Abstract
Background
The functional lumen imaging probe (FLIP) is a novel diagnostic tool that can be used to measure esophagogastric junction (EGJ) distensibility. In this study we performed intraoperative FLIP measurements during laparoscopic Heller myotomy (LHM) and peroral esophageal myotomy (POEM) for treatment of achalasia and evaluated the relationship between EGJ distensibility and postoperative symptoms.
Methods
Distensibility index (DI) (defined as the minimum cross-sectional area at the EGJ divided by distensive pressure) was measured with FLIP at two time points during LHM and POEM: 1) at baseline after induction of anesthesia, and 2) after operation completion.
Results
Measurements were performed in 20 patients undergoing LHM and 36 undergoing POEM. Both operations resulted in an increase in DI, although this increase was larger with POEM (7±3.1 vs. 5.1±3.4mm2/mmHg, p<.05). The two patients (both LHM) with the smallest increases in DI (1 and 1.6mm2/mmHg) both had persistent symptoms postoperatively and, overall, LHM patients with larger increases in DI had lower postoperative Eckardt scores. In the POEM group, there was no correlation between change in DI and symptoms; however, all POEM patients experienced an increase in DI of >3mm2/mmHg. When all patients were divided into thirds based on final DI, none in the lowest DI group (<6mm2/mmHg) had symptoms suggestive of reflux (i.e., GerdQ score >7), as compared with 20% in the middle third (6–9mm2/mmHg) and 36% in the highest third (>9mm2/mmHg). Patients within an “ideal” final DI range (4.5–8.5 mm2/mmHg) had optimal symptomatic outcomes (i.e. Eckardt≤1 and GerdQ≤7) in 88% of cases, compared with 47% in those with a final DI above or below that range (p<.05).
Conclusions
Intraoperative EGJ distensibility measurements with FLIP were predictive of postoperative symptomatic outcomes. These results provide initial evidence that FLIP has the potential to act as a useful calibration tool during operations for achalasia.
Keywords: achalasia, peroral endoscopic myotomy, laparoscopic Heller myotomy, functional lumen imaging probe, esophageal physiology, gastroesophageal reflux
Introduction
Achalasia is an immune-mediated disease of esophageal dysmotility that is characterized by a failure of esophagogastric junction (EGJ) relaxation and aperistalsis of the esophageal body in response to swallowing. These pathologies result in a functional outflow obstruction at the EGJ and ineffective food bolus transit, which cause symptoms of dysphagia and regurgitation1. Treatment modalities seek to disrupt the EGJ complex in order to facilitate passive bolus transit into the stomach and thus palliate symptoms. Current standard of care consists of either endoscopic pneumatic dilation or surgical myotomy (laparoscopic Heller myotomy (LHM)). While a recent randomized trial suggested similar outcomes between these two modalities2, there is substantial evidence that LHM provides more durable symptomatic relief3,4, while also resulting in less iatrogenic gastroesophageal reflux (GER) post-intervention5. Peroral esophageal myotomy (POEM) is a novel operation for the treatment of achalasia that combines the advantages of a completely endoscopic (i.e. incisionless) approach with the durability of a controlled surgical myotomy across the EGJ6. Thus far, POEM series with short to medium-term outcomes have reported excellent results in terms of both symptomatic relief and improvement in esophageal physiology6–8.
While high-resolution manometry (HRM) has been established as the gold-standard for confirming the diagnosis of achalasia, less is known regarding the physiologic evaluation of patients post-intervention. This is because a sizable subgroup of patients will have persistent achalasia symptoms after dilation or surgery, despite experiencing significant decreases in EGJ pressures, as measured by HRM9. A novel catheter-based tool, the functional lumen imaging probe (FLIP), uses impedance-planimetry to assess EGJ geometry and pressure in response to volume-controlled distension. These data are used to calculate EGJ distensibility, a metric that has recently been shown to better correlate with post-intervention symptoms than manometric pressure measurements9,10. We have additionally shown that FLIP can be used intraoperatively to measure distensibility in real-time and monitor the stepwise physiologic changes that occur at the EGJ during both LHM and POEM11. Thus if intraoperative distensibility measurements are shown to correlate with postoperative outcomes, FLIP could serve as an ideal tool for ensuring an adequate myotomy during operations for achalasia.
