Abstract
Chemokine (C-C motif) ligand 2 (CCL2), initially identified as monocyte chemoattractant protein-1 (MCP-1), recruits immune cells to the central nervous system (CNS) during autoimmune inflammation. CCL2 can be expressed by multiple cell types, but which cells are responsible for CCL2 function during acute and chronic phases of autoimmune disease is not known. We determined the role of CCL2 in astrocytes in vivo during experimental autoimmune encephalomyelitis (EAE) by using Cre-loxP gene deletion. Mice with a conditional gene deletion of CCL2 from astrocytes had less severe EAE late in disease while having a similar incidence and severity of disease at onset as compared to wild type (WT) control littermates. EAE mice devoid of CCL2 in astrocytes had less macrophage and T cell inflammation in the white matter of the spinal cord and less diffuse activation of astrocytes and microglia in both white and gray matter as well as less axonal loss and demyelination, compared to WT littermates. These findings demonstrate that CCL2 in astrocytes plays an important role in the continued recruitment of immune cells and activation of glial cells in the CNS during chronic EAE, thereby suggesting a novel cell specific target for neuroprotective treatments of chronic neuroinflammatory diseases.
Keywords: CCL2, Astrocytes, Multiple Sclerosis, EAE, T lymphocyte, Macrophage, Microglia, Neuroprotection
1. Introduction
Inflammation characterizes many disorders of the central nervous system (CNS), including neurodegenerative, traumatic and autoimmune disorders. In neurodegenerative and traumatic disorders, CNS inflammation is locally restricted and tends to resolve over time (Bush et al., 1999; Schnell et al., 1999; Donnelly and Popovich, 2008). In contrast, in autoimmune disorders, CNS inflammation is widespread and continuous or recurring (Raine et al., 1980; Lucchinetti et al., 2000; McFarland and Martin, 2007). Understanding which molecule on which cell regulates the continuous influx of inflammatory cells into the CNS during autoimmune disease is central to the development of treatment strategies aiming to block continuous inflammation.
Multiple sclerosis (MS) is an autoimmune disease of the CNS characterized by continuous inflammation, demyelination and axonal loss. Current treatment strategies are only partially effective at controlling peripheral immune responses, reduce relapse rates and delay, but do not halt, permanent disability accumulation and ongoing neurodegeneration (Hauser et al., 2013). Thus, there is a need to develop treatments that target molecules on CNS cells to achieve more direct neuroprotection. Experimental autoimmune encephalomyelitis (EAE) is the most widely used animal model for MS. It has been used for decades to study peripheral immune responses and was central to the development of many currently approved treatments in MS (Yednock et al., 1992; Yu et al., 1996; Aharoni et al., 1997; Webb et al., 2004; Prinz et al., 2008). More recently the chronic progressive EAE model in C57BL/6 mice has been used to study neurodegenerative aspects of the disease as a means toward finding a neuroprotective treatment (Bannerman et al., 2005; Rasmussen et al., 2007; Aharoni et al., 2008; Xu et al., 2008; MacKenzie-Graham et al., 2009; Ziehn et al., 2010; Rasmussen et al., 2011; MacKenzie-Graham et al., 2012; Ziehn et al., 2012; Mori et al., 2013).
Astrocytes are intimately associated with and signal to blood vessels (Iadecola and Nedergaard, 2007) and are recognized as playing important roles in regulating leukocyte trafficking and inflammation in the CNS. They produce a wide variety of pro-inflammatory chemokines and cytokines, as well as reactive oxygen species (ROS) in vitro, consistent with a pro-inflammatory role (Dong and Benveniste, 2001; Chen and Swanson, 2003; Farina et al., 2007; Nair et al., 2008). Conversely, astrocytes also produce anti-inflammatory cytokines and ROS scavengers, thereby suggesting a role in mitigating inflammation (Aloisi et al., 1997; Dong and Benveniste, 2001; Dringen and Hirrlinger, 2003; Nair et al., 2008). Astrocytes have been implicated in vivo in locally triggering innate pro-inflammatory responses after CNS trauma and stroke (Farina et al., 2007), while, on the other hand, scar-forming reactive astrocytes formed essential barriers that restricted leukocyte migration from areas of damaged tissue into neighboring healthy tissue (Bush et al., 1999; Faulkner et al., 2004; Myer et al., 2006; Okada et al., 2006; Herrmann et al., 2008; Li et al., 2008). Thus, astrocytes are thought to play complex roles in regulating leukocyte trafficking in the CNS (John et al., 2005). Reactive astrocytosis is a prominent feature of chronic inflammation of the CNS during EAE and MS (Eng et al., 1970; Liedtke et al., 1998; Eng et al., 2000), and transgenically targeted ablation of proliferating, scar-forming reactive astrocytes during EAE revealed loss of astrocyte barrier function resulting in widespread inflammation and worsening of EAE outcomes (Voskuhl et al., 2009).
Chemokine (C-Cmotif) ligand 2 (CCL2) is a chemokine initially identified as monocyte chemoattractant protein-1 (MCP-1). Upon tissue specific expression of CCL2, it can attract macrophages, T cells, dendritic cells, mast cells and basophils to tissue sites (Rollins, 1991, 1997). CCL2 expression in the CNS occurs during chronic relapsing EAE, with higher CCL2 expression correlating with relapses, and antibody mediated blocking decreasing disease severity and reducing macrophage infiltration (Kennedy et al., 1998). Global knockouts of CCL2 (Huang et al., 2001) and its receptor CCR2 (Fife et al., 2000) were resistant to EAE induction. Adoptive transfer of encephalitigenic cells that were devoid of CCL2 induced EAE in wild type recipients showing that CCL2 expression in the peripheral immune induction phase was not required. However, EAE was not induced when recipients of adoptively transferred wild type encephalitogenic cells were devoid of CCL2, thereby showing that CCL2 expression in the recipient was required for disease. CCL2 expression in the recipient could be required either in the recipient's CNS or in peripheral immune cells recruited during disease (Fife et al., 2000). Bone marrow chimeras using global CCL2 gene deletion separated out effects of CCL2 in the immune system versus the CNS. Specifically, CCL2 expression in the reconstituted immune system was not necessary for active EAE induction in chimeras, while CCL2 expression in the engrafted recipient was required (Dogan et al., 2008). Together these reports showed that CCL2 expression in a CNS cell is central to chronic EAE pathogenesis. Which CNS cell, however, remains unknown.
