/kg), as approved by the Institutional Animal Care and Use Committee of the University of Maryland. Dissociated nodose ganglion neurons (NGNs) were prepared as described previously (Leal-Cardoso et al., 1993) with the exception that sterile technique was used and the final neuronal pellet was re-suspended in Leibovitz L-15 medium containing 10% v/v FBS, 100 units/ml penicillin and 100 μg/ml streptomycin. The resulting cell suspension was plated in 0.2-mL aliquots onto 25-mm round No. 1 glass coverslips (Fisher Scientific, Newark, DE) coated with poly-D-lysine (0.1 mg/mL; MW 30 – 70 kD, Sigma Chemical Co., St. Louis, MO). NGNs were incubated in air at 37°C for 12 h, then maintained at room temperature to prevent neurite outgrowth, and used for experiments for up to 48 h.
For isolating cardiac ventricular myocytes, hearts were removed from adult Sprague-Dawley rats (250 – 275 g) after deep anesthesia had been induced by pentobarbital sodium (30 mg/kg ip; Abbott Laboratories, Chicago, IL), as approved by the Institutional Animal Care and Use Committee of the University of Maryland. The aorta was quickly cannulated for Langendorff perfusion, and ventricular myocytes were isolated, as previously described (DuBell et al., 1993), by perfusion of the coronary arteries with a buffer containing 50 μM Ca2+, collagenase (type II; Worthington Biochemical, Lakewood, NJ), and protease (type XIV). The isolated cells were suspended in HEPES-buffered DMEM, containing 10% v/v FBS, at room temperature. Immediately before microscopy experiments, myocytes were plated onto No. 1 glass coverslips coated with laminin (34 μg/ml aqueous solution; BD Biosciences, San Jose, CA) and bathed in HEPES-buffered normal Tyrode’s solution, which contained (mM): 140 NaCl, 5 KCl, 10 HEPES, 0.33 NaH2PO4, 1 MgCl2, 1.8 CaCl2, 10 glucose, at pH 7.4.
2.7. Data presentation
Experimental results are presented as mean ± standard deviation.
3. Results
3.1. KmTx 2 induces osmotic cell lysis
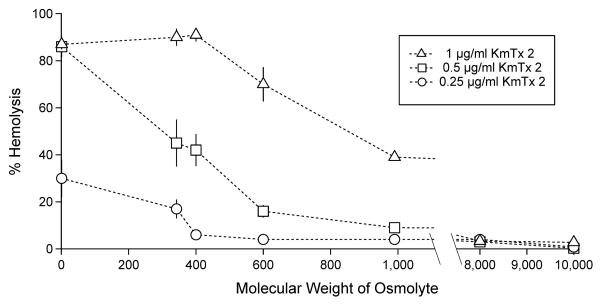
KmTx 2 is a lytic toxin that is highly active against erythrocytes, causing almost complete cell lysis at ~0.5 μg/ml (Fig. 2). Because typical cytosolic protein (“colloid”) concentrations are high, a toxin-induced increase in the permeability of the erythrocyte membrane to small ions would cause a net ionic influx driven by Donnan forces. The consequent osmotic movement of water then leads to cell swelling and lysis. This process is expected to be inhibited by extracellular osmolytes which, by balancing the osmotic effect of intracellular proteins, protect the cells from lysis. Therefore, erythrocytes were co-incubated with KmTx 2 and inert osmolytes, including sugars, poly(ethylene glycol)s, and dextrans. These solutes ranged in molecular weight from 342 to 10,000 Da. The osmolytes did indeed protect erythrocytes against KmTx 2-induced osmolysis, with osmolytes of higher molecular weights affording greater protection (Fig. 2). Thus, osmolytes with MW > 8,000 Da completely inhibited hemolysis even in the presence of 1 μg/ml KmTx 2 (Fig. 2). These results indicate that KmTx 2 induces cell lysis through colloid osmolysis presumably through membrane permeability changes.
Fig. 2. Extracellular osmolytes protect against KmTx 2 induced hemolysis.