With this in mind, in this study we sought to further investigate the utility of intraoperative FLIP distensibility measurements during both LHM and POEM for treatment of achalasia. We had three primary aims: 1) to evaluate the relationship between baseline (i.e. preoperative) EGJ distensibility and other markers of esophageal physiology, patient demographics and disease characteristics; 2) to evaluate and compare changes in EGJ distensibility that occur as a result of LHM and POEM; and 3) to assess the correlation between final intraoperative EGJ distensibility and eventual postoperative symptomatic outcomes, in order to evaluate the use of FLIP for intraoperative calibration and prognostication.
Methods
Patient selection
During the study period, patients presenting to a single institution for treatment of achalasia were counseled regarding the available options (pneumatic dilation, LHM, or POEM) and chose among them in consultation with their physicians. Patients undergoing LHM or POEM were approached regarding additionally having intraoperative FLIP measurements. Additional FLIP study eligibility criteria included age ≥18 years and a diagnosis of achalasia confirmed by esophageal manometry. All patients signed an informed consent for intraoperative FLIP measurements prior to their procedure and measurements were conducted according to a study protocol approved by the Northwestern Institutional Review Board.
Preoperative demographics and physiologic assessment
Demographic and disease-specific information including age, sex, duration of symptoms, Eckardt symptom score12, prior endoscopic treatment for achalasia, and body mass index was collected prospectively. Patients underwent preoperative high-resolution manometry to confirm the diagnosis of achalasia. Manometry data were evaluated using esophageal pressure topography13 and interpreted according to the Chicago Classification of esophageal motility disorders14. Patients also underwent a preoperative timed barium esophagram (TBE) using 200 ml of diluted contrast.
LHM operative technique
LHM operations were performed by one of two surgeons (E.H. and N.S.). Our operative technique for LHM has been previously described in detail15. Briefly, after creation of a pneumoperitoneum, the crura were opened widely and the esophagus was dissected high into the mediastinum. A myotomy of both the longitudinal and circular muscle layers was then performed from 6 cm proximal to the EGJ, to 3 cm distal to it onto the stomach. A sterile ruler or the distance between the open jaws of a laparoscopic grasper (2.5 cm) was used to measure myotomy length. A partial fundoplication was then performed. We prefer a posterior Toupet fundoplication, unless this configuration results in excessive anterior angulation of the EGJ, in which case an anterior Dor fundoplication was created.
POEM operative technique
POEM procedures were performed by one of the same two surgeons who performed LHM, or by both of these surgeons conjointly. Our operative technique closely follows that of Inoue and colleagues6, and has been previously described in detail7. A standard high-definition gastroscope with a transparent dissecting cap and CO2 insufflation was used. A saline solution was first injected into the anterior esophageal wall 12 cm proximal to the EGJ, in order to create a submucosal fluid bleb. A mucosotomy was then made overlying the fluid bleb and the scope maneuvered into the submucosal space. A submucosal tunnel was then dissected to at least 3 cm beyond the EGJ. A selective myotomy of the inner, circular muscle layer was then performed from 6 cm proximal to the EGJ to 3 cm distal to it. The endoscope shaft markings were used to measure myotomy distances in relation to the EGJ. After myotomy completion, the mucosotomy was closed with endoscopic clips.
FLIP
Intraoperative measurements were taken using a commercially available FLIP system (EndoFLIP; Crospon Ltd., Galway, Ireland) and probes (EF-325N; Crospon) that have previously been described in detail16,17. FLIP is a catheter-based probe that contains 17 ring-electrodes at 5 mm intervals, housed within a bag that can be variably inflated with saline solution. Impedance planimetry measurements are used to calculate bag cross-sectional areas (CSA) at the level of each electrode pair. Therefore, when the catheter is placed across the EGJ, these measurements represent the 16 CSAs at 5 mm intervals along an 8 cm segment of esophagus, EGJ, and stomach, giving a representation of luminal geometry. The probe also contains a solid-state pressure transducer that measures intra-bag pressure.
The EGJ distensibility index (DI) is defined as the minimum CSA (i.e. narrowest portion of the EGJ) divided by intra-bag pressure. The median value of minimum CSA and intra-bag pressure measurements were calculated using MATLAB software (MathWorks; Natick, MA), and used for subsequent analysis when determining the DI.
