Abstract
Inflammatory Breast Cancer (IBC) is a rare and aggressive form of breast cancer. Currently, multimodality treatment is recommended, but the optimal surgical management has not been fully elucidated. In this study, we investigated the long-term outcomes of utilizing breast conserving therapy in IBC patients undergoing neoadjuvant chemotherapy (NAC). 24 patients with IBC were treated from 2002 to 2006. NAC was initiated with doxorubicin and cyclophosphamide followed by paclitaxel. In addition, HER2/neu positive patients received trastuzumab while HER2/neu negative patients received bevacizumab. Clinical response was assessed by dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) prior to surgery and pathologic response following surgery. A partial mastectomy with sentinel lymph node biopsy and/or axillary lymph node dissection or a modified radical mastectomy was performed based on the surgeon's recommendations and patient's preference. All patients received adjuvant radiation. Of the 24 patients, 7 (29%) underwent a partial mastectomy and 17 (71%) underwent a mastectomy. The overall survival rate for partial mastectomy and for mastectomy patients was 59% and 57% (p-value= 0.49), respectively, at a median follow-up of 60 months (range 48-92 months). Breast conserving therapy can be considered in a selected group of patients who demonstrate a good response to NAC.
Introduction
Inflammatory breast cancer (IBC) is a rare and aggressive form of breast cancer. It accounts for approximately 1% to 6% of all breast cancer diagnoses in the United States (1). Currently, the diagnosis of IBC relies heavily on the initial clinical presentation of the patient. The American Joint Committee on Cancer (AJCC) defines IBC as a clinicopathologic entity characterized by diffuse erythema and edema, which has a peau d’orange appearance that involves the majority of the breast, but often without an underlying mass (2). Microscopically, dermal lymphatic invasion is typically seen, but it is not sufficient, nor necessary, for the diagnosis. Current IBC therapy is multimodal with Neoadjuvant Chemotherapy (NAC) subsequently followed by a modified radical mastectomy and adjuvant radiotherapy (3). The goal of NAC is to eliminate micrometastatic disease while making the primary tumor amenable to surgical resection. Dynamic Contrast Enhanced magnetic resonance imaging (DCE-MRI) can accurately visualize characteristic features of IBC, such as diffuse skin thickening and lymphatic invasion (4). Thus, DCE-MRI can be used to monitor the response of IBC to NAC, which can assist in surgical planning (5).
IBC therapy has not been fully elucidated and is still controversial. The purpose of this study was to investigate the long-term outcomes of breast conserving therapy as part of a multimodality approach in the treatment of IBC patients. We predicted that long-term survival rates would be comparable in patients managed with a mastectomy or breast conserving surgery.
Methods
After receiving institutional review board approval, a retrospective analysis of data collected from 2002 to 2006 was undertaken to identify patients diagnosed with IBC and treated at the University of California, Irvine Chao Family Comprehensive Cancer Center.
All patients received induction therapy consisted of bi-weekly dose-dense Doxorubicin and Cyclophosphamide (AC) as the first-line regimen. The second-line taxane based regimen consisted of Paclitaxel or Nab-paclitaxel combined with Carboplatin. In addition, patients who were HER2/neu positive received trastuzumab and HER2/neu negative patients received bevacizumab. The patient's response to NAC was monitored with serial DCE-MRI. The MRI was done on a 1.5 T MR scanner (Philips Medical Systems, Cleveland, Ohio). If the 1-dimenional size reduction after completing NAC was less than 30% compared to their pre-treatment size, the case was classified as a non-responder (NR). If residual tumor was present with greater than 30% size reduction, the case was classified as a partial response (PR). Cases in which no enhanced tissues were visible or with minimal enhancement magnitude equivalent to or lower than the normal breast tissue were classified as a complete clinical response (cCR) (Figure 1). Following completion of NAC, DCE-MRI was performed to guide surgical planning. A partial mastectomy with sentinel lymph node biopsy and/or axillary lymph node dissection or a modified radical mastectomy was performed based on the surgeon's recommendations and the patient's preference. Patients were examined histologically at the time of surgery for pathologic response. All patients received post-operative radiation appropriate for the selected surgical procedure.
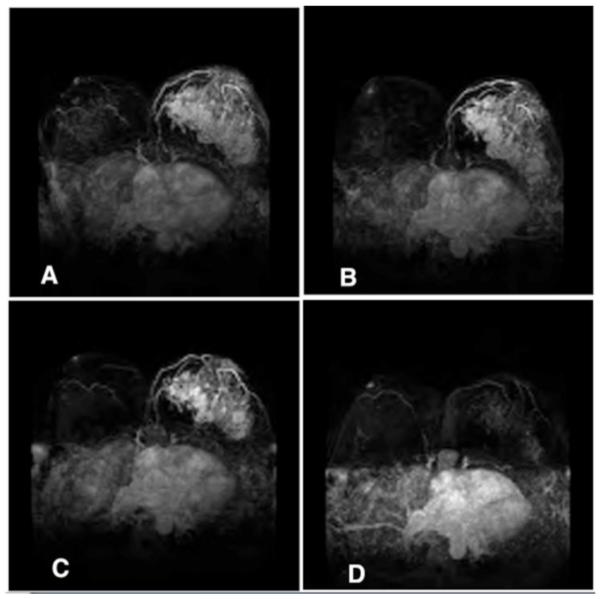
Fig. 1.