In this study we have ascertained whether CCL2 expression in astrocytes may be critical to EAE pathogenesis. We have created a conditional knock out of CCL2 in astrocytes and asked whether this affects the clinical course, focal macrophage and T cell infiltration, diffuse activation of astrocytes and microglial cells, as well as axonal and myelin loss during EAE.
2. Methods
2.1. Animals
CCL2 conditional gene deletion or knockouts (CKO) from astrocytes (astro-CCL2-CKO) were generated by crossing transgenic mice that express Cre-recombinase under regulation of the mouse glial fibrillary acid protein (mGFAP) promoter, mGFAP-Cre line 73.12 (Herrmann et al., 2008), with mice carrying a CCL2 gene flanked by loxP sites (CCL2flox/flox) (Shi et al., 2011). The CCL2flox/flox was a generous gift from Professor Eric G. Pamer (Sloan-Kettering Institute for Cancer Research). Reporter mice were generated by crossing mGFAP-Cre line 73.12with transgenic mice harboring ROSA-tdTomato under regulation of a loxP-flanked STOP cassette (JAX, Bar Harbor, ME). Animals were maintained under standard conditions in a 12 h dark/light cycle with access to food and water ad libitum. All procedures were done in accordance to the guidelines of the National Institutes of Health and the Chancellor's Animal Research Committee of the University of California, Los Angeles Office for the Protection of Research Subjects.
2.2. Active EAE induction and clinical scoring
Astro-CCL2-CKO and littermate control (WT) mice were immunized subcutaneously with myelin oligodendrocyte glycoprotein (MOG), amino acids 35–55 (200 µg/animal, American Peptides) emulsified in Complete Freund's Adjuvant, supplemented with Mycobacterium Tuberculosis H37Ra (400 µg/animal, Difco Laboratories), over two sites drained by left inguinal and auxiliary lymph nodes in a total volume of 0.1 ml/mouse. One week later, a booster immunization was delivered over contra lateral lymph nodes. The animals were daily monitored for EAE signs based on a standard EAE 0–5 scale scoring system: 0, healthy; 1, complete loss of tail tonicity; 2, loss of righting reflex; 3, partial paralysis; 4, complete paralysis of one or both hind limbs; and 5, moribund, as described (Voskuhl et al., 2009).
2.3. Histological preparation
Mice were exposed to a lethal dose of isoflurane and perfused transcardially with ice-cold 1X PBS for 8–10 min, followed by 10% formalin for 8–10 min. Spinal cords were dissected and submerged in 10% formalin overnight at 4 °C, followed by 30% sucrose for 24 hrs at 4 °C. Spinal cords were embedded in optimal cutting temperature compound (Tissue Tek) and stored in −80 °C after flash frozen in an isopentane bath cooled with liquid nitrogen. Spinal cord cross-sections were obtained 40 µm-thick with a microtome cryostat (model HM505E) at −20 °C. Tissues were collected serially and stored free floating in 1X PBS with 1% sodium azide in 4 °C for further analysis, as described (Spence et al., 2013).
2.4. Immunofluorescence
Prior to histological staining, 40-mm thick free-floating sections were thoroughly washed with 1X PBS to remove residual sodium azide. In the case of anti-MBP labeling, tissue sections were processed with an additional 2 h incubation with 5% glacial acetic acid in 100-proof ethanol at room temperature (RT). After washing tissue sections were permeabilized with 0.05% Tween20 and 2% normal goat serum in 1X PBS for 30 min at RT and blocked with 10% normal goat serum in 1X PBS for 1 hr. Without washing, tissues were then incubated with primary antibodies overnight in 4 °C. The following primary antibodies were used: anti-CD3 at 1:2000 (BD Biosciences PharMingen), anti-Iba-1 at 1:10,000 (Wako Chemicals), anti-GFAP at 1:40,000 (Dako), anti- MCP-1 (CCL2) at 1:200 (Torrey Pines Biolabs), anti-neurofilament- NF200 at 1:750 dilutions (Sigma), and anti-MBP at 1:750 (Sigma). The next day tissues were washed and incubated with secondary antibodies conjugated to Cy5 or Cy3 (Millipore) for 1 hr at RT. DAPI at 1:5000 (Molecular Probes) was used for nuclear stain. Sections were mounted on slides, allowed to semi-dry, and cover slipped in fluoromount G (Fisher Scientific) for confocal microscopy, as described (Spence et al., 2013).
2.5. Microscopy and image processing
Stained sections were examined and imaged using a confocal microscope (Leica TCS-SP) or a fluorescence microscope (BX51WI; Olympus) equipped with Plan Fluor objectives connected to a camera (DP70, Olympus). Images were processed using SlideBook™ 4.2 (Intelligent Imaging Innovations, Inc.) and assembled using Microsoft PowerPoint (Microsoft), as described (Spence et al., 2013).
2.6. Statistical analysis
Differences in EAE clinical scores were determined by repeated-measures one-way ANOVA. Immunofluorescence data were analyzed by one-way ANOVA. For these analyses, one-way ANOVA and Bonferroni post hoc analysis were performed on F-stat values, and significance was determined at the 95% confidence interval (Prism), as described (Spence et al., 2013).