Intraoperative FLIP protocol
Prior to probe insertion, an automated purge sequence was used to evacuate air from the FLIP probe and the pressure transducer was zeroed to atmospheric pressure. During both LHM and POEM, after induction of anesthesia, paralysis, and endotracheal intubation, an upper endoscopy was performed and the FLIP probe was advanced down the esophagus under direct endoscopic visualization. Probe position across the EGJ was confirmed endoscopically and by seeing an “hour glass” anatomic configuration on the FLIP monitor.
FLIP measurements were taken during LHM and POEM using a bag distension volume of 40 ml at two time points: 1) after induction of anesthesia, paralysis, and endotracheal intubation (subsequently referred to as the “baseline” measurement) and 2) after completion of the operation (subsequently referred to as the “final” measurement). During LHM, the final FLIP measurement was taken after deinsufflation of pneumoperitoneum.
Assessment of postoperative symptoms
Patients who were more than six months post-procedure were contacted via telephone in order to assess their current symptoms. The Eckardt symptom score, the elements of which are displayed in Table 1, was used to assess for persistence of achalasia symptoms. The GerdQ score was used to assess for symptoms suggestive of GER. A total GerdQ score > 7 was considered indicative of significant GER symptoms, in line with prior usage of the questionnaire18.
Table 1.
Determination of the Eckardt symptom score for achalasia. The final score is the sum of the four component scores, ranging from 0 to 12.
| Symptom | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Dysphagia | None | Occasional | Daily | With every meal |
| Regurgitation | None | Occasional | Daily | With every meal |
| Chest pain | None | Occasional | Daily | Several times a day |
| Weight loss (kg) | 0 | < 5 | 5–10 | > 10 |
Statistical analysis
Data analysis was performed using SPSS software (version 22; IBM, Armonk, NY). For continuous variables, comparisons between baseline and final DI measurements within the same procedure were performed using a paired t-test. Comparisons between procedure types were performed using an independent t-test. Categorical variables were compared using a Fisher exact test. Relationships between variables were assessed with a Spearman’s rank correlation coefficient. A two-tailed p-value of < .05 was used to determine statistical significance in all cases. Values throughout are presented as mean ± standard deviation.
Results
Intraoperative FLIP measurements were performed in 20 patients undergoing LHM and 36 undergoing POEM. Table 2 displays the patients’ preoperative demographics, disease characteristics, and HRM and TBE measures, all of which were similar between the LHM and POEM groups. Likewise, baseline DI was similar between groups. Of the LHM patients, 10 had a Toupet fundoplication and 10 a Dor.
Table 2.
Baseline patient demographics, disease characteristics, and measures of esophageal physiology.
| Demographics | LHM | POEM | p–value |
|---|---|---|---|
| Number | 20 | 36 | |
| Female | 11 (55%) | 11 (31%) | .09 |
| Age | 53 ± 14 | 50 ± 15 | .38 |
| Body mass index (kg/m2) | 28 ± 5 | 26 ± 7 | .49 |
| Disease characteristics | |||
| Symptom duration (years) | 6 ± 7 | 4 ± 5 | .29 |
| Eckardt score (scale 0–12) | 7 ± 3 | 7 ± 2 | .40 |
| Prior endoscopic treatment | 8 (40%) | 7 (19%) | .12 |
| High-resolution manometry | |||
| EGJ expiratory pressure (mmHg) | 21 ± 16 | 23 ± 12 | .79 |
| EGJ integrated relaxation pressure (mmHg) | 24 ± 14 | 28 ± 11 | .27 |
| Achalasia subtype | .95 | ||
| I | 33% | 31% | |
| II | 53% | 58% | |
| III | 7% | 3% | |
| EGJ outflow obstruction | 7% | 8% | |
| Time barium esophagram | |||
| Contrast column height at 5 min (cm) | 7 ± 3 | 7 ± 2 | .85 |
| Functional lumen imaging probe (FLIP) | |||
| Baseline EGJ distensibility (mm2/mmHg) | 1.5 ± 1.2 | 1.3 ± 1.4 | .58 |
Relationships between patient characteristics and baseline DI
There were no significant relationships between baseline DI and patient sex, age, or BMI. Table 3 displays the preoperative characteristics that were found to correlate with baseline DI. Patients who had undergone prior endoscopic treatment for achalasia and those with longer-standing symptoms had a higher baseline DI, but both only at a trend level (rs = .27, p = .07 and rs = .24, p = .07, respectively). Patients with higher preoperative Eckardt scores (i.e. more severe symptoms) had a lower baseline DI (rs = −.32, p < .05). On preoperative HRM, patients with higher EGJ resting pressures and EGJ relaxation pressures were found to have a lower baseline DI (rs = −.52, p < .001 and rs = −.47, p = .001, respectively). Patients with type I achalasia had a higher baseline DI than those with type II (2.1 ± 2 vs. 1.1 ± 0.6 mm2/mmHg, p = .01). Patients with type III achalasia and EGJ outflow obstruction had a lower baseline DI (0.5 ± 0.1 and 1.0 ± 0.3 mm2/mmHg respectively) but there were too few of these patients to warrant a statistical comparison with the other subtypes. Patients with higher contrast columns on preoperative TBE were also found to have a lower baseline DI.
