Abstract
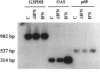
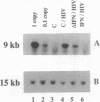
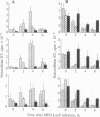
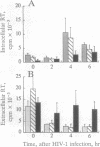
We are developing methods for somatic-cell gene therapy directed against infection with human immunodeficiency virus, by enhancing antiviral resistance of target cells through the constitutive production of autocrine interferon (IFN). Using the human IFN-beta coding sequence under the constitutive low-expression control of a 0.6-kb murine H-2Kb promoter-fragment, we have constructed a retroviral vector, HMB-KbHuIFN beta, and have transformed cells of the T98G human neuroblastoma line, the U-937 human promonocytic line, and the CEM human lymphocytic line. These human IFN-beta-transformed cell populations have acquired a low, constitutive production of human IFN, while replicating at a rate similar to that of untransformed cells and of cells transformed with the control vector carrying a human IFN-beta sequence encoding an inactive, mutated protein. In the three different cell populations tested, transformation with the HMB-KbHuIFN beta vector resulted in a 1.3-2.3 log10 reduction in the number of cells infected with a defective amphotropic MFG-LaZ retrovirus. A kinetic study of the fate of the MFG-LacZ retrovirus in the culture medium and intracellularly immediately after exposure of the cells to virus revealed a significant reduction of the appearance of intracellular virus in human IFN-beta-transformed cells. A similar effect was obtained by treating untransformed T98G, U-937, and CEM cells with exogenous human IFN-beta. The blocking effect of autocrine or exogenous human IFN-beta on viral entry was not limited to virus specific for the amphotropic receptor but was also obtained in murine IFN-beta-treated NIH 3T3 mouse fibroblasts infected with an ecotropic MFG-LacZ retrovirus. Infection of human IFN-beta-transformed CEM cells with human immunodeficiency virus type 1 gave comparable results. Immediately following exposure of the cells to human immunodeficiency virus, a kinetic study of the fate of the virus failed to reveal the appearance of intracellular virus and showed that the majority of the input virus remained in the extracellular medium. We conclude that low autocrine IFN-beta synthesis, or exposure of cells to exogenous IFN-beta, prevents virus from getting inside the cells, regardless of the virus receptor involved.
Full text
PDF

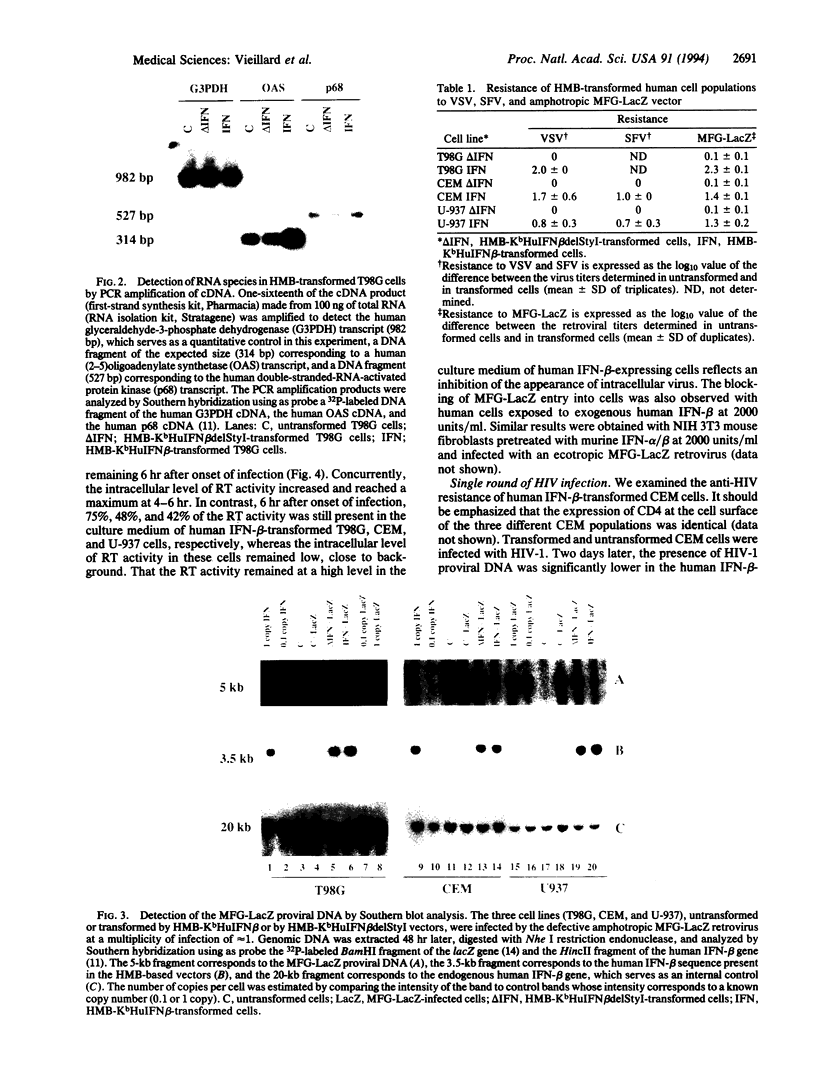
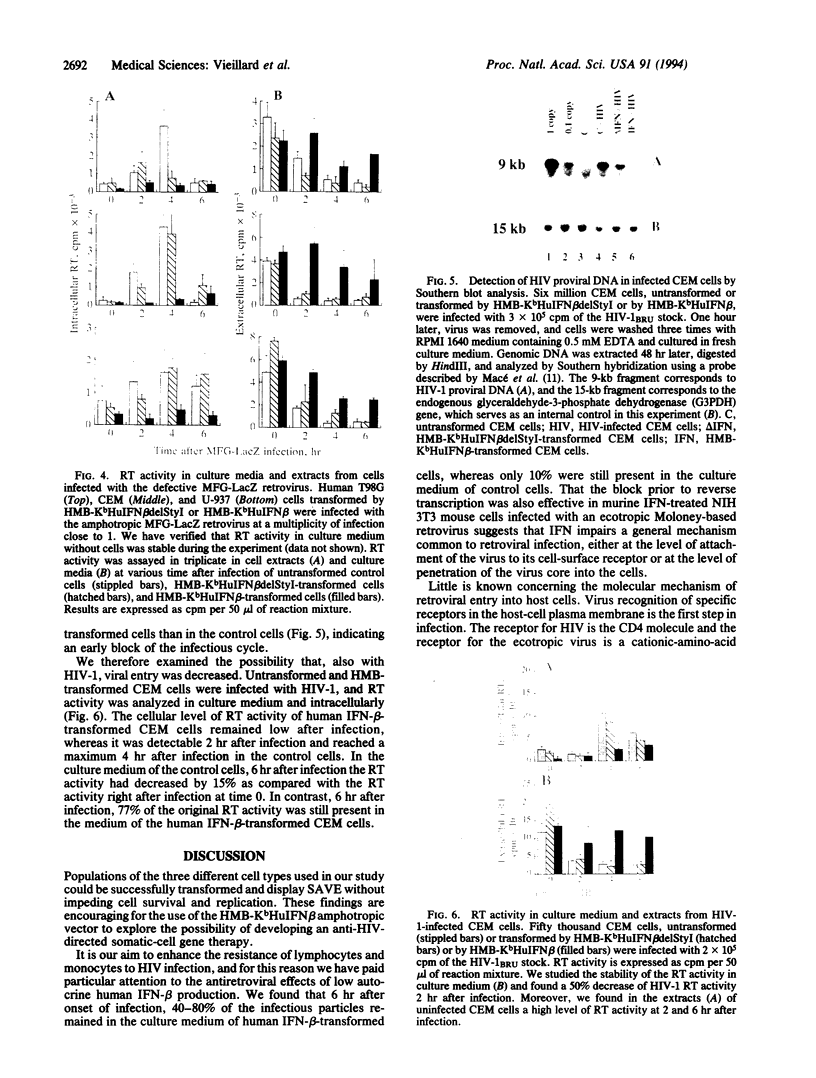

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Shoor R., Salzberg S. An effect of interferon on the uncoating of murine leukaemia virus not related to the antiviral state. J Gen Virol. 1980 Dec;51(Pt 2):425–429. doi: 10.1099/0022-1317-51-2-425. [DOI] [PubMed] [Google Scholar]
