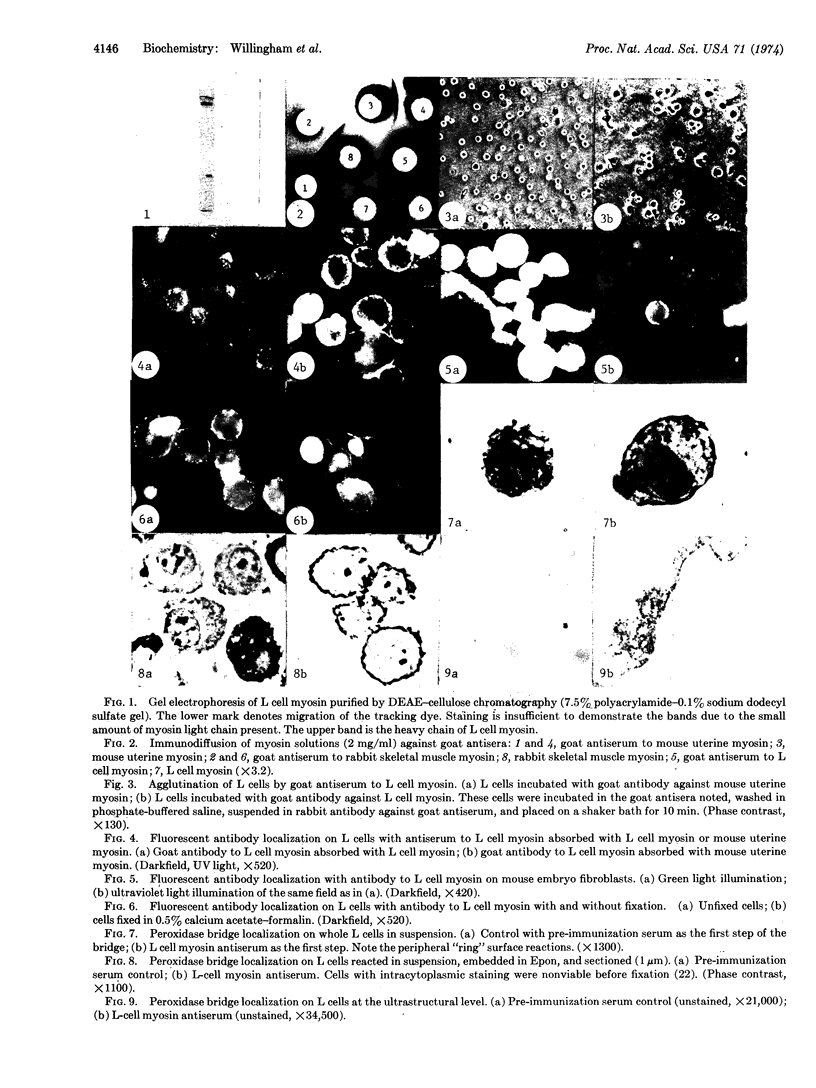
Abstract
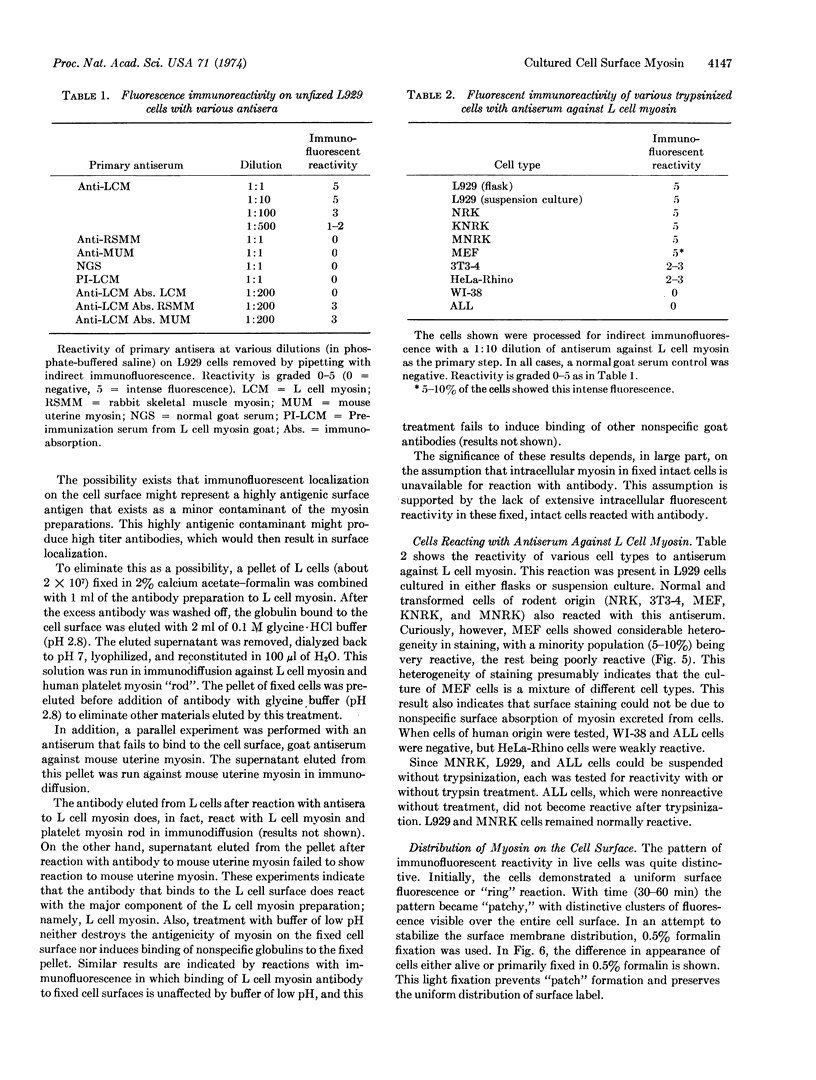
Antisera to myosin from mouse L929 cells, mouse uterus, and rabbit skeletal muscle have been prepared in goats. Each antiserum shows specific reaction by immunodiffusion only to the myosin against which it was prepared. Antiserum against L cell myosin agglutinates L929 cells and shows surface localization in unfixed cells by indirect immunofluorescence. This reaction is prevented by immunoabsorption with L cell myosin, but not by absorption with mouse uterine or rabbit skeletal muscle myosins. Elution of the antibody from L cell myosin antiserum that absorbs to fixed L cells yields antibody reactive to L cell myosin in immunodiffusion. At the ultrastructural level, surface localization is also evident with a peroxidase bridge immunocytologic method as a uniform distribution of surface label. Antiserum against L cell myosin also shows localization on the surface of other cells of rodent origin (3T3-4, NRK, MNRK, KNRK, and MEF) and HeLa cells, but not with some other cells of human origin (ALL and WI-38). The reactivity with antibody of the plasma membrane myosin is unaffected by trypsin treatment or fixation with 0.5-2.0% formalin. These results strongly suggest that myosin in cultured nonmuscle cells is a component of the plasma membrane, a finding of possible significance in studying the mechanisms regulating the motility, morphology, and growth of these cells.
Keywords: immunofluorescence, peroxidase, plasma membrane, agglutination, actomyosin, immunoabsorption, L929 cells, immunodiffusion
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res. 1970 Oct;62(2):389–398. doi: 10.1016/0014-4827(70)90570-7. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Conti M. A., Johnson G. S., Pastan I., Pollard T. D. Isolation and characterization of myosin from cloned mouse fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3693–3697. doi: 10.1073/pnas.69.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Archer F. L., Beck J. S., Melvin J. M. Localization of smooth muscle protein in myoepithelium by immunofluorescence. Am J Pathol. 1971 Apr;63(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Bettex-Galland M., Lüscher E. F. Thrombosthenin, the contractile protein from blood platelets and its relation to other contractile proteins. Adv Protein Chem. 1965;20:1–35. doi: 10.1016/s0065-3233(08)60387-3. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Kirkland W. L. Actin detected in mouse neuroblastoma cells by binding of heavy meromyosin. Nat New Biol. 1972 Oct 25;239(95):244–246. doi: 10.1038/newbio239244a0. [DOI] [PubMed] [Google Scholar]