3. Results
3.1. CCL2 is specifically deleted from astrocytes in astro-CCL2-CKO mice
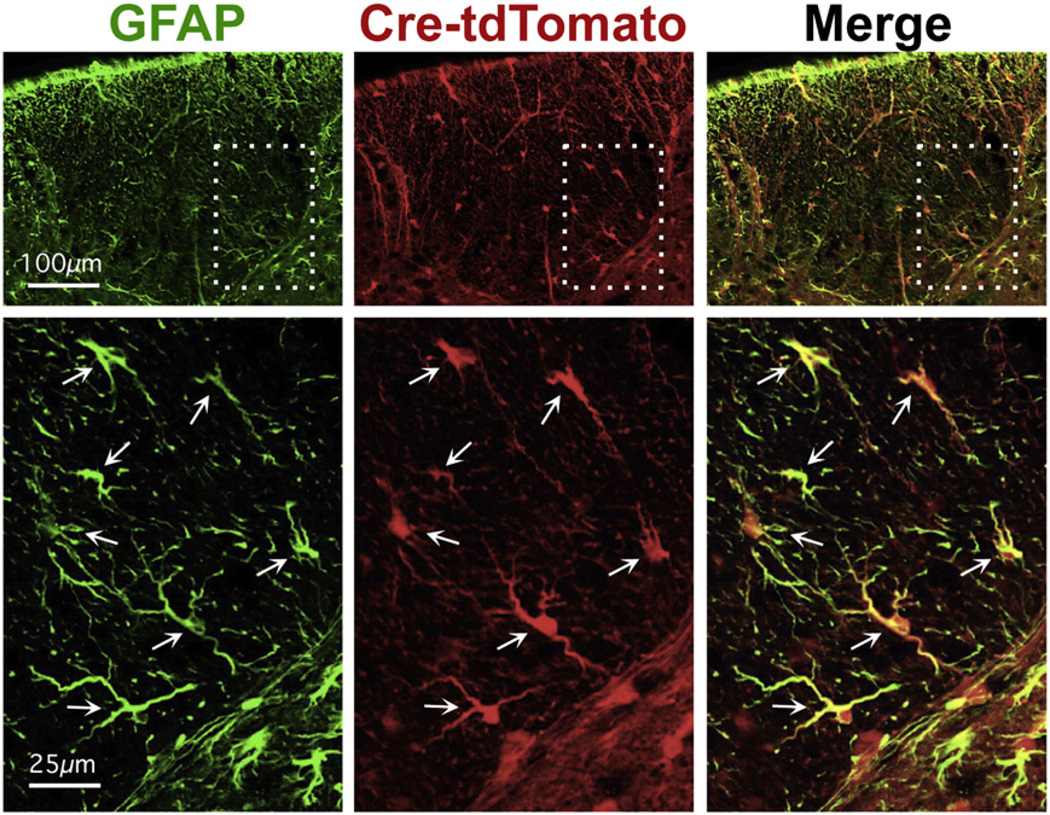
To target the deletion of CCL2 to astrocytes we used a well-characterized mGFAP-Cre line previously shown to target Cre activity selectively in astrocytes in the CNS (Herrmann et al., 2008; Spence et al., 2011, 2013). This mGFAP-Cre line targets 98% of all astrocytes in the spinal cord, the tissue evaluated in this study, with no targeting of other cells, such as oligodendrocytes, microglia, and neurons (Herrmann et al., 2008). First, we confirmed the targeting of Cre activity to essentially all astrocytes and only to astrocytes in the spinal cord of healthy, untreated young adult mice of this transgenic line by showing complete overlap in the expression of GFAP with the highly sensitive reporter protein tdTomato whose expression requires Cre expression activity (Fig. 1). We then crossed mGFAP-Cre mice with CCL2-loxP mice (CCL2flox/flox mice) (Shi et al., 2011). To confirm the selectivity of CCL2 deletion in astrocytes during EAE, we assessed CCL2 expression by immunohistochemistry using double staining for CCL2 (green) and GFAP (red). Genetically unmanipulated C57BL/6 control mice with EAE exhibited readily detectable immunoreactive CCL2, with some co-localization of CCL2 with GFAP and some CCL2 not colocalizing with GFAP (Fig. 2A, top row), thereby demonstrating CCL2 expression both in astrocytes as well as in other cells during EAE. In contrast, astro-CCL2-CKO mice with EAE no longer showed co-localization of CCL2 with GFAP, with CCL2 staining limited to non-GFAP staining cells (Fig. 2A, middle row). Astro-CCL2-CKO littermate WT mice with EAE (Fig. 1A, bottom row) were the same as C57BL/6 mice with EAE (Fig. 2A, top row). CCL2 staining in dorsal column of thoracic spinal cord as shown in Fig. 2B, and quantified in Fig. 2C, confirmed that deletion of CCL2 from astrocytes in our astro-CCL2-CKO mice resulted in overall partial deletion of CCL2 in cord during EAE. Specifically, astro-CCL2-CKO mice had removal of CCL2 from astrocytes, but not other cells, during EAE.
Fig. 1.
Verification of extent and selectivity of Cre targeting to astrocytes. Immunofluorescence shows complete GFAP (green) with tdTomato reporter protein (red) in healthy, untreated young adult mice mGFAP-Cre-tdTomato reporter mice. Merged images (GFAP+ tdTomato) show co-localization in yellow. Survey images (top row) show dorsal column white matter. Scale bar =100 µm. Boxed areas indicate areas shown in detail below. Scale bar = 25 µm. Arrows indicate double-labeled astrocytes. All detectable astrocytes express both GFAP and tdTomato.
Fig. 2.
Verification of CCL2 deletion specificity in GFAP+ astrocytes during EAE. A, Immunofluorescence shows co-localization of CCL2 (green) with GFAP (red) in C57BL/6 wildtype (top row) and CCL2 wildtype littermate controls (CCL2 WT bottom row), but not in mice with astrocyte CCL2 conditional gene deletion (astro-CCL2-CKO middle row) during EAE. Merged images (GFAP + CCL2) show co-localization in yellow. Scale bar, 20 µm. B, Immunofluorescence shows significantly reduced CCL2 expression in the spinal cord dorsal column of astro-CCL2-CKO mice compared to CCL2 WT mice during EAE. Scale bar, 100 µm. C, Quantification shows reduced % CCL2 expression area in the spinal cord dorsal column of astro-CCL2-CKO mice. *p < 0.05 versus CCL2 WT (mean ± SEM).