Erythrocytes were treated with [○] 0.25, [□] 0.5 or, [◇] 1.0 μg/ml KmTx 2 together with 30 mM of either sucrose (MW 342.3), poly(ethylene glycol)s (MW 400, 600, 8,000), maltohexaose (MW 990.0), or dextran (MW 10,000). Error bars = 1 s.d. (n=3). x-axis broken between MW 1,000 and 8,000 as no osmolytes were tested in this range.
3.2. KmTx 2 alters membrane ionic permeability
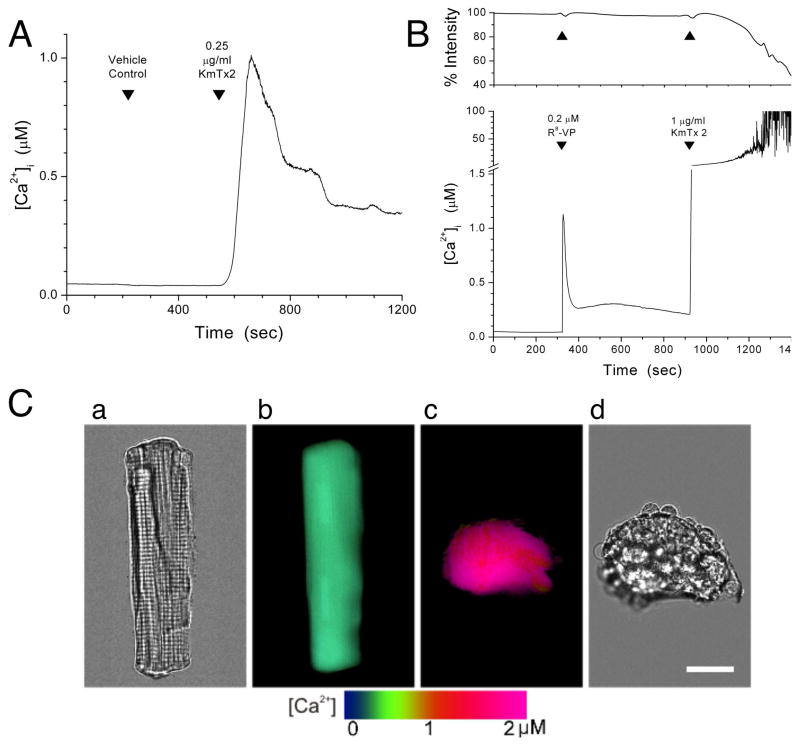
KmTx 2-induced changes in membrane ionic permeability were assessed by applying the toxin to rat embryo fibroblasts (REF52 line) loaded with either fura-2 or SBFI, fluorescent indicators for measuring intracellular free Ca2+ ([Ca2+]i) or Na+ ([Na+]i) concentration, respectively (Grykiewicz et al., 1985; Minta and Tsien, 1989). Increased cellular permeability to Mn2+ was indicated by a decrease in intracellular fura-2 fluorescence due to the displacement of Ca+ by Mn2+, which has a higher affinity for fura-2 and which, being a high-spin transition metal ion, quenches the fluorescence of the indicator (Raimondi et al. 2000). Steady-state bath application of 0.25 μg/ml KmTx 2 elicited an increase in [Ca2+]i, which then subsided (Fig. 3A); this behavior was observed in all 20 cells tested. In marked contrast, a 4-fold higher concentration of KmTx 2 (1.0 μg/ml) evoked a sharp and irreversible rise in [Ca2+]i (Fig. 3B, lower panel), and progressive cell lysis, as evidenced by the accelerated loss of fura-2 fluorescence from the cells (Fig. 3B, upper panel). The lytic action of high-dose KmTx 2 was consistently observed in ten of ten cells tested. In comparison, addition of 0.2 μM Arg8-vasopressin, which activates phosphoinositide signaling, evoked a transient rise in [Ca2+]i with no change in intracellular indicator content (Fig. 3B, upper and lower panel). Further, the toxin-induced rise in [Ca2+]i due to KmTx 2 was attributable to Ca2+ influx, and not release from intracellular Ca2+ stores, because in Ca-free medium, the change in [Ca2+]i was abolished (data not shown). We obtained essentially identical results when the above experiments were repeated with several other mammalian cell types, including rat intestinal epithelial cells, rabbit vagal sensory neurons, human T-lymphocytes, as well as rat ventricular cardiac myocytes. Fig. 3C shows the effect of KmTx 2 on a rat cardiac myocyte loaded with fura-2. At rest, the myocyte displayed normal relaxed morphology (brightfield micrograph, Fig. 3Ca) and low resting [Ca2+]i (pseudocolor-coded image, Fig. 3Cb). Steady-state bath application of 0.25 μg/ml KmTx 2 caused [Ca2+]i to be dramatically elevated (Fig. 3Cc), and the myocyte was irreversibly contorted by hypercontraction (Fig. 3Cd).