Table 3.
Correlations between preoperative patient variables and baseline EGJ distensibility as measured by FLIP.
| Variable | rs | p-value |
|---|---|---|
| Duration of symptoms | .24 | .07 |
| Prior endoscopic treatment | 27 | .07 |
| Eckardt symptom score | −.32 | <.05 |
| EGJ expiratory pressure | −.52 | <.001 |
| EGJ integrated relaxation Pressure | −.47 | .001 |
| Esophagram contrast column height | −.37 | <.05 |
Operative changes in DI
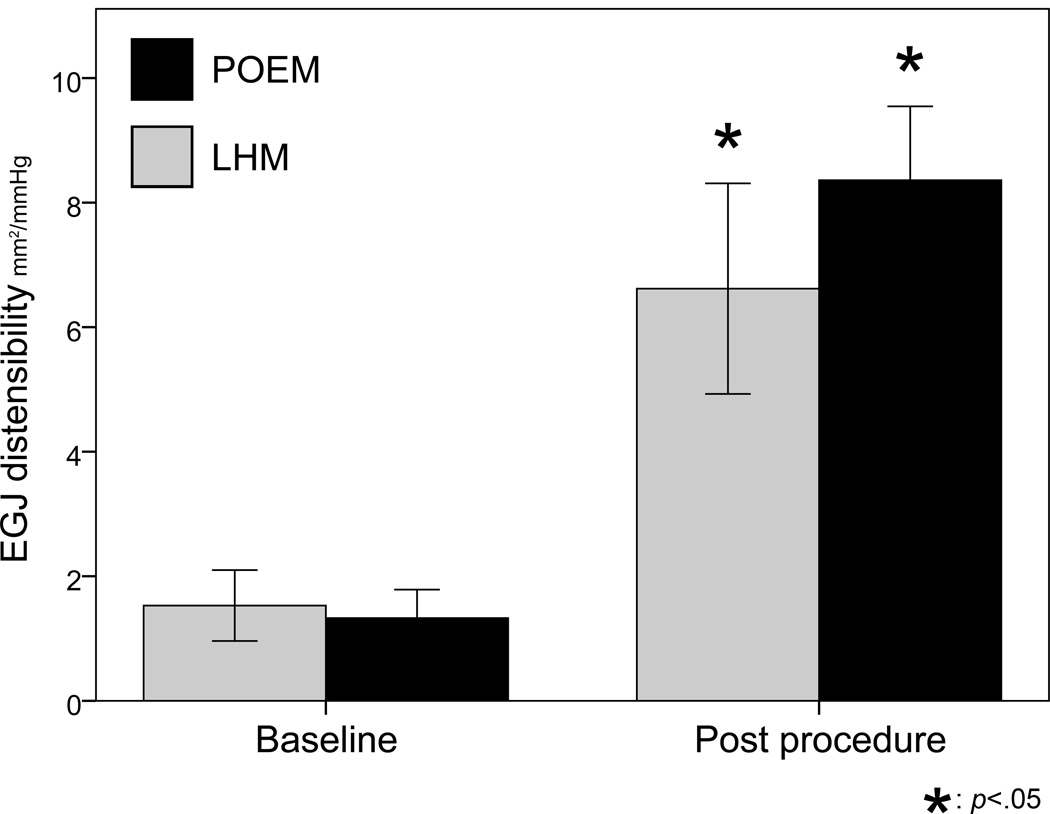
Figure 1 displays baseline and final DI according to procedure type. Both LHM and POEM resulted in a significant increase in DI. All 56 patients experienced an increase in DI, although the range of DI change was fairly large (total range 1 to 17.2 mm2/mmHg, interquartile range 4.3 to 8 mm2/mmHg). The mean increase in DI was larger in patients undergoing POEM, as compared to those undergoing LHM (mean DI increase 7 ± 3.1 vs. 5.1 ± 3.4 mm2/mmHg, p < .05).