3.2. CCL2 deletion from astrocytes during EAE provides clinical disease protection late in disease
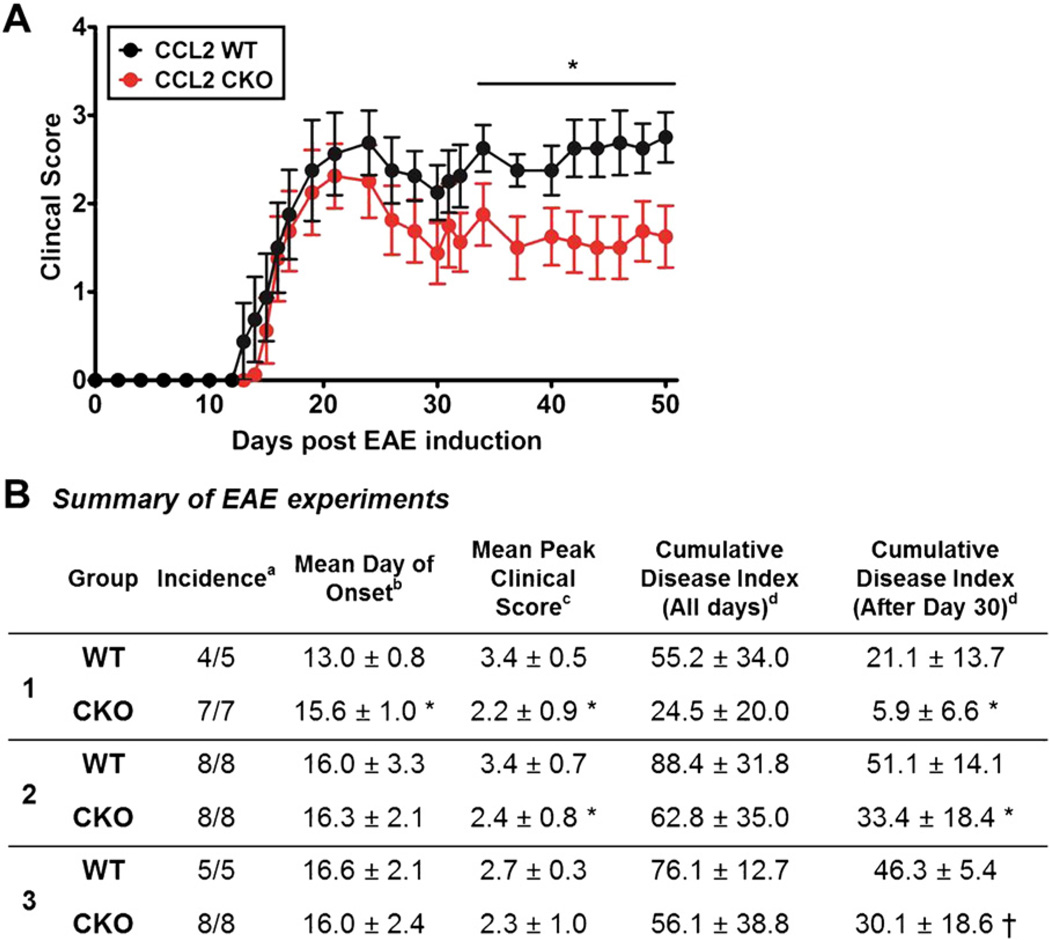
To determine whether CCL2 from astrocytes plays a role in EAE, we compared standard EAE clinical scores in astro-CCL2-CKO versus WT littermates. Astro-CCL2-CKO mice had significantly less severe EAE late in disease, while having a similar incidence and severity of disease at onset (Fig. 3). This result showed a role for CCL2 from astrocytes in mechanisms related to disease progression, but not in acute disease onset.
Fig. 3.
Astrocyte CCL2 CKO, as compared to WT littermates, has less severe EAE late in disease. A, Astro-CCL2-CKO mice had significantly better clinical EAE scores late (after EAE day 30) compared with CCL2 WT mice. *p < 0.05 (repeated-measures ANOVA with post hoc Bonferroni pairwise analysis). Graphical representation of experiment #2 from Table. B, Table showing clinical assessments in three separate experiments. Statistical analysis shows significant decrease in the cumulative disease score index after 30 days of EAE induction in astro-CCL2-CKO compared to CCL2 WT. aIncidence is the number of mice that developed EAE signs from the total number of mice in each group. bMean day (±SEM) of onset was assessed when mice showed first clinical disease signs (score of >1) after EAE induction. cMean peak clinical score (±SEM) was assessed when each mouse reached its maximum clinical score. dCumulative disease index (±SEM) was calculated by summing the daily clinical scores of each mouse and averaging them within each group. “All days” signifies day 0 of EAE induction to day of sacrifice, while “After Day 30” is from day 30 of EAE to day of sacrifice. *p < 0.05, †p < 0.08 versus CCL2 WT (mean ± SEM).
