Abstract
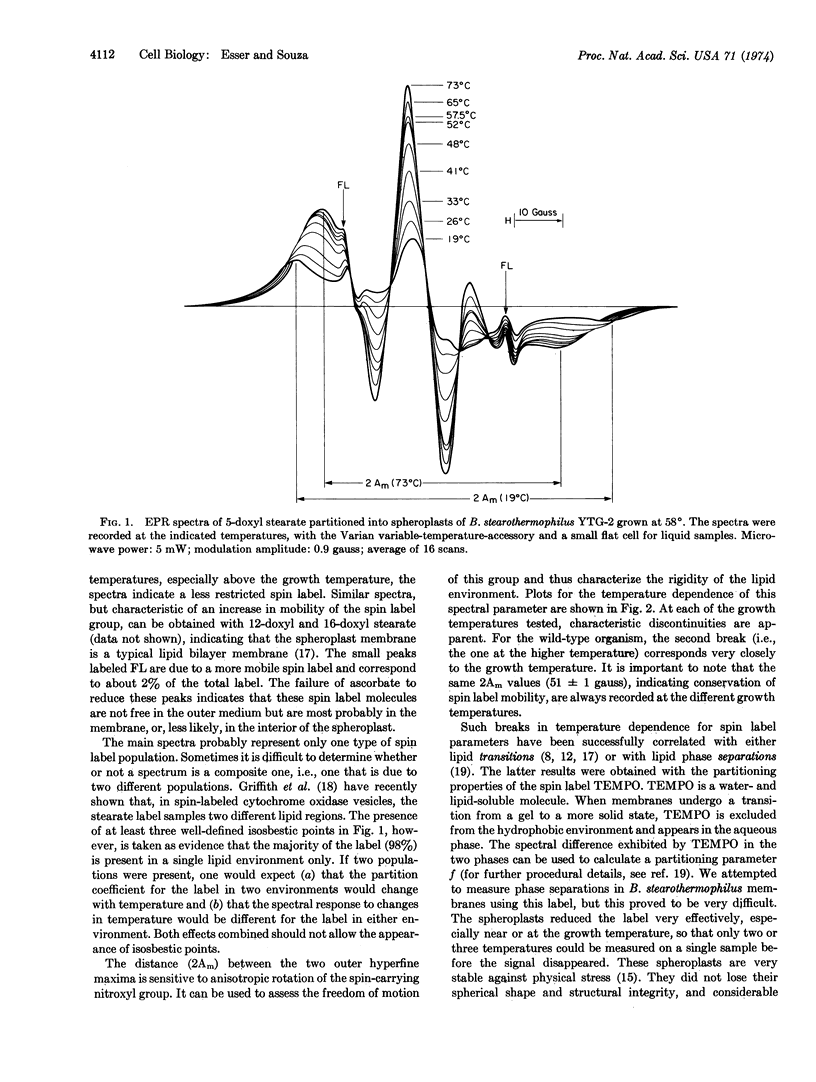
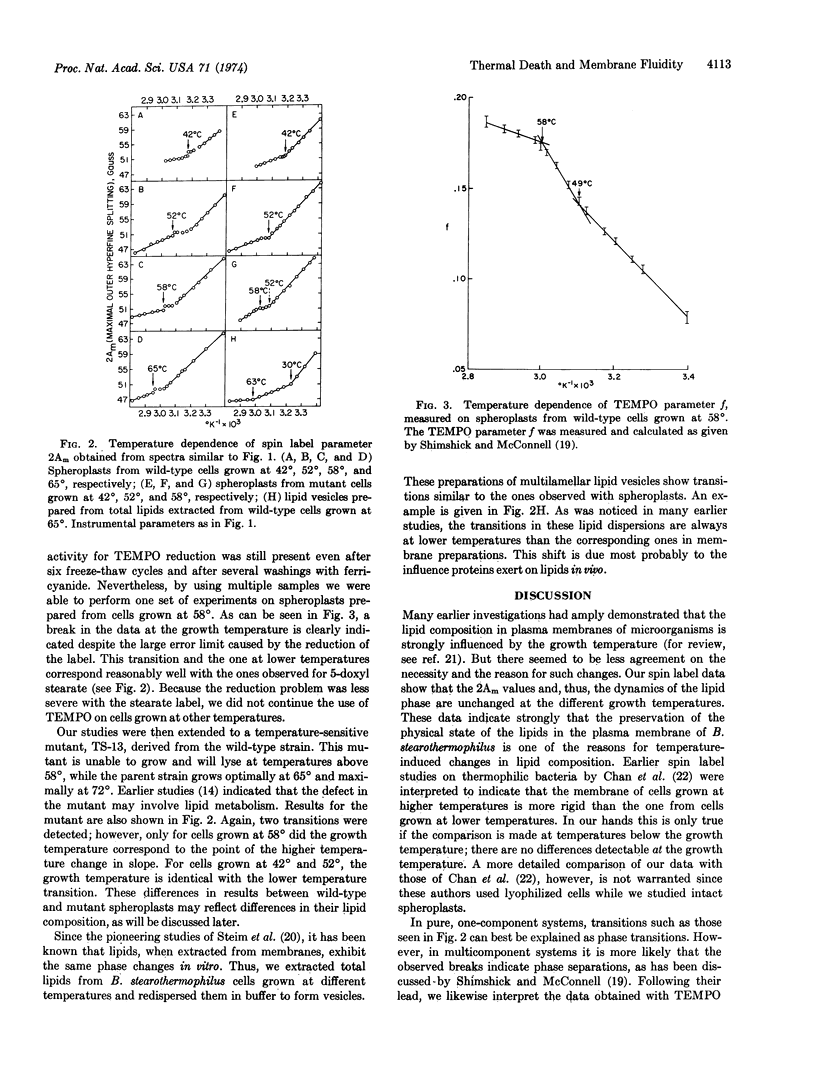
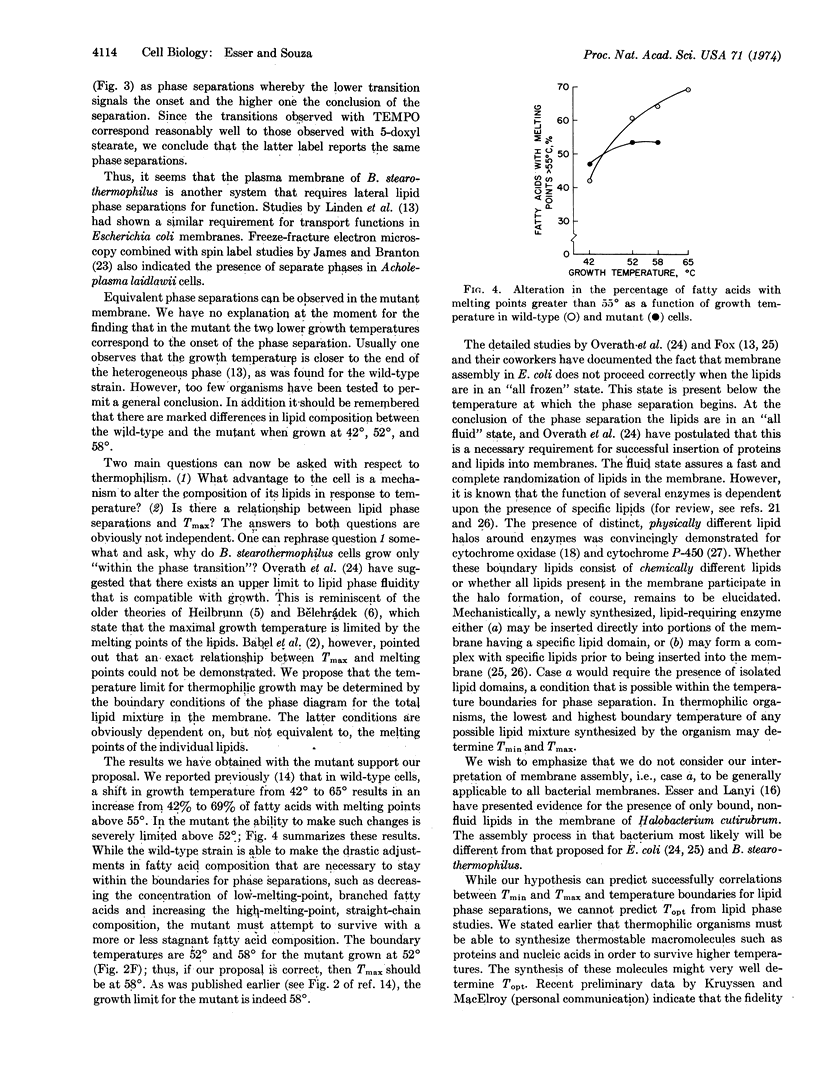
Paramagnetic resonance spectra of spin labels partitioned into spheroplast membranes of Bacillus stearothermophilus indicate lateral lipid phase separations. Cells adjust their lipid composition in response to temperature changes so that the same change of state in membrane phospholipids is achieved at the respective growth temperature. A temperature-sensitive mutant that fails to change its lipid composition above a certain temperature can survive only up to the higher temperature boundary for lateral phase separation. These data are interpreted to indicate that the maximal and minimal growth temperatures of thermophiles are regulated by the onset and conclusion of phase separations of the particular lipid composition they synthesize. It is suggested that isolated lipid domains are required for functional membrane assembly.