Fig. 3. Calcium influx is a cellular consequence of KmTx 2 action.
A. KmTx 2 (0.25 μg/ml) caused a transient rise in [Ca2+]i in REF52 fibroblasts; vehicle (0.025% DMSO) had no effect (n = 20). B. Simultaneous monitoring of [Ca2+]i (lower panel) and total intracellular fura-2 fluorescence (upper panel) in REF52 fibroblasts. Arg8-vasopressin (0.2 μM) activates phosphoinositide signaling and evoked only a transient rise in [Ca2+]i, with no change in intracellular indicator content. In contrast, 1.0 μg/ml KmTx 2 caused a sharp and irreversible [Ca2+]i rise (note y-axis break), and concomitant decrease in intracellular indicator fluorescence, indicating loss of fura-2 indicator from cells (n = 10). C. Effect of KmTx 2 on cardiac myocytes. Before KmTx 2 treatment, the rat myocyte showed normal relaxed morphology (image a) and low resting [Ca2+]i (pseudocolor-coded image b). Ninety-five seconds after KmTx 2 application (0.25 μg/ml), the myocyte showed dramatically increased [Ca2+]i (c) and hyper-contraction (d). Bar = 20 μm.
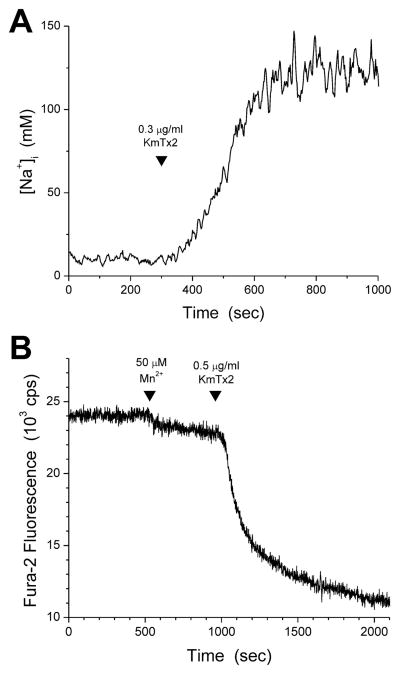
The increased membrane permeability induced by KmTx 2 was not selective for Ca2+. Through methods used previously to characterize microbial toxins (Raimondi et al., 2000), it was determined that KmTx 2 promoted the permeation of cations as different as Na+ and Mn2+, in addition to Ca2+. Application of 0.3 μg/ml KmTx 2 to REF52 fibroblasts pre-loaded with SBFI resulted in a marked increase in [Na+]i (Fig. 4A) indicating increased permeability of the cell membrane to extracellular Na+; such a [Na+]i rise occurred in all three cells tested. In separate experiments, application of 0.5 μg/ml KmTx 2 to fura-2-loaded REF52 fibroblasts in the presence of 50 μM extracellular Mn2+ accelerated the decline of intracellular fura-2 fluorescence, reflecting the displacement of Ca+ by Mn2+ and the consequent quenching of fura-2 fluorescence by the bound Mn2+ (Fig. 4B); this was observed in all three cells studied with this protocol. Together, these results indicate that the increase in membrane permeability caused by KmTx 2 is not ion-specific.
Fig. 4. Membrane permeability increase caused by KmTx 2 is not ion-specific.
A. Exposure of REF52 cells loaded with the fluorescent Na+ indicator SBFI to 0.3 μg/ml KmTx 2 cause a large increase in [Na+]i (n = 3). B. Mn2+ (50 μM) was added to the medium bathing REF52 cells (first arrowhead). Gradual Mn2+ entry into the intact cells leads to a slow but detectable decline in fura-2 fluorescence, as Mn2+ binds to fura-2 and quenches its fluorescence (see main text for more detailed mechanistic discussion). Subsequent application of 0.5 μg/ml KmTx 2 accelerated the decline in fluorescence, reflecting increased influx of Mn2+ (n = 3).