3.3. CCL2 deletion from astrocytes reduces macrophage and T cell infiltrates during EAE
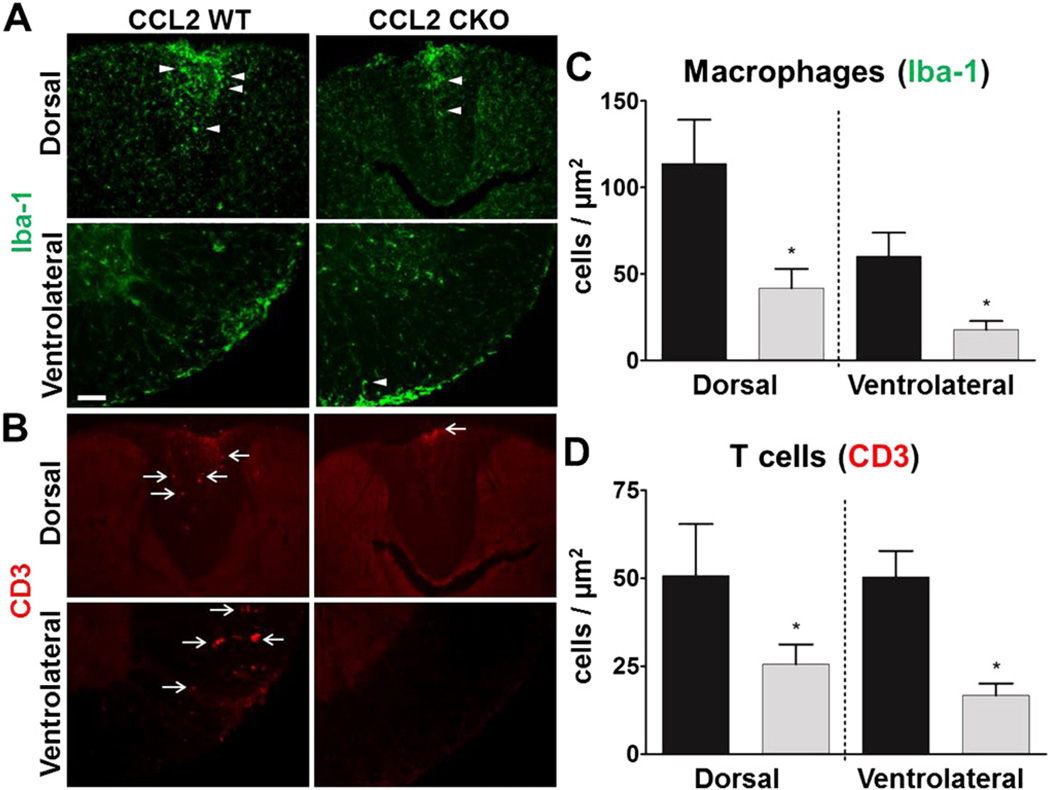
To understand how CCL2 in astrocytes may affect the progression of EAE, we sacrificed mice during late disease and assessed immune cell infiltrates by immunofluorescence using a T lymphocyte marker (anti-CD3) and macrophage marker (Iba-1).We used immunohistochemical identification and a stereological procedure (StereoInvestigator ®) to quantify these cell types in the spinal cord dorsal and ventral columns. To identify macrophages, distinct from microglia, we used immunohistochemistry for Iba-1 and counted cells with a globoid shape, as previously described (Voskuhl et al., 2009). We found that astro-CCL2-CKO mice, compared to WT littermate controls, had a significant reduction in both macrophage and T cell infiltrates in both the dorsal and ventrolateral white matter of spinal cords (Fig. 4). Representative images of astro-CCL2-CKO and WT EAE mice showed the differences in infiltrates with anti-Iba-1 (arrowhead) and anti-CD3 (arrow) staining (Fig. 4A,B), while quantitative analysis confirmed the significant reduction of macrophages (Fig. 4C) and T cells (Fig. 4D) in astro-CCL2-CKO mice. These results showed a role for CCL2 in astrocytes in the recruitment of macrophages and T cells to the CNS during progressive EAE.
Fig. 4.
Astrocyte CCL2 CKO mice with EAE have a reduction in macrophage and T cell infiltrates in both dorsal and ventral white matter of the spinal cord during EAE. Representative immunofluorescence images of A, Iba-1 (green) globoid macrophage (arrowhead) and B, CD3 (red) T cell (arrow) infiltrates, each reduced in the dorsal (top rows) and ventralateral (bottom rows) white matter of spinal cords during EAE. Scale bar, 100 µm. Quantitative analysis show that astro-CCL2-CKO mice have a significant reduction in C, Iba-1 globoid macrophages and D, CD3 T cells in the dorsal and ventralateral white matter of the spinal cord during EAE. *p < 0.05 versus CCL2 WT (mean ± SEM).
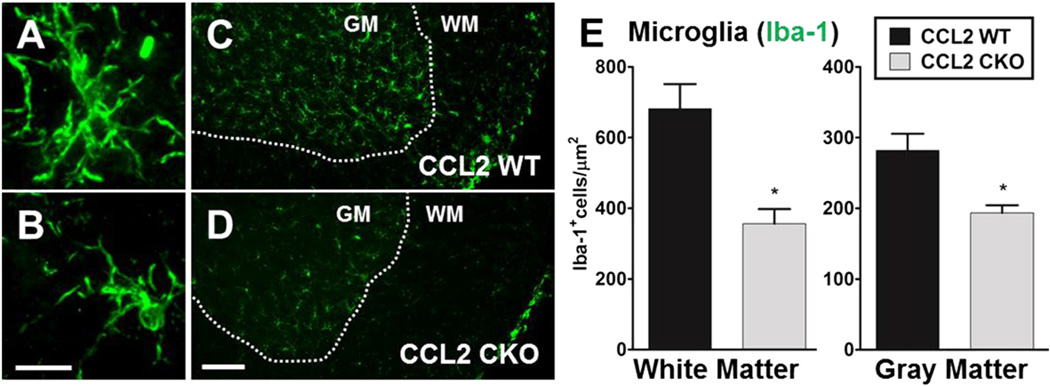
3.4. CCL2 deletion from astrocytes results in less activation of astrocytes and microglial diffusely during EAE
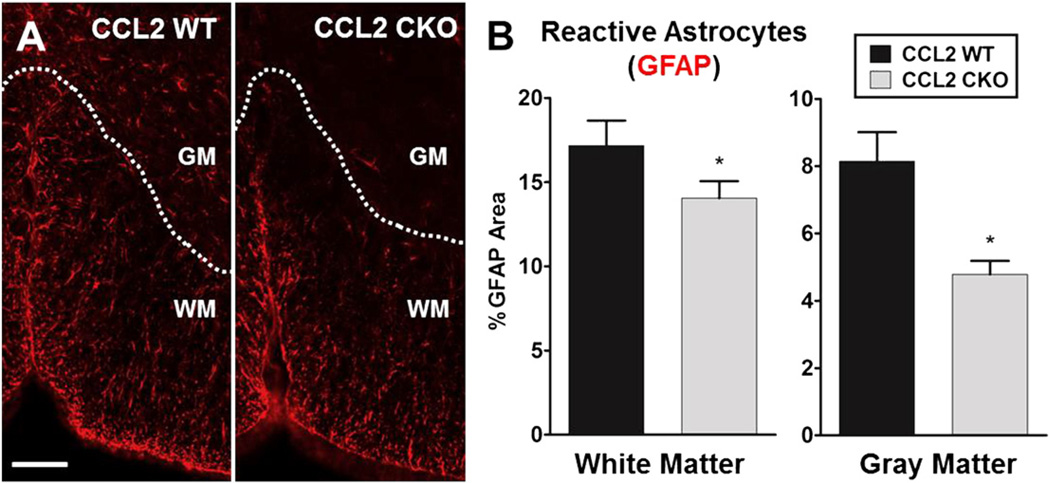
Astrocytes are known to become reactive and increase in number, not only in focal lesions, but also diffusively in both white and gray matter throughout the CNS during EAE (Liedtke et al., 1998; Voskuhl et al., 2009). In addition, microglial cells are known to change their morphology to a more ramified state and increase in number diffusely during EAE (Rasmussen et al., 2007). Thus, we next went beyond classic focal white matter lesions containing macrophage and T cell infiltrates to assess a role for CCL2 in astrocytes on diffuse glial activation in both white and gray matter of spinal cords during progressive EAE. Astro-CCL2-CKO mice showed a significant reduction in reactive astrocytes as compared to WT littermates in white matter and gray matter (Fig. 5). Also, less ramified/activated microglia were observed when comparing astro-CCL2-CKO versus WT littermates with EAE, and quantitative analysis showed fewer microglia in both white and gray matter of the spinal cords of astro-CCL2-CKO EAE mice (Fig. 6). Together these results demonstrated a role of CCL2 in astrocytes on diffuse glial activation in the CNS during progressive EAE.