- Aboud M., Wolfson M., Hassan Y., Huleihel M. Rapid purification of extracellular and intracellular Moloney murine leukemia virus. Arch Virol. 1982;71(3):185–195. doi: 10.1007/BF01314870. [DOI] [PubMed] [Google Scholar]
- Aloia R. C., Tian H., Jensen F. C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrabose K., Cuatrecasas P., Pottathil R. Changes in fatty acyl chains of phospholipids induced by interferon in mouse sarcoma S-180 cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):661–668. doi: 10.1016/0006-291x(81)91165-7. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Myers M. W., Wong P. K., Friedman R. M. The inhibitory effect of interferon on a temperature-sensitive mutant of Moloney murine leukemia virus. Virology. 1977 Apr;77(2):625–636. doi: 10.1016/0042-6822(77)90487-1. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Cheung H. C., Hunter E. Interferon inhibits Sendai virus-induced cell fusion: an effect on cell membrane fluidity. Proc Natl Acad Sci U S A. 1982 Feb;79(3):835–839. doi: 10.1073/pnas.79.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcayre A. X., Lotz M., Lernhardt W. Inhibition of Epstein-Barr virus-mediated capping of CD21/CR2 by alpha interferon (IFN-alpha): immediate antiviral activity of IFN-alpha during the early phase of infection. J Virol. 1993 May;67(5):2918–2921. doi: 10.1128/jvi.67.5.2918-2921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Sabourin L. A., Hawley T. S. An improved retroviral vector for gene transfer into undifferentiated cells. Nucleic Acids Res. 1989 May 25;17(10):4001–4001. doi: 10.1093/nar/17.10.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kornbluth R. S., Oh P. S., Munis J. R., Cleveland P. H., Richman D. D. The role of interferons in the control of HIV replication in macrophages. Clin Immunol Immunopathol. 1990 Feb;54(2):200–219. doi: 10.1016/0090-1229(90)90082-2. [DOI] [PubMed] [Google Scholar]
- Kumar R., Sen G. C. Interferon-mediated inhibition of retroviral infection: use of a defective retrovirus carrying a drug-resistance gene. Virus Res. 1989 Aug;13(4):295–302. doi: 10.1016/0168-1702(89)90075-0. [DOI] [PubMed] [Google Scholar]
- Lauret E., Riviere I., Rousseau V., Vieillard V., De Maeyer-Guignard J., De Maeyer E. Development of methods for somatic cell gene therapy directed against viral diseases, using retroviral vectors carrying the murine or human interferon-beta coding sequence: establishment of the antiviral state in human cells. Hum Gene Ther. 1993 Oct;4(5):567–577. doi: 10.1089/hum.1993.4.5-567. [DOI] [PubMed] [Google Scholar]
- Mace K., Seif I., Anjard C., De Maeyer-Guignard J., Dodon M. D., Gazzolo L., De Maeyer E. Enhanced resistance to HIV-1 replication in U937 cells stably transfected with the human IFN-beta gene behind an MHC promoter fragment. J Immunol. 1991 Nov 15;147(10):3553–3559. [PubMed] [Google Scholar]