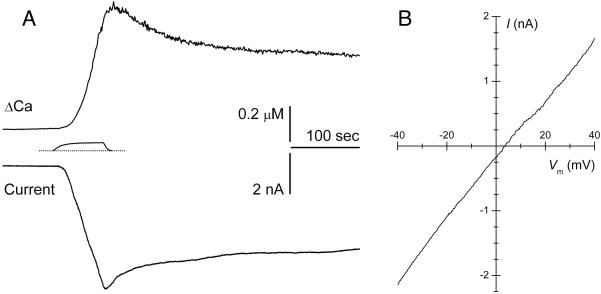
A change in membrane ionic permeability is detectable electrically. The whole-cell patch-clamp technique was used to characterize the electrophysiological effect of KmTx 2 on vagal sensory (nodose) neurons isolated from adult rabbits. To enable simultaneous monitoring of [Ca2+]i, fura-2 was included in the patch pipette (intracellular) solution. At a holding potential of −50 mV, a 60-sec transient application of KmTx 2 (1 μg/ml) consistently caused a large inward current to develop (Fig. 4A, lower trace). A corresponding rise in [Ca2+]i was also observed (Fig. 5A, upper trace). Notably, the time course of the rise in [Ca2+]i is somewhat delayed relative to the ionic current. This suggested that ions other than Ca2+ might be the principal carriers of the toxin-evoked current; this is consistent with the above findings that the KmTx 2-induced permeability increase is not ion-specific. Using analytical techniques previously applied to these neurons (Hoesch et al. 2001), it was estimated that less than 4% of the total current was carried by Ca2+, as suspected. From voltage-ramp experiments, current-voltage (I-V) relations were constructed for the KmTx 2-induced current (Fig. 5B). The reversal potential of Erev = +3.6 ± 5.4 mV (n = 7) is consistent with what has been observed for non-selective cation currents. Using a modified form of the Goldman-Hodgkin-Katz equation (Lewis, 1979) enabled estimation of the relative permeabilities for K+ and Na+: PK/PNa = 0.79. These results indicate that, under these conditions, KmTx 2 acted principally to increase membrane permeability to Na+ and K+, while causing a more modest, but very physiologically significant, increase in Ca2+ permeability. It is important to note that in a living system, in addition to Na+, K+ and Ca2+, physiologically relevant ions that occur at high concentrations include Mg2+, Cl− and HxPO43-x (phosphate in various protonation states). Owing to the chemical similarity of Ca2+ and Mg2+, it is highly likely that the membrane permeability caused by KmTx 2 would admit Mg2+ as well. While no direct measurement was made to determine whether KmTx 2 increases membrane permeability to physiological anions such as phosphate and chloride, a rational inference is possible. As shown in Fig. 3B (upper panel), KmTx 2-induced permeability enables rapid leakage of fura-2 indicator from cells. Fura-2 is a penta-anion with a molecular weight of 636.5. The fact that a large, highly negatively-charged molecule like fura-2 can leak out rapidly following KmTx 2 application strongly suggests that much smaller physiological anions such as phosphate and chloride would be permeant as well.
Fig. 5. Electrophysiological measurements indicate KmTx 2 non-selectively increases the ionic permeability of cell membranes.
A. Simultaneous measurement of [Ca2+]i and whole-cell membrane current in a rabbit nodose neuron at −50 mV. Transient application of KmTx 2 (1 μg/ml) induced a large inward ionic current and a concomitant [Ca2+]i rise. The precise KmTx 2 concentration-vs-time profile, estimated using a fluorescent tracer in the flow system that irrigates the cells, is shown between the traces. B. Current-voltage (I-V) relation for the KmTx 2-evoked ionic current. Averaged data from 7 neurons shown; reversal potential is 3.6 ± 5.4 mV.