- Chang C. M., Goldman R. D. The localization of actin-like fibers in cultured neuroblastoma cells as revealed by heavy meromyosin binding. J Cell Biol. 1973 Jun;57(3):867–874. doi: 10.1083/jcb.57.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Cyclic AMP and growth of fibroblasts: effect of environmental pH. Nat New Biol. 1973 Mar 21;242(116):78–80. doi: 10.1038/newbio242078a0. [DOI] [PubMed] [Google Scholar]
- Farrow L. J., Holborow E. J., Brighton W. D. Reaction of human smooth muscle antibody with liver cells. Nat New Biol. 1971 Aug 11;232(2):186–187. doi: 10.1038/newbio232186a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Role of 3',5'-adenosine monophosphate in regulation of morphology and growth of transformed and normal fibroblasts. J Natl Cancer Inst. 1972 May;48(5):1377–1387. [PubMed] [Google Scholar]
- Kemp R. B., Jones B. M., Gröschel-Stewart U. Aggregative behaviour of embryonic chick cells in the presence of antibodies directed against actomyosins. J Cell Sci. 1971 Jul;9(1):103–122. doi: 10.1242/jcs.9.1.103. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Mason T. E., Phifer R. F., Spicer S. S., Swallow R. A., Dreskin R. B. An immunoglobulin-enzyme bridge method for localizing tissue antigens. J Histochem Cytochem. 1969 Sep;17(9):563–569. doi: 10.1177/17.9.563. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Perdue J. F. The distribution, ultrastructure, and chemistry of microfilaments in cultured chick embryo fibroblasts. J Cell Biol. 1973 Aug;58(2):265–283. doi: 10.1083/jcb.58.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Acanthamoeba myosin. I. Isolation from Acanthamoeba castellanii of an enzyme similar to muscle myosin. J Biol Chem. 1973 Jul 10;248(13):4682–4690. [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Filaments of Amoeba proteus. II. Binding of heavy meromyosin by thin filaments in motile cytoplasmic extracts. J Cell Biol. 1971 Jan;48(1):216–219. doi: 10.1083/jcb.48.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Carchman R. A., Pastan I. H. A mutant of 3T3 cells with cyclic AMP metabolism sensitive to temperature change. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2906–2910. doi: 10.1073/pnas.70.10.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Spicer S. S., Graber C. D. Immunocytologic labeling of calf and human lymphocyte surface antigens. Lab Invest. 1971 Sep;25(3):211–219. [PubMed] [Google Scholar]