Fig. 5.
Selective deletion of CCL2 from astrocytes results in less activation of astrocytes diffusively in the spinal cords during EAE. A, Representative immunofluorescence images show that astro-CCL2-CKOmice have less reactive GFAP+ astrocytes (red) in the gray matter (GM) and white matter(WM) of the spinal cord compared to CCL2 WT during EAE. Scale bar, 100 µm. B, Quantitative analysis shows that astro-CCL2-CKO mice have a significant reduction of GFAP expression in both white and gray matter of the spinal cord compared to CCL2 WT. *p < 0.05 versus CCL2 WT (mean ± SEM).
Fig. 6.
Selective deletion of CCL2 from astrocytes results in less microglial activation diffusively in the spinal cords during EAE. Representative immunofluorescence image of A, ramified/activated and B, resting microglial cell using Iba-1 staining (green). Scale bar, 10 µm. C and D, astro-CCL2-CKO mice show a reduced number of ramified/activated microglial cells in white matter (WM) and gray matter (GM) of the spinal cord compared to CCL2 WT mice during EAE. Scale bar, 100 µm. E, Quantitative analysis confirms that astro-CCL2-CKO mice have reduced activated microglial cells in the white and gray matter of the spinal cord during EAE. *p < 0.05 versus CCL2 WT (mean ± SEM).
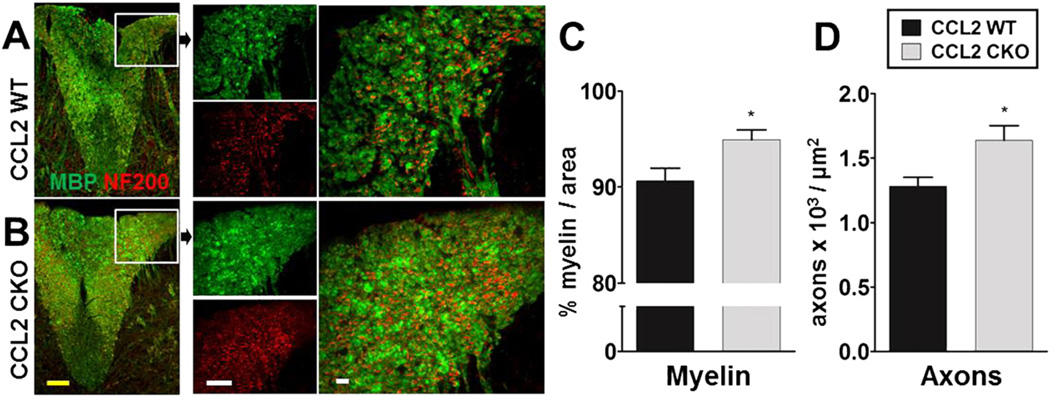
3.5. CCL2 deletion from astrocytes reduces demyelination and axonal loss during EAE
We evaluated EAE neuropathology, namely demyelination and axonal loss in spinal cord white matter, using immunofluorescence staining with anti-myelin basic protein (MBP) and anti-neurofilament (NF200) antibody staining, respectively. Astro-CCL2-CKO mice with EAE had more myelin (green) and more axonal (red) staining as compared to WT littermates with EAE (Fig. 7A,B). Quantitative analysis of myelin (Fig. 7C) and axons (Fig. 7D) confirmed the significant preservation myelin and axons in astro-CCL2-CKO spinal cords as compared to WT. These results are consistent with differences in EAE clinical scores of astro-CCL2-CKO mice versus WT (Fig. 3), since axonal loss has been shown to correlate with clinical disease severity (Wujek et al., 2002). Together, these results confirm the functional significance of CCL2 in astrocytes during the later progressive phase of EAE.
Fig. 7.
Astrocyte CCL2 CKO, as compared to WT littermates, has less demyelination and axonal loss during EAE. A and B, Immunofluorescence images of MBP+ myelin (green) and NF200+ axon (red) in the spinal cord dorsal column of astro-CCL2-CKO and CCL2 WT mice induced with EAE. Left images show entire dorsal column of the spinal cord, with images in boxes shown in higher magnification. Astro-CCL2-CKO, compared to CCL2 WT, has more myelin and axons in the dorsal column shown in individual (left) and merged (right) images. Scale bars; yellow, 100 µm: white, 20 µm. Quantitative analysis shows that astro-CCL2-CKO mice have more C, myelin and D, axons in the dorsal column of the spinal cord compared to CCL2 WT during EAE. *p < 0.05 versus CCL2 WT (mean ± SEM).