4. Discussion
In the first description of the karlotoxins (Deeds et al., 2002), it was shown that the addition of a high dose (2 μg/ml) of KmTx 1 (then known as Tox A) to a rat pituitary tumor cell line (GH4C1) resulted in 100% release of the cytosolic marker lactate dehydrogenase (LDH), suggesting that KmTx 1 causes cell lysis. Wassler et al. (1990) showed that the release of LDH from saponin-treated mammalian hepatocytes could be inhibited through the addition of osmotically active substances to the culture media. To confirm the hypothesis that KmTx 2 also kills vertebrate cells by colloid osmotic lysis, it is shown here that co-incubation of rainbow trout erythrocytes with KmTx 2 and a range of inert osmolytes, including sugars, poly(ethylene glycol)s, and dextrans (342.3 – 10,000 MW), causes a progressive inhibition of lysis, with higher MW osmolytes affording greater protection against lysis. High-MW osmolytes (~8000 MW) completely prevent lysis, even in the presence of a high concentration of KmTx 2 (1 μg/ml). These results confirm that KmTx 2 causes colloid osmotic lysis if applied in sufficient doses.
Single-cell microfluorimetric measurements with fluorescent ion indicators using five differentiated mammalian cell types, including rat embryo fibroblasts, human T-lymphocytes, rat intestinal epithelial cells, rabbit vagal sensory neurons, and rat ventricular cardiac myocytes, show here that KmTx 2 causes a pre-lytic increase in the permeability of the plasma membrane to cations such as Na+, Ca2+, and Mn2+. Electrophysiological studies with the whole-cell patch-clamp technique further confirmed that KmTx 2 permeabilizes the plasma membrane to cations non-selectively; permeability to Ca2+ is increased modestly but significantly, while permeability to Na+ and K+ is increased very markedly. These results clarify the microscopic mechanisms that underlie the pre-lytic action of KmTx 2. Membrane permeability is altered to allow ions to flow freely across vertebrate cells. It is presumed that water follows down these same pathways leading to increased osmotic stress.
Despite the fact that KmTx 2 in this study was equally active against a range of vertebrate cell types, the primary harmful effect associated with this organism in nature has been ichthyotoxicity. Previous studies revealed that KmTx 2 concentrations of ~0.5 μg/ml were acutely toxic (< 1 hr) to zebrafish (Danio rerio), and even exposures to 0.1 μg/ml caused severe damage to gill epithelia (Deeds et al., 2006). In water collected during fish kills, KmTx 2 has been measured at 0.1 – 0.8 μg/ml (Deeds et al., 2004). These results indicate that fish in the wild can be exposed to toxic levels of KmTx 2, and provide strong evidence for the ichthytoxicity of K. veneficum in the environment. This damage was consistent with effects on fish exposed to KmTx 2 or K. veneficum both reported earlier (Deeds et al., 2006) and previously in the literature (Nielsen, 1993). It should be noted that the cell lines used in this study were chosen to represent physiologically important vertebrate cell types (connective tissue, immune system, epithelia, as well as the nervous and muscular systems). Additional insight could be gained into the exact time course and concentrations required to cause fish kills at naturally occurring concentrations of karlotoxins through the further study of model fish gill and skin cell lines.
The present work shows K. veneficum’s karlotoxins target the cell membrane to cause membrane permeability changes. This is similar to the reported mode of action for the structurally similar amphidinols. Several reports have shown that amphidinol 3 (AM3) enhances the permeability of the biological membrane by forming pores or lesions in lipid bilayers, (Houdai et al., 2004, 2005; Morsy et al., 2008; Murata et al., 1999; Oishi et al., 2008; Paul et al. 1997) which is thought to be responsible for their potent antifungal activity.