Representative example of complete radiographic response to neoadjuvant chemotherapy (NAC) on MRI. Tumor prior to NAC (A), after 2 cycles of dose-dense Doxorubicin and Cyclophosphamide (B), after 4 cycles (C), and at completion of NAC (D). Patient went on to have a mastectomy with pathology showing a 1 mm focus of ductal carcinoma in situ.
Results
24 patients were diagnosed with IBC during the data collection period. 17 (71%) patients underwent a modified radical mastectomy and 7 (29%) patients underwent a partial mastectomy. Table 1 summarizes the patient characteristics for each treatment cohort. The median age was 51 years and 49 years for the mastectomy and partial mastectomy groups, respectively. The average tumor size was 7.7 cm and 4.4 cm, respectively. The predominant tumor type was invasive ductal carcinoma for each cohort. The majority of patients were estrogen receptor negative (ER-), progesterone receptor negative (PR-), and HER2/neu positive. Patients were stage IIIB-IV. Table 2 summarizes the treatment outcomes for each cohort. Histologically, 58% of mastectomy and 57% of partial mastectomy patients had a complete pathological response (pCR) at the time of surgery (p-value= 0.50). DCE-MRI identified a cCR for 65% of mastectomy and 71% of partial mastectomy patients (p-value=0.49). Overall survival (OS) was 59% for the mastectomy cohort and 57% for the partial mastectomy cohort (p-value= 0.49). The median follow-up was 60 months with a range of 48-92 months for both cohorts.
Table 1.
Patient Characteristics
| Mastectomy | Partial Mastectomy | |
|---|---|---|
| N=17 | N=7 | |
| Age | ||
| Median | 51 years | 49 years |
| Range | 31-68 years | 32-76 years |
| Tumor Size | ||
| Average | 7.7 cm | 4.4 cm |
| Range | 2.5-16 cm | 2.9-7.1 cm |
| Tumor Type | ||
| IDC | 15 (88%) | 4 (57%) |
| IDC with metaplastic tumor | 2 (12%) | 1 (14%) |
| Mixed IDC/ILC | 0 (0%) | 2 (29%) |
| Biomarkers | ||
| ER Positive | 5 (29%) | 2 (29%) |
| ER Negative | 12 (71%) | 5 (71%) |
| PR Positive | 4 (34%) | 3 (33%) |
| PR Negative | 13 (76%) | 4 (57%) |
| HER2/neu Positive | 10 (59%) | 5 (71%) |
| HER2/neu Negative | 7 (41%) | 2 (29%) |
| Stage | IIIB-IV | IIIB-IV |
IDC Invasive Ductal Carcinoma
ILC Invasive Lobular Carcinoma
ER Estrogen Receptor
PR Progesterone Receptor
Table 2.
Treatment Outcome
| Mastectomy (N=17) |
Partial Mastectomy (N=7) |
p-value | |
|---|---|---|---|
| Complete pathological response (pCR) |
10 (58%) | 4 (57%) | 0.50 |
| Incomplete pathological response |
7 (42%) | 3 (43%) | 0.50 |
| Complete clinical response (cCR) on DCE-MRI |
11 (65%) | 5 (71%) | 0.49 |
| Partial response (PR) on DCE- MRI |
5 (29%) | 2 (29%) | 1.00 |
| Non-responder (NR) on DCE- MRI |
1 (6%) | 0 (0%) | 0.49 |
| Overall Survival | 59% | 57% | 0.49 |
| Median Follow up | 60 (48-92 months) | 60 (48-92 months) |
Discussion
IBC is a locally aggressive disease characterized by dermal lymphatic involvement, rapid tumor growth, and early development of distant metastases. Although the current standard of care incorporates the use of NAC, the inclusion of surgery and extent of it are controversial.
The goals of surgery are to maintain local control and improve survival. In the case of IBC, achieving local control is an important quality-of-life issue. Historically, IBC was treated by a mastectomy, if resectable, followed by radiotherapy. Predictably, this type of treatment plan resulted in a dismal 5-year OS rate of 4% (1, 6, 7). The addition of NAC, however, improved the OS rate for this aggressive disease, but a debate arose as to whether surgery still had a role in the treatment of IBC. To address this issue, several studies in the 1990’s showed an improved average 5-year OS rate of 46% and average disease free survival rate of 40% with a surgical component. In contrast, studies without surgical intervention yielded an average 5-year OS of 31% and average disease free survival rate of 21% (8).