3.6. CCL2 deletion from astrocytes reduces had no effect during early EAE
Lastly, given our findings of effects of CCL2 on astrocytes during late EAE, we ascertained whether such effects might also be observed early during EAE. Thus, in additional experiments, mice were sacrificed early in EAE, before clinical disease scores began to differ between astro-CCL2-CKO and WT littermates, and assessed for potential differences in immune cell (T cell and macrophage) infiltrates, diffuse glial (astrocytes and microglia), demyelination and axonal loss. In contrast to differences observed at late in EAE, such differences were not found early (Fig. 8). This revealed that effects of CCL2 in astrocytes that were observed late in EAE were not merely secondary to early differences, but rather were due to effects of CCL2 in astrocytes during late disease. This is consistent with our conclusion that CCL2 in astrocytes is important for T cell and macrophage infiltration into the CNS during the late chronic phase of the disease.
Fig. 8.
No differences were found between astro-CCL2-CKO mice as compared to WT littermates during the early phase of EAE. A, Astro-CCL2-CKO mice have similar clinical disease scores as WT mice early during EAE. B, Representative immunofluorescence images and quantitative analysis show that astro-CCL2-CKO and WT have similar levels of Iba-1 (green) globoid macrophage (arrowhead) and CD3 (red) T cell (arrow) infiltrates in the spinal cord dorsal column. Scale bar, 100 µm. Quantitative analyses confirm that astro-CCL2-CKO mice and CCL2 WT mice also have similar levels of diffuse activation of C, reactive astrocytes and D, activated microglial cells in the white and gray matter of the spinal cord early during. E, Quantitative analyses show that astro-CCL2-CKO mice and CCL2 WT mice have similar levels of myelin and axons in the dorsal column of the spinal cord.
4. Discussion
By selectively removing CCL2 from astrocytes, we found that CCL2 expression in astrocytes is functionally significant during EAE. It plays a role in focal macrophage and T cell infiltration in spinal cord white matter as well as diffuse activation of astrocytes and microglia in both white and gray matter, together consistent with the more severe EAE clinical scores, demyelination and axonal loss observed in wild type littermates as compared to astro-CCL2-CKOs.
Seminal work on the important role of CCL2 in the pathogenesis of EAE was done by the Ransohoff group (Huang et al., 2001) and the Karpus group (Kennedy et al., 1998; Fife et al., 2000; Dogan et al., 2008). While originally, CCL2 was thought to primarily be involved in the generation of encephalitogenic immune responses, their elegant work involving knock outs during adoptive EAE, as well as induction of active EAE in bone marrow chimeras, together revealed that it was CCL2 expression in the CNS that was critical in mediating macrophage and T cell infiltrates during EAE. Specifically, CCL2 production from resident CNS was thought to contribute to the recruitment of myeloid dendritic cells and macrophages expressing TNFα and iNOS during EAE (Dogan et al., 2008). Until now, the CNS resident cell with functionally significant CCL2 expression during EAE has remained unknown. Here, we created mice with a specific deletion of CCL2 in astrocytes and showed effects on the recruitment of macrophages and T cells to the CNS during EAE, thereby showing functional significance of CCL2 expression by astrocytes in mediating inflammation. This is consistent with previous reports showing a correlation between higher astrocyte CCL2 expression and worse disease severity in a variety of CNS injury models (Brambilla et al., 2005; Conductier et al., 2010; Carrillo-de Sauvage et al., 2012; Hamby et al., 2012), and that CCL2 is expressed in astrocytes surrounding lesions in MS patients (McManus et al., 1998; Simpson et al., 1998). That said, our finding of a role for CCL2 in astrocytes is not mutually exclusive of an additional role for CCL2 in other CNS resident cells. Indeed, we found that astro-CCL2-CKO mice had reduced EAE severity late in disease while having a similar incidence and severity of disease at onset as compared to wild type (WT) control littermates. In this context it is important to note that our analysis of reporter gene expression demonstrates Cre-recombinase activity in essentially all spinal cord astrocytes in healthy, uninjured young adult mGFAP-Cre transgenic mice, indicating that CCL2 mediated deletion from astrocytes would have occurred prior to the onset of EAE. Given previous reports that global CCL2 knockouts were resistant to disease induction (Fife et al., 2000), together this may suggest that CCL2 on astrocytes is critical to ongoing, continuing inflammation late in chronic EAE, while CCL2 expression on other CNS resident cells, such as endothelial cells (Berman et al., 1996; dos Santos et al., 2005; Ge et al., 2009), may be important in initial early inflammatory processes at disease onset. Distinguishing between earlier versus later inflammatory mechanisms may be important in developing the most appropriate treatment strategies for various phases of MS.
Reactive astrocytosis with production of NFkappaB (NFκB)-dependent proinflammatory molecules including CCL2 are thought to play a role in continued inflammation and secondary injury processes not only in EAE and MS, but also in other neurological disorders. This neuroinflammatory pathway may be therefore an important mechanism to target with candidate neuroprotective treatments. Indeed, the mechanism of estradiol as a neuroprotective treatment involves this neuroinflammatory pathway. Initially, estradiol treatment was shown to reduce EAE clinical disease severity and decrease CNS inflammation through its effects on peripheral immune responses, with global estrogen receptor (ER)α knock outs and selective ERα ligand treatments showing that this was mediated through ERα (Liu et al., 2003; Polanczyk et al., 2003; Morales et al., 2006). Astrocytes were then shown to be a direct target of ERα ligand treatment using conditional deletion of ERα selectively removed from astrocytes. Mice with astro-ERα-CKO exhibited significantly reduced estrogen mediated clinical disease protection and less CNS anti-inflammatory effects (Spence et al., 2011) as well as complete loss of the estrogen mediated reduction in astrocyte CCL2 expression (Spence et al., 2013). Another complementary study showed that ERα ligand treatment ameliorated EAE and reduced astrocyte CCL2 production in vivo and inhibited TNFα induced CCL2 expression in vitro with direct suppression of NFκB recruitment to the CCL2 enhancer (Giraud et al., 2010). Finally, in both spinal cord injury and EAE, selective inactivation of NFκB in astrocytes lead to an improvement in clinical outcomes, with less white matter injury and reduced expression of proinflammatory chemokines and cytokines including CCL2 (Brambilla et al., 2005, 2009). Together these reports reinforce the conclusion that a promising approach for neuroprotective therapies would be to target the astrocyte's ability to upregulate CCL2 and other chemokines.