For several cytolytic pore-forming compounds (including microalgal derived karlotoxins, amphidinols, and prymnesins), membrane sterols appear to play a critical role in toxicity. For example, the polyene antibiotic, amphotericin B, can bind to membranes devoid of sterols, but sterols in the target membrane are reported to be required for permeabilization leading to cell lysis (Gary-Bobo, 1987). Similarly, incubation with either cholesterol or ergosterol inhibited the activity of prymnesins, potent hemolytic and ichthyotoxic polyketides produced by the haptophyte Prymnesium parvum (Igarashi et al., 1998), while the presence of cholesterol in liposomes enhanced the activity of amphidinols (Paul et al., 1997). Leblond and Chapman (2002) found that the sterol profile of K. veneficum is dominated by (24S)-4-methyl-5α-ergosta-8(14),22-dien-3β-ol (gymnodinosterol). This profile is almost unique—being shared only by the closely-related dinoflagellates in the Kareniaceae (Leblond and Chapman, 2000; Mooney et al., 2007). It was previously shown that this unique sterol likely plays a role in protecting K. veneficum from the membrane-disrupting effects of its own toxin. Co-incubation with cholesterol or ergosterol inhibited KmTx 2 hemolytic activity (Deeds and Place, 2006). In contrast, co-incubation with gymnodinosterol had no effect on hemolysis. It was inferred from these results that cholesterol and ergosterol can complex with KmTx 2, sequestering the toxin away from erythrocyte membranes, and thus afford protection against hemolysis. In contrast, simple phosopholipids and gymnodinosterol cannot complex KmTx 2 and, therefore, offer no protection (Deeds and Place, 2006). These observations suggest that by utilizing a sterol that is structurally different from sterols found in the membranes of heterologous species, K. veneficum renders itself immune to the pore-forming properties its own toxins.
Despite the information provided here and previously suggesting that karlotoxins non-specifically increase permeability in vertebrate (cholesterol containing) membranes leading to colloid osmotic lysis, the structure of the pore itself has yet to be determined. Houdai et al. (2005) found evidence for a hairpin structure in amphidinols and that membrane permeabilization was hardly influenced by the bilayer thickness (Morsy et al., 2008). Based on their structural similarities, a hairpin-like structure is also expected for the karlotoxins. Although the conformation of C1–C20 remains unelucidated due to the high flexibility, AM3 is predicted to take an umbrella-like or a T-shaped structure in target membranes, with the bent polyol portion having a large cross-sectional area and the extended polyene chain having a smaller cross-section (Matumori and Murata, 2010). This suggested to the authors that a barrel-stave pore was not the most likely model for the amphidinol pore because the stability of the latter generally depends on the membrane thickness. Instead a toroidal pore model was proposed, where the lipid monolayer curves continuously from the outer leaflet to the inner in the fashion of a toroidal hole, where the pore is lined by both the pore-formers and the lipid head-groups. Future work with karlotoxins will determine whether a similar model can be developed and what role different membrane sterols play in the pore-forming process.
Highlights.
Karlotoxin 2 (KmTx 2) non-selectively alters vertebrate membrane permeability.
KmTx 2 induces cell lysis through colloid osmolysis.
KmTx 2 induces a marked increase in permeability to Na+ and K+.
KmTx 2 induces a modest but significant increase in cytosolic Ca2+.
Cytosolic Ca2+ increase is due to influx and not release from intracellular stores.
Acknowledgments
The authors wish to acknowledge Frederick Fry Jr. (US FDA) for the creation of Figure 1. This work was funded in part by grants from ECOHAB and NIEHS (to A.R.P.) and NIGMS (to J.P.Y.K.). This is contribution #4974 from the University of Maryland Center for Environmental Science (UMCES), #14-140 for the Institute of Marine and Environmental Technology (IMET), and #709 from the Ecology and Oceanography of Harmful Algal Blooms (ECOHAB) program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott BC, Ballantine D. The toxin from Gymnodinium veneficum Ballantine. J Mar Biol Assoc U K. 1957;36:169–189. [Google Scholar]
