Abstract
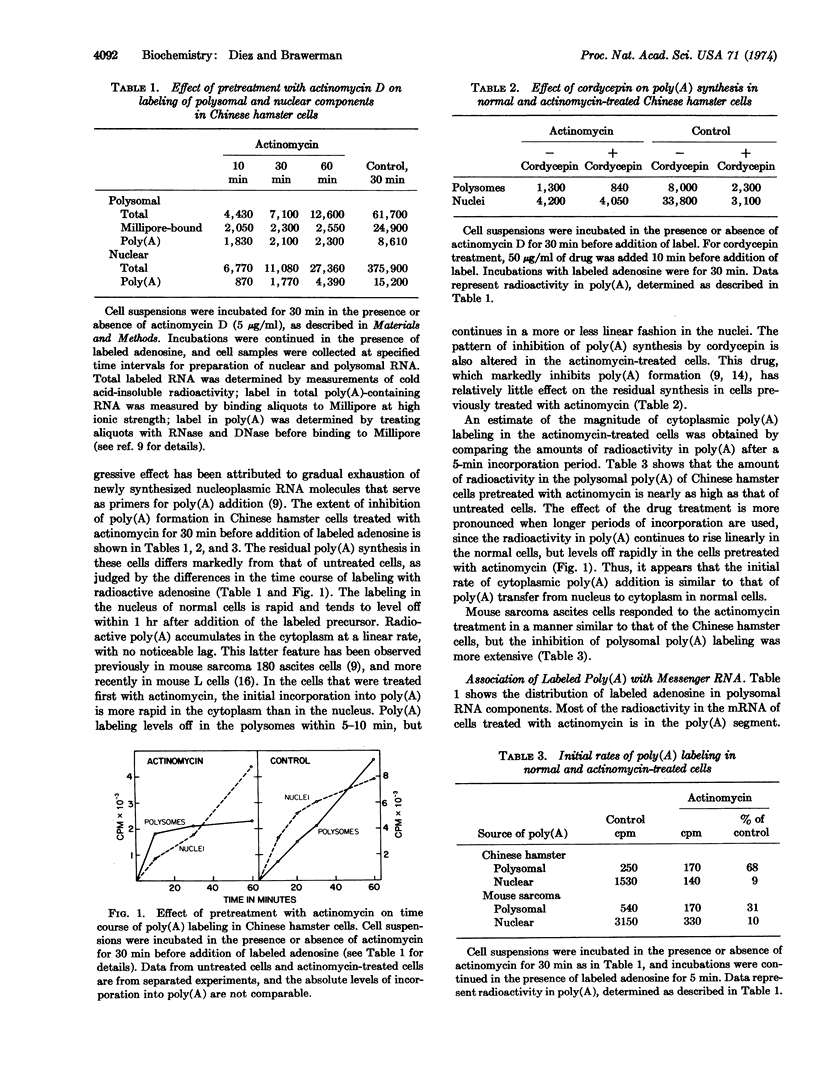
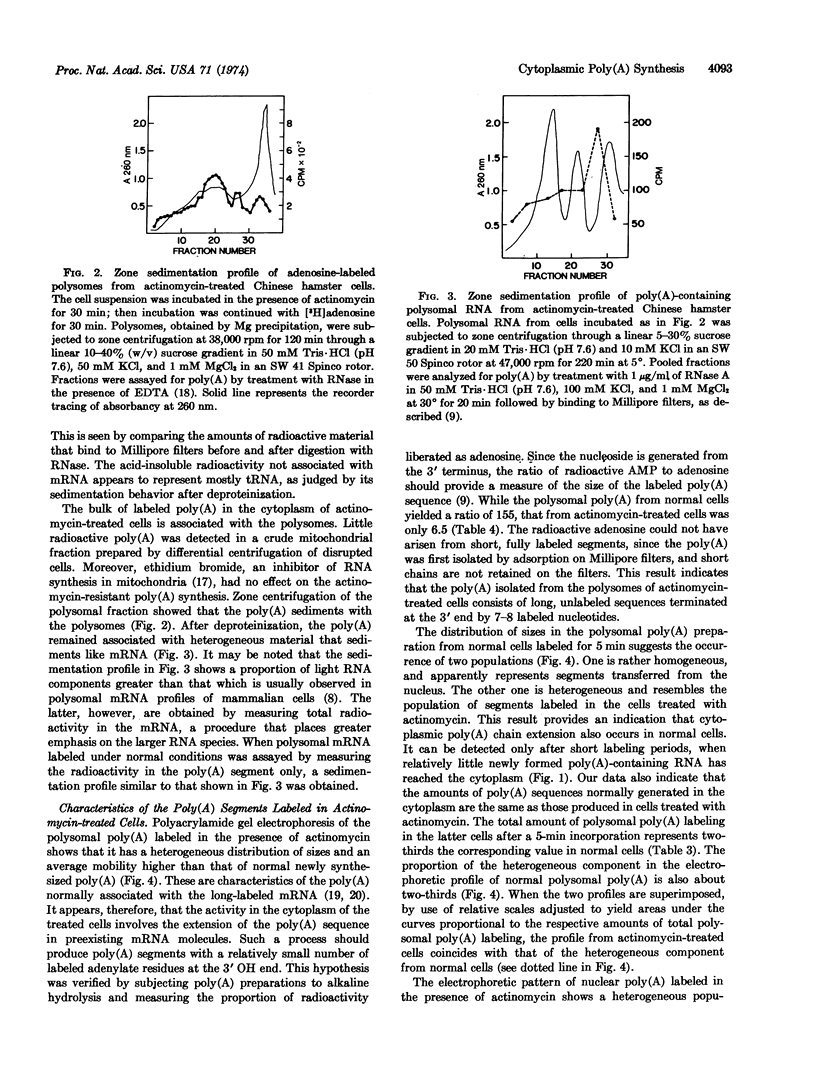
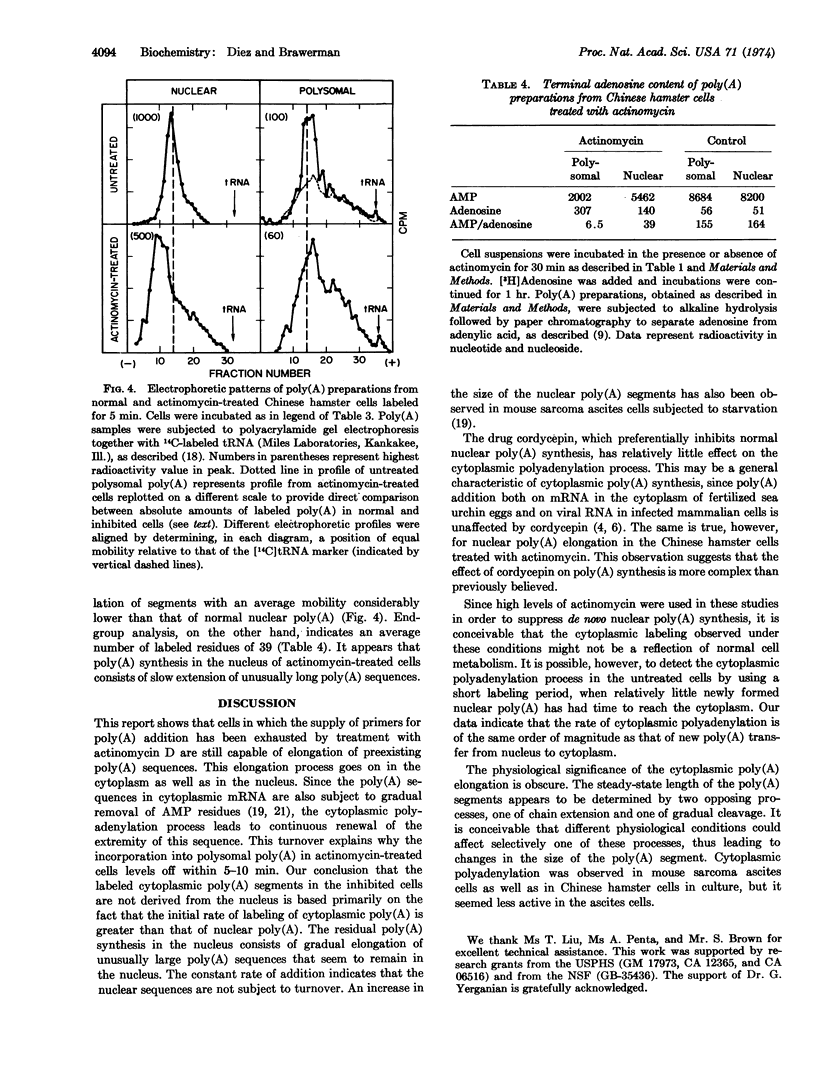
Chinese hamster and mouse sarcoma 180 ascites cells, treated with high levels of actinomycin D, still carry out limited poly(A) synthesis. The residual activity, which consists of poly(A) chain extension in the cytoplasm as well as in the nucleus, is essentially insensitive to cordycepin. Nuclear polyadenylation proceeds linearly and involves the gradual extension of unusually long poly(A) sequences. Cytoplasmic poly(A) labeling is initially more rapid than in the nucleus, but levels off within 5-10 min. It consists of addition of seven to eight AMP residues to the poly(A) sequences in preexisting mRNA molecules. The levelling off can be accounted for by a rapid turnover of the extremity of the poly(A) chain in the mRNA. Cytoplasmic poly(A) chain extension can be detected in cells not subjected to the actinomycin treatment. The rate of this process is of the same order of magnitude as that of new poly(A) transfer from nucleus to cytoplasm. It could serve to control the length of the poly(A) sequence in mRNA.
Keywords: poly(A) synthesis, actinomycin D, cordycepin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz D. M., Kakefuda T., Sporn M. A simple and rapid method for the isolation of enzymatically active HeLa cell nuclei. J Cell Biol. 1969 Sep;42(3):851–854. doi: 10.1083/jcb.42.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. The isolation and characterization of steady-state labeled messenger RNA from L-cells. Biochim Biophys Acta. 1972 Dec 6;287(2):361–366. doi: 10.1016/0005-2787(72)90386-3. [DOI] [PubMed] [Google Scholar]
- Kwan S. W., Brawerman G. A particle associated with the polyadenylate segment in mammalian messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3247–3250. doi: 10.1073/pnas.69.11.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Brawerman G. Pulse-labeled ribonucleic acid complexes released by dissociation of rat liver polysomes. Biochemistry. 1971 Feb 2;10(3):510–516. doi: 10.1021/bi00779a025. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Initiation of polysome formation in mouse sarcoma 180 ascites cells. Utilization of cytoplasmic messenger ribonucleic acid. Biochemistry. 1971 Mar 2;10(5):895–900. doi: 10.1021/bi00781a026. [DOI] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Slater I., Gillespie D., Slater D. W. Cytoplasmic adenylylation and processing of maternal RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):406–411. doi: 10.1073/pnas.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria M., Huang A. S. Association of polyadenylic acid with messenger RNA of vesicular stomatitis virus. J Mol Biol. 1973 Jul 5;77(3):449–455. doi: 10.1016/0022-2836(73)90450-6. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]