Designing neuroprotective treatments for neurological disorders is particularly challenging since many molecules, including chemokines, are expressed in the CNS, the immune system and other organ systems (Ransohoff, 2009). Thus, targeting specific molecules in the CNS can be confounded by off target binding with unexpected results. Targeting specific CNS cells is complicated by the various roles that a given cell may have during a disease process. Depending on the condition and the timing, astrocytes may have pro- or anti-inflammatory effects as well as neuroprotective effects mediated through glutamate uptake or neurotrophic factor production (Anderson et al., 2014; Sofroniew, 2014). In the MS model, EAE, CCL2 on astrocytes plays a role in attracting immune cell infiltrates to form CNS lesions, while the barrier function of scar forming astrocytes prevents focal inflammation from becoming widespread (Voskuhl et al., 2009). Thus, the challenge of future neurotherapeutics will be to design both molecule specific and cell specific treatments to be delivered at the optimal phase of an evolving disease process.
Acknowledgements
This work was supported by the National Institutes of Health [grant K24NS052117] and from the National Multiple Sclerosis Society [grants RG4033 and RG4364, to RRV], as well as funding from the Skirball Foundation, the Conrad N. Hilton Foundation and the Sherak Family Foundation. RK was supported in part by the UCLA Laboratory of Neuroendocrinology (LNE), funded by the National Institutes of Health [grant T32 HD07228-26]. The authors would like to acknowledge Penelope Kim-Lim for assistance with this work.
References
- Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Herschkovitz A, Eilam R, Blumberg-Hazan M, Sela M, Bruck W, et al. Demyelination arrest and remyelination induced by glatiramer acetate treatment of experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11358–11363. doi: 10.1073/pnas.0804632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Cerase J, Menendez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 1997;159:1604–1612. [PubMed] [Google Scholar]
- Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman PG, Hahn A, Ramirez S, Morley M, Bonnemann C, Yu S, et al. Motor neuron pathology in experimental autoimmune encephalomyelitis: studies in THY1-YFP transgenic mice. Brain. 2005;128:1877–1886. doi: 10.1093/brain/awh550. [DOI] [PubMed] [Google Scholar]
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J. Immunol. 1996;156:3017–3023. [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J. Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Carrillo-de Sauvage MA, Gomez A, Ros CM, Ros-Bernal F, Martin ED, Perez-Valles A, et al. CCL2-expressing astrocytes mediate the extravasation of T lymphocytes in the brain. Evidence from patients with glioma and experimental models in vivo. PLoS One. 2012;7:e30762. doi: 10.1371/journal.pone.0030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Dogan RN, Elhofy A, Karpus WJ. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J. Immunol. 2008;180:7376–7384. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos AC, Barsante MM, Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis–an intravital microscopy study. J. Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol. Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Eng LF, Gerstl B, Vanderhaeghen JJ. A study of proteins in old multiple sclerosis plaques. Trans. Am. Soc. Neurochem. 1970;1:42. [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem. Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Murugesan N, Pachter JS. Astrocyte- and endothelial-targeted CCL2 conditional knockout mice: critical tools for studying the pathogenesis of neuroinflammation. J. Mol. Neurosci. 2009;39:269–283. doi: 10.1007/s12031-009-9197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J. Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: prospects and promise. Ann. Neurol. 2013;74:317–327. doi: 10.1002/ana.24009. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J. Neuroimmunol. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, et al. Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Chiu FC, Kucherlapati R, Raine CS. Experimental autoimmune encephalomyelitis in mice lacking glial fibrillary acidic protein is characterized by a more severe clinical course and an infiltrative central nervous system lesion. Am. J. Pathol. 1998;152:251–259. [PMC free article] [PubMed] [Google Scholar]
- Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J. Immunol. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Tiwari-Wooddruff S, Sharma G, Aguilar C, Vo KT, Strickland LV, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48:637–651. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Rinek GA, Avedisian A, Gold SM, Frew AJ, Aguilar C, et al. Cortical atrophy in experimental autoimmune encephalomyelitis: in vivo imaging. Neuroimage. 2012;60:95–104. doi: 10.1016/j.neuroimage.2011.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J. Neuroimmunol. 1998;86:20–29. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J. Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Rossi S, Piccinin S, Motta C, Mango D, Kusayanagi H, et al. Synaptic plasticity and PDGF signaling defects underlie clinical progression in multiple sclerosis. J. Neurosci. 2013;33:19112–19119. doi: 10.1523/JNEUROSCI.2536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell. Mol. Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am. J. Pathol. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Schmidt H, Mildner A, Knobeloch K-P, Hanisch U-K, Raasch J, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Raine CS, Barnett LB, Brown A, Behar T, McFarlin DE. Neuropathology of experimental allergic encephalomyelitis in inbred strains of mice. Lab. Investig. 1980;43:150–157. [PubMed] [Google Scholar]
- Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Wang Y, Kivisakk P, Bronson RT, Meyer M, Imitola J, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing remitting experimental autoimmune encephalomyelitis. Brain. 2007;30:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Imitola J, Ayuso-Sacido A, Wang Y, Starossom SC, Kivisakk P, et al. Reversible neural stem cell niche dysfunction in a model of multiple sclerosis. Ann. Neurol. 2011;69:878–891. doi: 10.1002/ana.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ. JE/MCP-1: an early-response gene encodes a monocyte-specific cytokine. Cancer Cells. 1991;3:517–524. [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur. J. Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J. Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, et al. Neuroprotection mediated through estrogen receptor-{alpha} in astrocytes. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J. Neurosci. 2013;33:10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, et al. Sphingo-sine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J. Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, et al. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- Xu J, Sun SW, Naismith RT, Snyder AZ, Cross AH, Song SK. Assessing optic nerve pathology with diffusion MRI: from mouse to human. NMR Biomed. 2008;21:928–940. doi: 10.1002/nbm.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yu M, Nishiyama A, Trapp BD, Tuohy VK. Interferon-beta inhibits progression of relapsing-remitting experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1996;64:91–100. doi: 10.1016/0165-5728(95)00160-3. [DOI] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab. Investig. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Dervin SM, Umeda EA, O'Dell TJ, Voskuhl RR. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J. Neurosci. 2012;32:12312–12324. doi: 10.1523/JNEUROSCI.2796-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]