Abstract
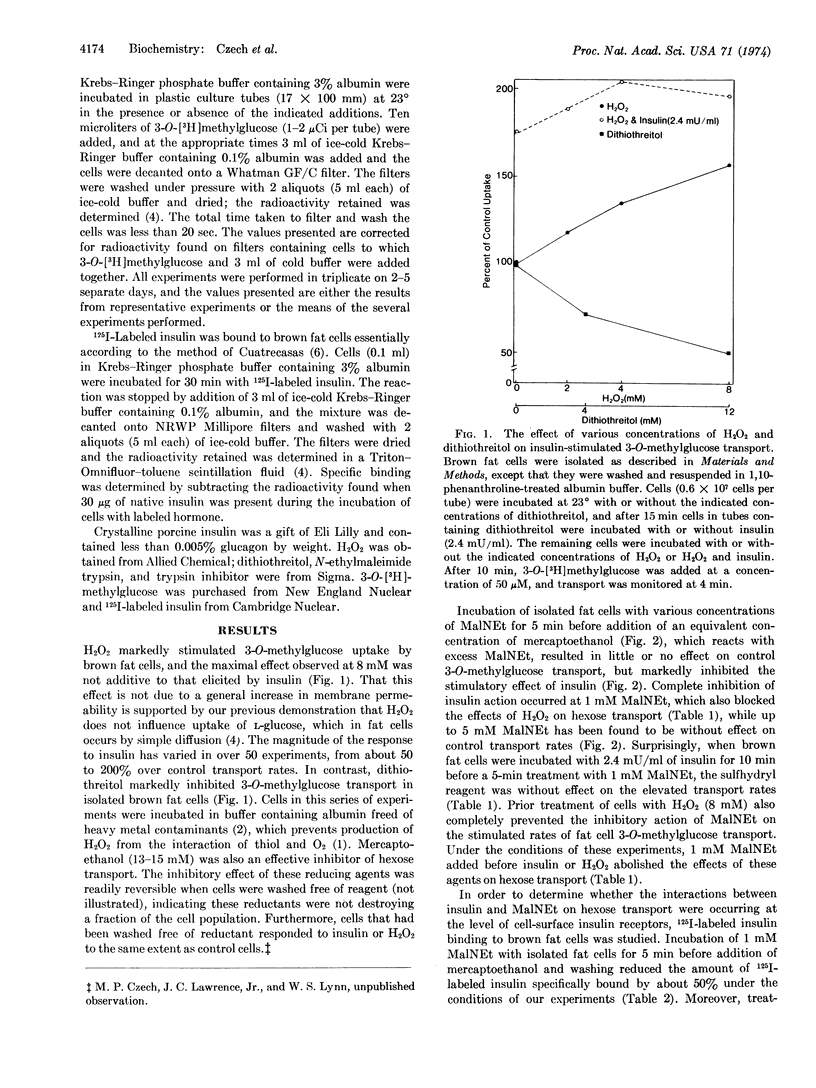
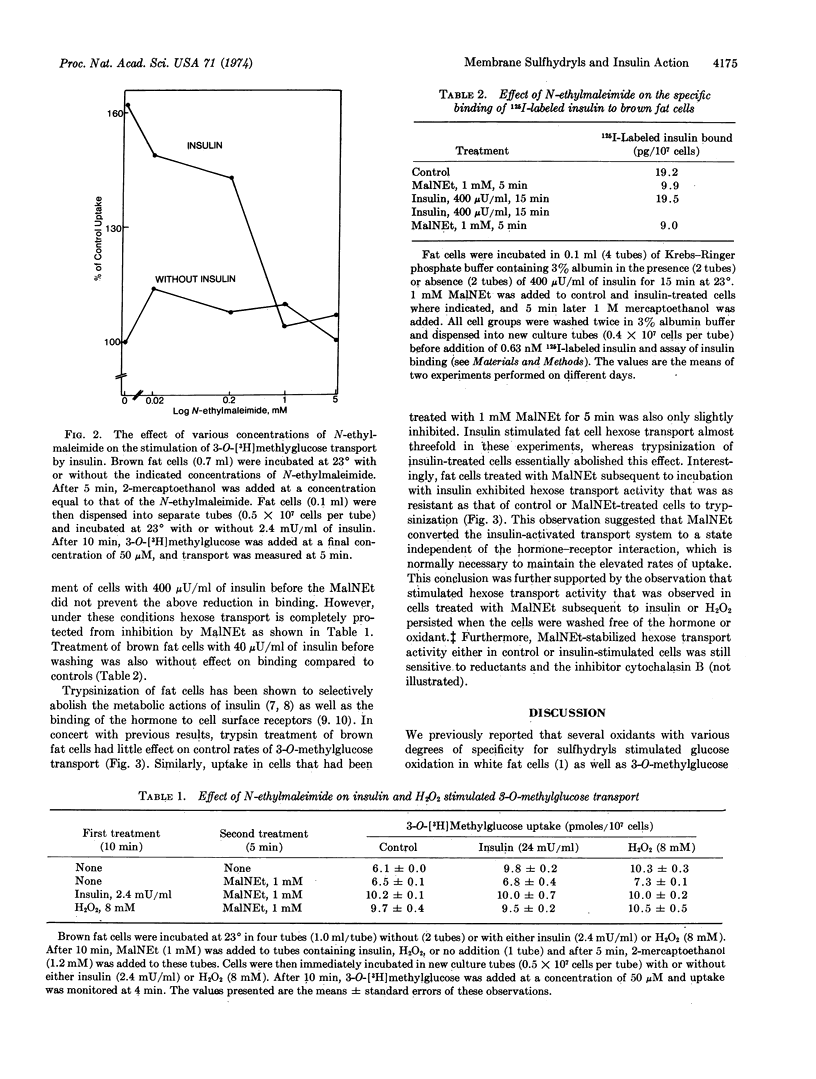
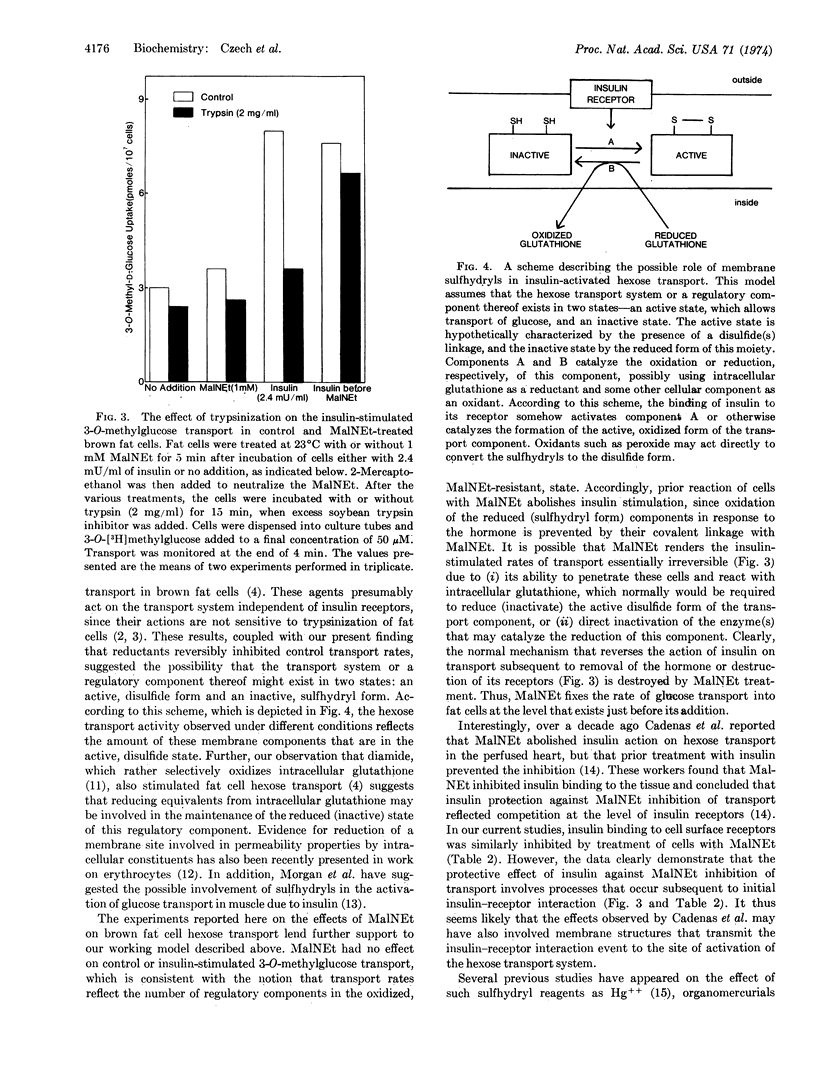
Previous studies have shown that the oxidants Cu++, H2O2, and diamide mimic the stimulatory effect of insulin on 3-O-methylglucose transport in isolated fat cells. The present experiments were designed to determine whether sulfhydryl oxidation plays a key role in the activation of the glucose transport system. It was found that reductants such as dithiothreitol inhibited 3-O-methylglucose transport rates and that this effect was reversible when cells were washed free of reducing agent. Treatment of cells with 1 mM N-ethylmalcimide for 5 min completely blocked the actions of insulin and oxidants on hexose transport without affecting control transport system activity. Under these conditions, binding of 125I-labeled insulin to fat cell surface receptors was inhibited by only about 50%. Addition of insulin or oxidants to fat cells for 10 min before addition of N-ethylmaleimide completely prevented the inhibitory effect of N-ethylmaleimide on the activated transport system. This protective effect on transport rates appears to reside at a site that is altered by insulin subsequent to hormone-receptor interaction, since prior treatment of fat cells with insulin did not prevent the partial inhibitory effect of N-ethylmaleimide on insulin receptors. Furthermore, treatment of cells with N-ethylmaleimide after incubation with insulin prevented the elevated transport rates from returning to control levels when either the cells were washed free of hormone or insulin binding to its receptors was disrupted by trypsin digestion. However, transport rates in these cells treated with N-ethylmaleimide remained sensitive to cytochalasin B, phlorizin, and reductants. These data suggest that a component of the glucose transport system in isolated fat cells must be maintained in its disulfide state for expression of transport activity. Further, the results are consistent with the concept that the binding of insulin to cell surface receptors triggers sulfhydryl oxidation in this component, which prevents its reaction with N-ethylmaleimide.
