Abstract
A specific lipoprotein of the E. coli outer membrane has been synthesized in a cell-free system directed by purified messenger RNA. The mRNA for the lipoprotein was purified as 7S RNA about 250-fold from exponentially growing cells. Protein synthesis of the cell-free system was totally dependent upon the addition of the purified mRNA. The product of the cell-free system was identified as the specific lipoprotein by immunoprecipitation and by peptide mapping.
Keywords: immunoassay, BrCN cleavage, protein identification
Full text
PDF
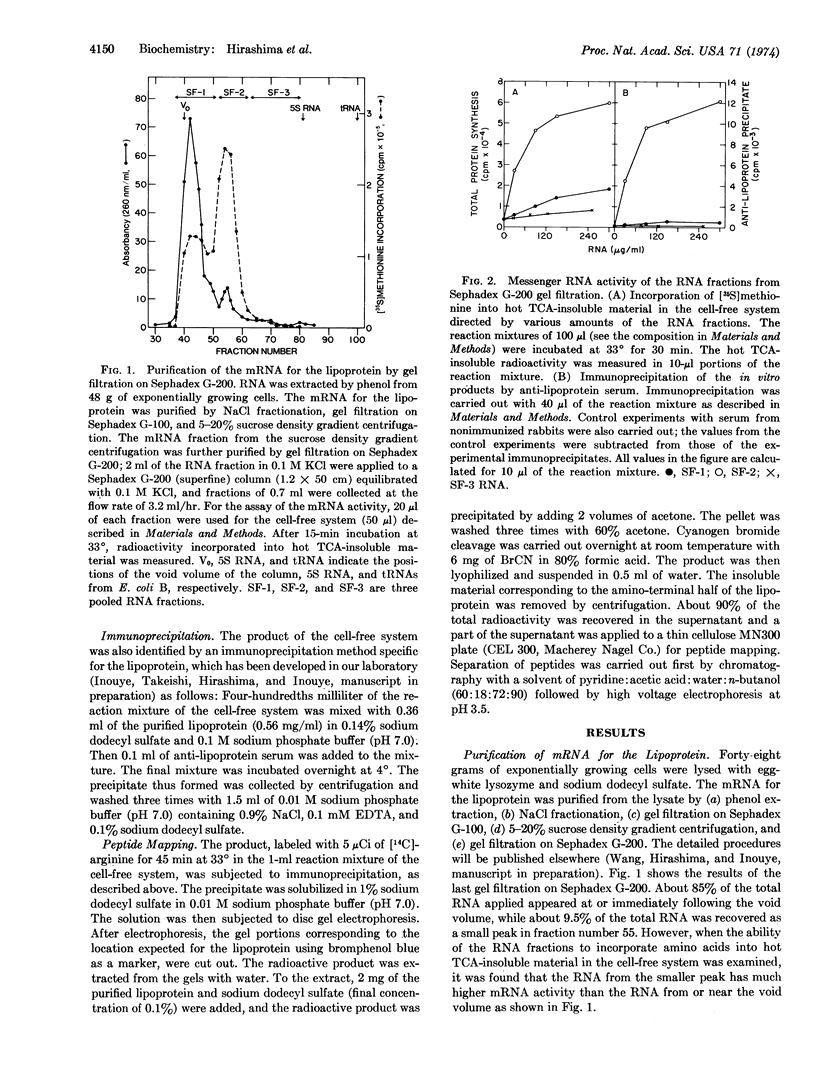
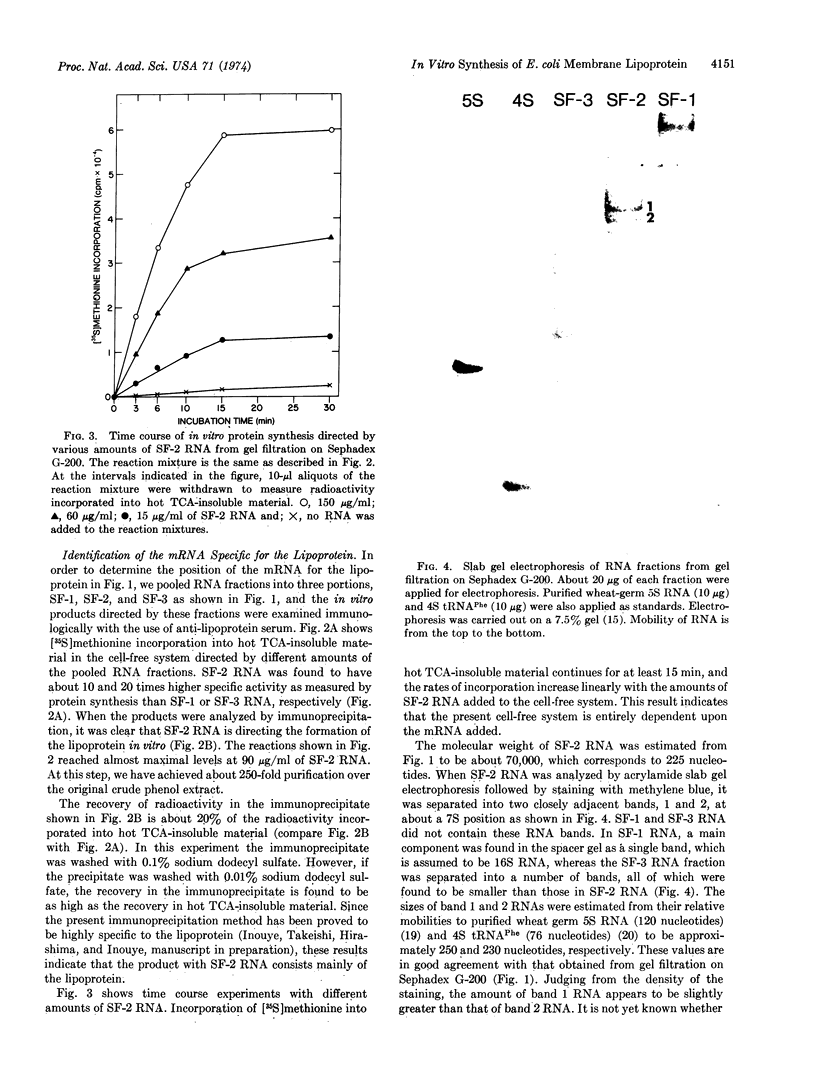

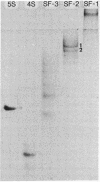
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M. On the release of the formyl group from nascent protein. J Mol Biol. 1968 May 14;33(3):571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- Bosch V., Braun V. Distribution of murein-lipoprotein between the cytoplasmic and outer membrane of Escherichia coli. FEBS Lett. 1973 Aug 15;34(2):307–310. doi: 10.1016/0014-5793(73)80818-x. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Squires C., Yanofsky C. Nucleotide sequences from tryptophan messenger RNA of Escherichia coli: the sequence corresponding to the amino-terminal region of the first polypeptide specified by the operon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2335–2339. doi: 10.1073/pnas.70.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudock B. S., Katz G., Taylor E. K., Holley R. W. Primary structure of wheat germ phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):941–945. doi: 10.1073/pnas.62.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Gausing K. Efficiency of protein and messenger RNA synthesis in bacteriophage T4-infected cells of Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):529–545. doi: 10.1016/s0022-2836(72)80021-4. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Inouye M. Specific biosynthesis of an envelope protein of Escherichia coli. Nature. 1973 Apr 6;242(5397):405–407. doi: 10.1038/242405a0. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Lee N., Inouye M. Outer membrane proteins of Escherichia coli: biosynthesis and assembly. FEBS Lett. 1974 Feb 15;39(2):167–170. doi: 10.1016/0014-5793(74)80043-8. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]