- Bachvaroff TR, Adolf JE, Place AR. Strain variation in Karlodinium veneficum (Dinophyceae): Toxin profiles, pigments, and growth characteristics. J Phyc. 2009;45:137–153. doi: 10.1111/j.1529-8817.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- Bachvaroff TR, Adolf JE, Squier AH, Harvey HR, Place AR. Characterization and quantification of karlotoxins by liquid chromatography-mass spectrometry. Harmful Algae. 2008;7:473–484. [Google Scholar]
- Deeds JR, Kibler SR, Tester PA, Place AR. Geographic strain variation in toxin production in Karlodinium micrum (Dinophyceae) from Southeastern estuaries of the United States. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; St. Petersburg, FL, USA: 2004. pp. 145–147. [Google Scholar]
- Deeds JR, Terlizzi DE, Adolf JE, Stoecker DK, Place AR. Toxic activity from cultures of Karlodinium veneficum (=Gyrodinium galatheanum) (Dinophyceae) - A dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae. 2002;1:169–189. [Google Scholar]
- Deeds JR, Reimschuessel R, Place AR. Histopathological effects in fish exposed to the toxins from Karlodinium micrum. J Aq Anim Health. 2006;18:136–148. [Google Scholar]
- Deeds JR, Place AR. Sterol specific membrane interactions with the toxins from Karlodinium micrum (Dinophyceae) – A strategy for self-protection. Afr J Mar Sci. 2006;28(2):421–427. [Google Scholar]
- DuBell WH, Lewartowski B, Spurgeon HA, Silverman HA, Lakatta EG. Repletion of sarcoplasmic Ca after ryanodine in rat ventricular myocytes. Am J Physiol. 1993;265:3440–3450. doi: 10.1152/ajpheart.1993.265.2.H604. [DOI] [PubMed] [Google Scholar]
- Echigoya R, Rhodes L, Oshima Y, Satake M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae. 2005;4:383–389. [Google Scholar]
- Gary-Bobo CM. Polyene-sterol interaction and selective toxicity. Biochimie. 1989;71:37–47. doi: 10.1016/0300-9084(89)90129-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3340–3350. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved parch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Houdai T, Matsuoka S, Matsumori N, Murata M. Membrane-permeabilizing activities of amphidinol 3, polyene-polyhydroxy antifungal from a marine dinoflagellate. Biochim Biophys Acta. 2004;1667:91–100. doi: 10.1016/j.bbamem.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Houdai T, Matsuoka S, Morsy N, Matsumori N, Satake M, Murata M. Hairpin conformation of amphidinols possibly accounting for potent membrane permeabilizing activities. Tetrahedron. 2005;61:2795–2802. [Google Scholar]
- Houdai T, Matsuoka S, Murata M, Satake M, Ota S, Oshima Y, Rhodes LL. Acetate labeling patterns of dinoflagellate polyketides, amphidinols 2, 3 and 4. Tetrahedron. 2001;57:5551–5555. [Google Scholar]
- Hoesch RE, Weinreich D, Kao JPY. A novel Ca2+ influx pathway in mammalian sensory neurons is activated by caffine. J Neurophys. 2001;86:190–196. doi: 10.1152/jn.2001.86.1.190. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Aritake S, Yasumoto T. Biological activities of prymnesin-2 isolated from a red tide alga Prymnesium parvum. Nat Toxins. 1998;6:35–41. doi: 10.1002/(sici)1522-7189(199802)6:1<35::aid-nt7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kao JYP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Meth Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- Kao JPY, Li G, Auston DA. Practical aspects of measuring intracellular calcium signals with fluorescent indicators. Meth Cell Biol. 2010;99:113–52. doi: 10.1016/B978-0-12-374841-6.00005-0. [DOI] [PubMed] [Google Scholar]
- Kempton JW, Lewitus AJ, Deeds JR, Law JM, Place AR. Toxicity of Karlodinium veneficum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae. 2002;1:233–241. [Google Scholar]
- Leal-Cardoso H, Koschorke GM, Taylor G, Weinreich D. Electrophysiological properties and chemosensitivity of acutely isolated nodose ganglion neurons of the rabbit. J Auto Nerv Sys. 1993;45:29–39. doi: 10.1016/0165-1838(93)90359-3. [DOI] [PubMed] [Google Scholar]
- Leblond JD, Chapman PJA. Survey of the sterol composition of the marine dinoflagellates Karenia brevis, Karenia mikimotoi, and Karlodinium micrum: Distribution of sterols within other members of the class Dinophyceae. J Phycol. 2002;38:670–682. [Google Scholar]
