Abstract
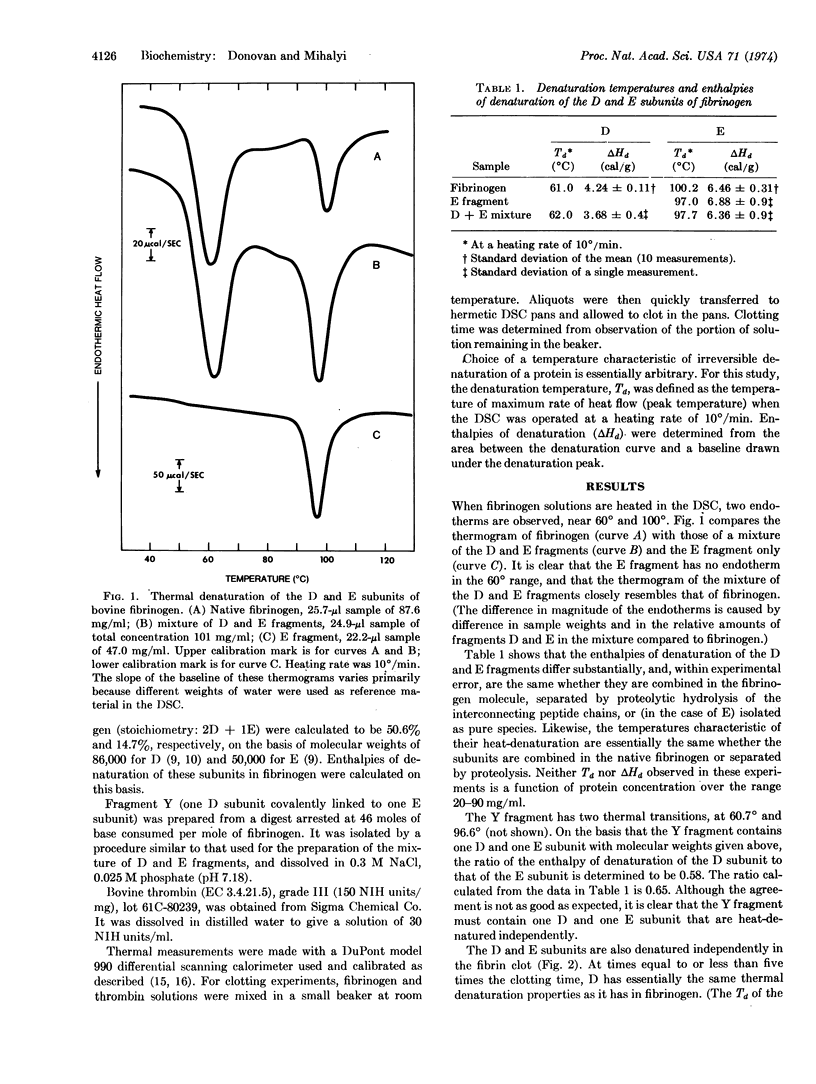
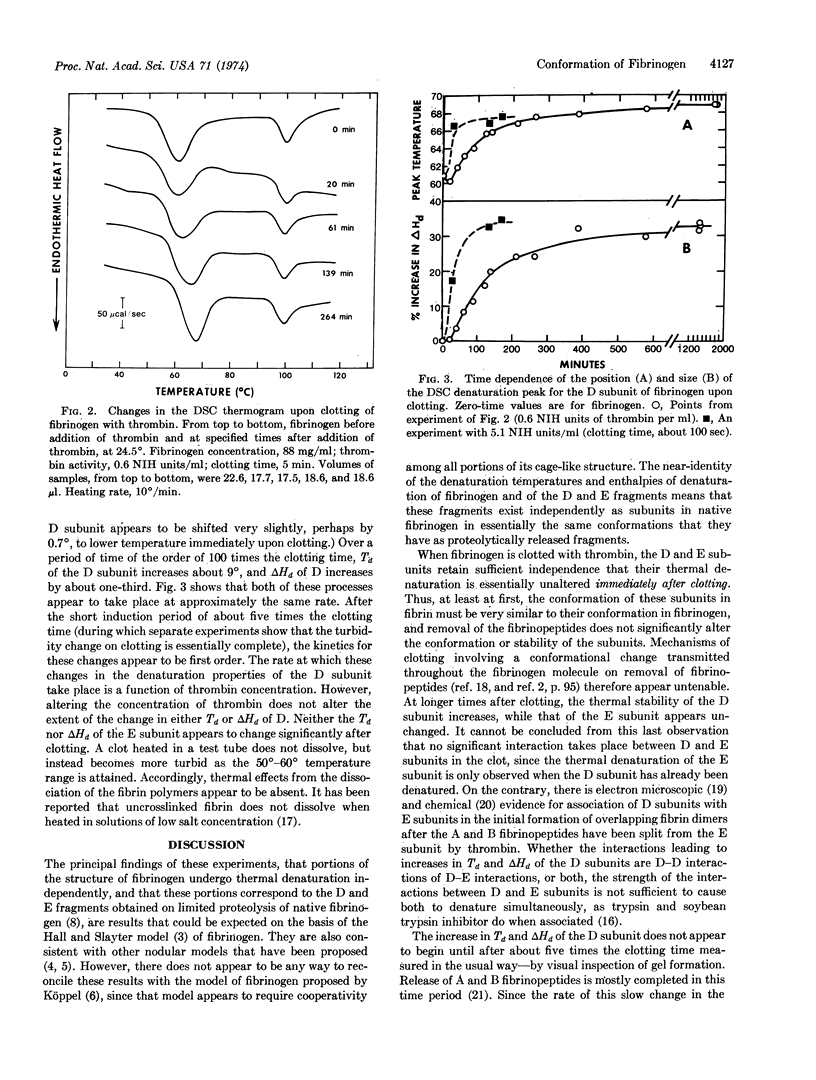
Solutions of fibrinogen show two endothermal (denaturing) transitions, at 61° and at 100°, when heated in a differential scanning calorimeter. Similar transitions are observed for a mixture of the fragments D and E obtained by limited proteolysis of fibrinogen. Isolated fragment E shows only a single transition, at 97°. The independent thermal denaturation of these portions of fibrinogen supports the three-nodular model proposed for fibrinogen. The D and E subunits retain their characteristic denaturation behavior when fibrinogen is clotted by thrombin addition, but over a period of about one hundred times the clotting time, the denaturation temperature of the D subunit increases by 9° and its enthalpy of denaturation by one-third. Since this change takes place in the absence of Factor XIII activity, and its rate is proportional to thrombin concentration, it is presumed to be mediated by a proteolytic cleavage distinct from those which liberate the A and B fibrinopeptides.