- Meylan P. R., Guatelli J. C., Munis J. R., Richman D. D., Kornbluth R. S. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology. 1993 Mar;193(1):138–148. doi: 10.1006/viro.1993.1110. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Burke D. C. An interferon-sensitive early step in the establishment of infection of murine cells by murine sarcoma/leukaemia virus. J Gen Virol. 1979 Apr;43(1):173–181. doi: 10.1099/0022-1317-43-1-173. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Kwok B. C., Landsberger F. R., Tamm I. Interferon stimulates cholesterol and phosphatidylcholine synthesis but inhibits cholesterol ester synthesis in HeLa-S3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2417–2421. doi: 10.1073/pnas.82.8.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Landsberger F. R., Tamm I. Beta-interferon-induced time-dependent changes in the plasma membrane lipid bilayer of cultured cells. J Interferon Res. 1981;1(4):613–620. doi: 10.1089/jir.1981.1.613. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon effects on microfilament organization, cellular fibronectin distribution, and cell motility in human fibroblasts. J Cell Biol. 1980 Apr;85(1):9–17. doi: 10.1083/jcb.85.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon inhibits the redistribution of cell surface components. J Exp Med. 1980 Aug 1;152(2):469–474. doi: 10.1084/jem.152.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., De Maeyer E., Riviere I., De Maeyer-Guignard J. Stable antiviral expression in BALB/c 3T3 cells carrying a beta interferon sequence behind a major histocompatibility complex promoter fragment. J Virol. 1991 Feb;65(2):664–671. doi: 10.1128/jvi.65.2.664-671.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi Y., Pitha P. M. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J Virol. 1992 Mar;66(3):1321–1328. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube W., Jungwirth C., Ziemiecki A., Jockusch B. M. Vinculin and 36 kDa protein are not tyrosine-phosphorylated in Rous sarcoma virus infected cells which have been treated with interferon. Eur J Cell Biol. 1985 Sep;38(2):226–233. [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wang E., Pfeffer L. M., Tamm I. Interferon increases the abundance of submembranous microfilaments in HeLa-S3 cells in suspension culture. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6281–6285. doi: 10.1073/pnas.78.10.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Wells D. E., Chatterjee S., Mulligan M. J., Compans R. W. Inhibition of human immunodeficiency virus type 1-induced cell fusion by recombinant human interferons. J Virol. 1991 Nov;65(11):6325–6330. doi: 10.1128/jvi.65.11.6325-6330.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]