Keywords: brown fat cells, 3-O-methylglucose uptake, insulin effector system, membranes, insulin receptors
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CADENAS E., KAJI H., PARK C. R., RASMUSSEN H. Inhibition of the insulin effect on sugar transport by N-ethylmaleimide. J Biol Chem. 1961 Sep;236:PC63–PC64. [PubMed] [Google Scholar]
- Carter J. R., Martin D. B. The effect of sulfhydryl blockade on insulin action and glucose transport in isolated adipose tissue cells. Biochim Biophys Acta. 1969 May 6;177(3):521–526. doi: 10.1016/0304-4165(69)90314-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Cutrecasas P. Perturbation of the insulin receptor of isolated fat cells with proteolytic enzymes. Direct measurement of insulin-receptor interactions. J Biol Chem. 1971 Nov;246(21):6522–6531. [PubMed] [Google Scholar]
- Czech M. P., Fain J. N. Cu ++ -dependent thiol stimulation of glucose metabolism in white fat cells. J Biol Chem. 1972 Oct 10;247(19):6218–6223. [PubMed] [Google Scholar]
- Czech M. P., Fain J. N. Insulin protection against fat cell receptor inactivation by trypsin. Endocrinology. 1970 Jul;87(1):191–194. doi: 10.1210/endo-87-1-191. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Evidence for electron transfer reactions involved in the Cu2+ -dependent thiol activation of fat cell glucose utilization. J Biol Chem. 1974 Feb 25;249(4):1001–1006. [PubMed] [Google Scholar]
- Fain J. N., Loken S. C. Response of trypsin-treated brown and white fat cells to hormones. Preferential inhibition of insulin action. J Biol Chem. 1969 Jul 10;244(13):3500–3506. [PubMed] [Google Scholar]
- Fain J. N., Reed N., Saperstein R. The isolation and metabolism of brown fat cells. J Biol Chem. 1967 Apr 25;242(8):1887–1894. [PubMed] [Google Scholar]
- George J. M. Effect of mercury on response of isolated fat cells to insulin and lipolytic hormones. Endocrinology. 1971 Dec;89(6):1489–1498. doi: 10.1210/endo-89-6-1489. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- Kosower E. M., Correa W., Kinon B. J., Kosower N. S. Glutathione. VII. Differentiation among substrates by the thiol-oxidizing agent, diamide. Biochim Biophys Acta. 1972 Mar 30;264(1):39–44. doi: 10.1016/0304-4165(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Minemura T., Crofford O. B. Insulin-receptor interaction in isolated fat cells. I. The insulin-like properties of p-chloromercuribenzene sulfonic acid. J Biol Chem. 1969 Oct 10;244(19):5181–5188. [PubMed] [Google Scholar]
- Morgan H. E., Neely J. R., Wood R. E., Liébecq C., Liebermeister H., Park C. R. Factors affecting glucose transport in heart muscle and erythrocytes. Fed Proc. 1965 Sep-Oct;24(5):1040–1045. [PubMed] [Google Scholar]
- Zipursky A., Stephens M., Brown E. J., Larsen P. Sulfhydryl groups of the erythrocyte membrane and their relation to glycolysis and drug-induced hemolytic anemia. J Clin Invest. 1974 Mar;53(3):805–812. doi: 10.1172/JCI107619. [DOI] [PMC free article] [PubMed] [Google Scholar]