Keywords: thermophiles, spin labels, lateral lipid phase separation, membrane biogenesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babel W., Rosenthal H. A., Rapoport S. A unified hypothesis on the causes of the cardinal temperatures of microorganisms; the temperature minimum of Bacillus stearothermophilus. Acta Biol Med Ger. 1972;28(4):565–576. [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Chan M., Virmani Y. P., Himes R. H., Akagi J. M. Spin-labeling studies on the membrane of a facultative thermophilic bacillus. J Bacteriol. 1973 Jan;113(1):322–328. doi: 10.1128/jb.113.1.322-328.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Daron H. H. Fatty acid composition of lipid extracts of a thermophilic Bacillus species. J Bacteriol. 1970 Jan;101(1):145–151. doi: 10.1128/jb.101.1.145-151.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser A. F., Lanyi J. K. Structure of the lipid phase in cell envelope vesicles from Halobacterium cutirubrum. Biochemistry. 1973 May 8;12(10):1933–1939. doi: 10.1021/bi00734a016. [DOI] [PubMed] [Google Scholar]
- Griffith O. H., Jost P., Capaldi R. A., Vanderkooi G. Boundary lipid and fluid bilayer regions in cytochrome oxidase model membranes. Ann N Y Acad Sci. 1973 Dec 31;222:561–573. doi: 10.1111/j.1749-6632.1973.tb15287.x. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D. Membrane properties of saturated fatty acid mutants of yeast revealed by spin labels. Chem Phys Lipids. 1971 Dec;7(4):245–265. doi: 10.1016/0009-3084(71)90004-1. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- JOHNSTON P. V., ROOTS B. I. BRAIN LIPID FATTY ACIDS AND TEMPERATURE ACCLIMATION. Comp Biochem Physiol. 1964 Mar;11:303–309. doi: 10.1016/0010-406x(64)90111-2. [DOI] [PubMed] [Google Scholar]
- James R., Branton D. Lipid- and temperature-dependent structural changes in Acholeplasma laidlawii cell membranes. Biochim Biophys Acta. 1973 Oct 25;323(3):378–390. doi: 10.1016/0005-2736(73)90183-1. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza K. A., Kostiw L. L., Tyson B. J. Alterations in normal fatty acid composition in a temperature-sensitive mutant of a thermophilic bacillus. Arch Microbiol. 1974 Apr 19;97(2):89–102. doi: 10.1007/BF00403049. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenesh J., Holazo A. A. Studies of the ribosomal ribonucleic acid from mesophilic and thermophilic bacteria. Biochim Biophys Acta. 1967 Apr 18;138(2):286–295. doi: 10.1016/0005-2787(67)90489-3. [DOI] [PubMed] [Google Scholar]
- Stier A., Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta. 1973 Jul 6;311(3):400–408. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Transport system assembly and the mobility of membrane lipids in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2822–2829. doi: 10.1021/bi00739a008. [DOI] [PubMed] [Google Scholar]
- Wisdom C., Welker N. E. Membranes of Bacillus stearothermophilus: factors affecting protoplast stability and thermostability of alkaline phosphatase and reduced nicotinamide adenine dinucleotide oxidase. J Bacteriol. 1973 Jun;114(3):1336–1345. doi: 10.1128/jb.114.3.1336-1345.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