Keywords: fibrin, blood clotting, differential scanning calorimetry, protein subunits, thermal denaturation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donovan J. W., Ross K. D. Increase in the stability of avidin produced by binding of biotin. A differential scanning calorimetric study of denaturation by heat. Biochemistry. 1973 Jan 30;12(3):512–517. doi: 10.1021/bi00727a024. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S., Wallén P., Gröndahl N. J., Henschen A., Blombäck B. On the primary structure of human fibrinogen. Isolation and characterization of N-terminal fragments from plasmic digests. Eur J Biochem. 1969 Mar;8(2):189–199. doi: 10.1111/j.1432-1033.1969.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Johnson P., Mihalyi E. Physicochemical studies of bovine fibrinogen. II. Depolarization of fluorescence studies. Biochim Biophys Acta. 1965 Jul 22;102(2):476–486. doi: 10.1016/0926-6585(65)90138-x. [DOI] [PubMed] [Google Scholar]
- KITZINGER C., LAKI K. Heat changes during the clotting of fibrinogen. Nature. 1956 Nov 3;178(4540):985–985. doi: 10.1038/178985a0. [DOI] [PubMed] [Google Scholar]
- Kay D., Cuddigan B. J. The fine structure of fibrin. Br J Haematol. 1967 May;13(3):341–347. doi: 10.1111/j.1365-2141.1967.tb08749.x. [DOI] [PubMed] [Google Scholar]
- Krakow W., Endres G. F., Siegel B. M., Scheraga H. A. An electron microscopic investigation of the polymerization of bovine fibrin monomer. J Mol Biol. 1972 Oct 28;71(1):95–103. doi: 10.1016/0022-2836(72)90403-2. [DOI] [PubMed] [Google Scholar]
- Kudryk B. J., Collen D., Woods K. R., Blombäck B. Evidence for localization of polymerization sites in fibrinogen. J Biol Chem. 1974 May 25;249(10):3322–3325. [PubMed] [Google Scholar]
- Köppel G. Elektronenmikroskopische Untersuchungen zur Gestalt und zum makromolekularen Bau des Fibrinogen molekïls und der Fibrinfasern. Z Zellforsch Mikrosk Anat. 1967;77(4):443–517. [PubMed] [Google Scholar]
- LAKI K. The polymerization of proteins; the action of thrombin on fibrinogen. Arch Biochem Biophys. 1951 Jul;32(2):317–324. doi: 10.1016/0003-9861(51)90277-9. [DOI] [PubMed] [Google Scholar]
- MIHALYI E., GODFREY J. E. Digestion of fibrinogen by trypsin. I. Kinetic studies of the reaction. Biochim Biophys Acta. 1963 Jan 8;67:73–89. doi: 10.1016/0006-3002(63)91798-0. [DOI] [PubMed] [Google Scholar]
- MIHALYI E., GODFREY J. E. Digestion of fibrinogen by trypsin. II. Characterization of the large fragment obtained. Biochim Biophys Acta. 1963 Jan 8;67:90–103. doi: 10.1016/0006-3002(63)91799-2. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Mihalyi E. Physicochemical studies of bovine fibrinogen. IV. Ultraviolet absorption and its relation to the structure of the molecule. Biochemistry. 1968 Jan;7(1):208–223. doi: 10.1021/bi00841a026. [DOI] [PubMed] [Google Scholar]
- Mills D., Karpatkin S. The non-plasmin, proteolytic origin of human fibrinogen heterogeneity. Biochim Biophys Acta. 1971 Oct;251(1):121–125. doi: 10.1016/0005-2795(71)90069-9. [DOI] [PubMed] [Google Scholar]
- NUSSENZWEIG V., SELIGMANN M., PELMONT J., GRABAR P. [The products of degradation of human fibrinogen by plasmin. I. Separation and physicochemical properties]. Ann Inst Pasteur (Paris) 1961 Mar;100:377–389. [PubMed] [Google Scholar]
- Shen L. L., McDonagh R. P., McDonagh J., Hermans J., Jr Fibrin gel structure: influence of calcium and covalent cross-linking on the elasticity. Biochem Biophys Res Commun. 1974 Feb 4;56(3):793–798. doi: 10.1016/0006-291x(74)90675-5. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Triantaphyllopoulos D. C., Chandra S. Digestion of fibrin by thrombin. Biochim Biophys Acta. 1973 Nov 11;328(1):229–232. doi: 10.1016/0005-2795(73)90349-8. [DOI] [PubMed] [Google Scholar]