As the importance of surgical intervention became established as part of multimodality treatment of IBC, the question arose as to the ideal extent of the surgery involved. A mastectomy including most of the overlying skin is the traditional approach. The reason for choosing this aggressive procedure was that clinical response, judged by physical examination, standard mammography and ultrasound, underestimated the extent of residual disease in more than 60% of patients (9, 10).
In this study, we looked at the characteristics of 24 patients who underwent NAC, but chose different surgical procedures at the end of their treatment. Overall, both groups had similar tumor profiles, except for size: the mastectomy group tended to have a larger overall tumor size than the partial mastectomy group. Other characteristics, such as post-NAC final pathological response and DCE-MRI clinical response, were similar in both groups. There was no significant difference between the two groups in the OS rate after being followed for 60 months post-treatment.
The observational result of this study questions the standard technique of performing a mastectomy on every IBC patient post-NAC. It appears that in certain instances, an IBC patient may safely choose to undergo a partial mastectomy instead of a traditional mastectomy and suffer no adverse effect on her OS rate. The problem will be to identify this select group of patients who can be offered this choice. One selection factor might be identifying the patients who have a favorable response to NAC as evidenced in the clinical response seen on DCE-MRI prior to surgery. From Table 2, it can be seen that the cCR seen on DCE-MRI correlates well with the final pathological response, which also correlates with the OS rate. A proposed scenario is to monitor a patient’s response to NAC with serial DCE-MRI, identify patients with a cCR on MRI, and then use the images to plan out the optimal area of resection if the patient decides on a partial mastectomy.
It must be noted that there are several limitations in this study, including a small sample size and retrospective design. In addition, the clinical diagnosis of IBC represents a challenge because of its subjective nature. Specifically, patients with other forms of locally advanced breast cancer with skin edema may have been included in our study population, giving an enhanced OS rate.
Conclusions
IBC, historically treated with surgical resection and radiotherapy alone, is now managed through a multimodality approach utilizing NAC, surgery and radiation therapy. A modified radical mastectomy has been the standard surgical procedure of choice. Presently, as novel chemotherapeutic regiments have resulted in improved tumor response and with the use of DCE-MRI to accurately monitor the response of treatment, the role for breast conserving surgery is being evaluated as a viable option. Our findings suggest that there was not a significant OS difference in performing a mastectomy versus a partial mastectomy in selected IBC patients (patients with a cCR). Future larger and randomized trials need to be conducted to better define the characteristic of these appropriate patients, the rate of local recurrence, and how these affect the quality of life.
Acknowledgments
This work was supported in part by NIH Grant Number: R01 CA127927
References
- 1.Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–75. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge S. AJCC Cancer Staging Manual. 7. Springer; New York NY: 2011. [Google Scholar]
- 3.Kell MR, Morrow M. Surgical aspects of inflammatory breast cancer. Breast Dis. 2005;22:67–73. doi: 10.3233/bd-2006-22108. [DOI] [PubMed] [Google Scholar]
- 4.Ojeda-Fournier H, de Guzman J, Hylton N. Breast magnetic resonance imaging for monitoring response to therapy. Magn Reson Imaging Clin N Am. 2013;21:533–46. doi: 10.1016/j.mric.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen JH, Bahri S, Mehta RS, et al. Impact of factors affecting the residual tumor size diagnosed by MRI following neoadjuvant chemotherapy in comparison to pathology. J Surg Oncol. 2013 Oct 28; doi: 10.1002/jso.23470. doi: 10.1002/jso.23470. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low JA, Berman AW, Steinberg SM, et al. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22:4067–74. doi: 10.1200/JCO.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Angulo AM, Hennessy BT, Broglio K, et al. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12:904–12. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 8.Singletary SE. Surgical management of inflammatory breast cancer. Semin Oncol. 2008;35:72–7. doi: 10.1053/j.seminoncol.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Hortobagyi G, Singletary SE, Strom EA. Treatment of locally advanced and inflammatory breast cancer. Lippincott, Williams & Wilkins; Philadelphia, PA: 2000. pp. 645–60. [Google Scholar]
- 10.Vlastos G, Fornage BD, Mirza NW, et al. The correlation of axillary ultrasonography with histological breast cancer downstaging after induction chemotherapy. Am J Surg. 2000;179:446–52. doi: 10.1016/s0002-9610(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 11.Fleming RY, Asmar L, Buzdar AU, et al. Effectiveness of mastectomy by response to induction chemotherapy for control in inflammatory breast carcinoma. Ann Surg Oncol. 1997;4:452–61. doi: 10.1007/BF02303668. [DOI] [PubMed] [Google Scholar]
- 12.Curcio LD, Rupp E, Williams WL, et al. Beyond palliative mastectomy in inflammatory breast cancer--a reassessment of margin status. Ann Surg Oncol. 1999;6(3):249–54. doi: 10.1007/s10434-999-0249-3. [DOI] [PubMed] [Google Scholar]