- Lewis CA. Ion concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori N, Murata M. 3D structures of membrane-associated small molecules as determined in isotropic bicells. Nat Prod Rep. 2010;27:1480–1492. doi: 10.1039/c0np00002g. [DOI] [PubMed] [Google Scholar]
- Mooney BD, Nichols PD, De Salas MF, Hallegraeff GM. Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta): Chemotaxonomy, and putative lipid phycotoxins. J Phycol. 2007;43:101–111. [Google Scholar]
- Morsy N, Matsuoka S, Houdai T, Matsumori N, Adachi S, Murata M, Iwashita T, Fujita T. Isolation and structure elucidation of a new amphidinol with a truncated polyhydroxyl chain from Amphidinium klebsii. Tetrahedron. 2005;61:8606–8610. [Google Scholar]
- Morsy N, Houdai T, Konoki K, Matsumori N, Oishi T, Murata M. Effects of lipid constituents on membrane-permeabilizing activity of amphidinols. Bioorg Med Chem. 2008;16:3084–3090. doi: 10.1016/j.bmc.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Murata M, Matsuoka S, Matsumori N, Paul GK, Tachibana K. Absolute configuration of amphidinol 3, the first complete structure determination from amphidinol homologues: application of a new configuration analysis based on carbon-hydrogen spin-coupling constants. J Am Chem Soc. 1999;121:870–871. [Google Scholar]
- Nielsen MV. Toxic effect of the marine dinoflagellate Gymnodinium galatheanum on juvenile cod Gadus morhua. Mar Ecol-Prog Ser. 1993;95:273–277. [Google Scholar]
- Oishi T, Kanemoto M, Swasono R, Matsumori N, Murata M. Combinatorial synthesis of the 1,5-polyol system based on cross metathesis: Structure revision of amphidinol 3. Organ Lett. 2008;10(22):5203–5206. doi: 10.1021/ol802168r. [DOI] [PubMed] [Google Scholar]
- Paul GK, Matsumori N, Konoki K, Murata M, Tachibana K. Chemical structures of amphidinols 5 and 6 isolated from marine dinoflagellate Amphidinium klebsii and their cholesterol-dependent membrane disruption. J Mar Biotech. 1997;5:124–128. [Google Scholar]
- Paul GK, Matsumori N, Murata M, Tachibana K. Isolation and chemical structure of amphidinol 2, a potent hemolytic compound from marine dinoflagellate Amphidinium klebsii. Tetra Lett. 1995;36:6279–6282. [Google Scholar]
- Peng J, Place AR, Yoshida W, Anklin C, Hamann MT. Structure and absolute configuration of karlotoxin-2, an ichthyotoxin from the marine dinoflagellate Karlodinium veneficum. J Am Chem Soc. 2010;132:3277–3279. doi: 10.1021/ja9091853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place AR, Bowers HA, Bachvaroff TR, Adolf JE, Deeds JR, Sheng J. Karlodinium veneficum - The little dinoflagellate with a big bite. Harmful Algae. 2012;14:179–195. [Google Scholar]
- Raimondi F, Kao JPY, Fiorentini C, Fabbri A, Donelli G, Gasparini N, Rubino A, Fasano A. Enterotoxicity and cytotoxicity of Vibrio parahaemolyticus thermostable direct hemolysin in in vitro systems. Infec Immun. 2000;68:3180–3185. doi: 10.1128/iai.68.6.3180-3185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M, Murata M, Yasumoto T. Amphidinol, a polyhydroxypolyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J Am Chem Soc. 1991;113:9859–9861. [Google Scholar]
- Swasono RT, Mouri R, Morsy N, Matsumori N, Oishi T, Murata M. Sterol effect on interaction between amphidinol 3 and liposomal membrane as evidenced by surface plasmon resonance. Bioorg Med Chem Lett. 2010;20:2215–2218. doi: 10.1016/j.bmcl.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Van Wagoner RM, Deeds JR, Satake M, Ribeiro AA, Place AR, Wright JLC. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetra Lett. 2008;49:6457–6461. doi: 10.1016/j.tetlet.2008.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner RM, Deeds JR, Tatters AO, Place AR, Tomas CR, Wright JL. Structure and relative potency of several karlotoxins from Karlodinium veneficum. J Nat Prod. 2010;73:1360–1365. doi: 10.1021/np100158r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Deeds JR, Place AR, Belas R. Dinoflagellate community analysis of a fish kill using denaturing gradient gel electrophoresis. Harmful Algae. 2005;4:151–162. [Google Scholar]
- Wassler M, Westman J, Fries E. Permeabilization of hepatocytes by a saponin and the effects of dextran. Eur J Cell Biol. 1990;51:252–258. [PubMed] [Google Scholar]