Figure 1.
EGJ distensibility is shown before and after surgery for patients undergoing laparoscopic Heller myotomy (LMH) and POEM. Both operations resulted in an increase in distensibility, but this increase was larger in the POEM group.
Relationships between intraoperative DI and postoperative symptoms
Eleven LHM patients and 21 POEM patients were more than 6 months post-procedure and were contacted to obtain current symptom scores. The mean postoperative follow-up interval was similar between groups (LHM: 12 months, POEM: 11 months, p = ns). Both procedure groups experienced a significant and similar decrease in mean Eckardt symptom scores after surgery (LHM: pre 5.7 vs. post 1, p < .05, POEM: pre 7.2 vs. post 0.7, p < .001).
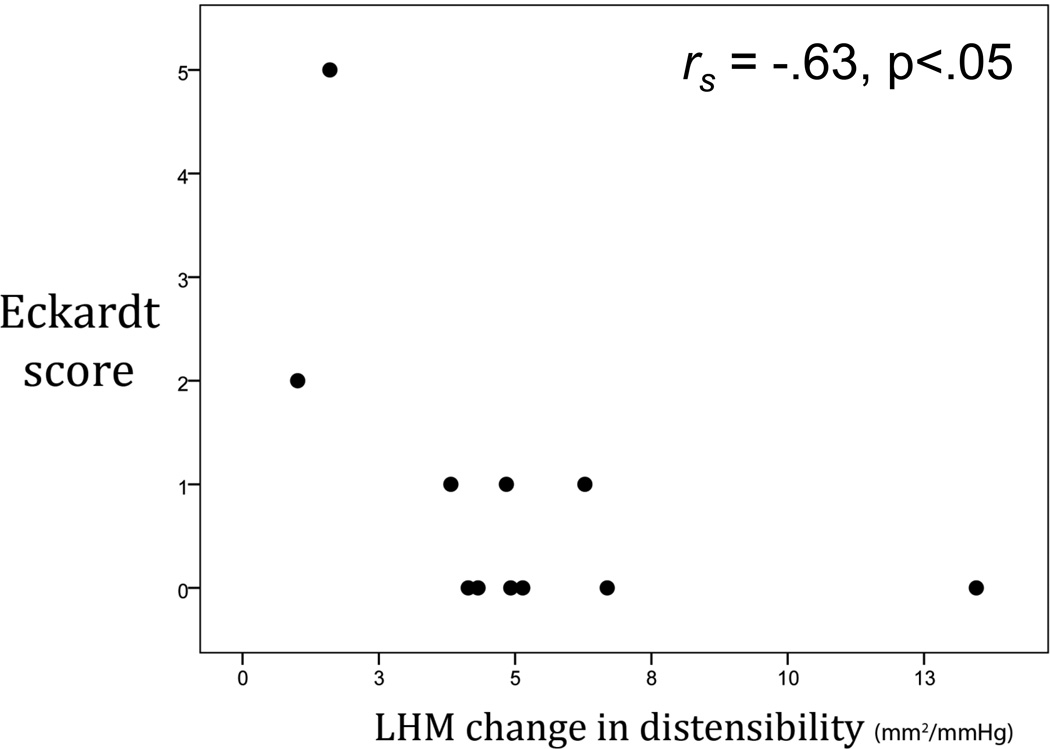
In the LHM group, 2 (18%) patients had a postoperative Eckardt score > 1, signifying daily dysphagia or occasion dysphagia in addition to regurgitation or chest pain. These two patients also had the smallest increases in intraoperative DI of the entire cohort (1 and 1.6 mm2/mmHg). Following from this, Figure 2 shows that overall, LHM patients with larger operative increases in DI had lower postoperative Eckardt scores (rs = −.63, p < .05). In the POEM group, there was no correlation between change in DI and postoperative Eckardt score; however, all POEM patients experienced an increase in DI of at least 3 mm2/mmHg as a result of surgery.
Figure 2.
In patients undergoing laparoscopic Heller myotomy, change in EGJ distensibility was inversely correlated with postoperative Eckardt score. That is, patients with the least change had the most severe symptoms after surgery. In the POEM group, there was no correlation between change in DI and postoperative Eckardt score; however, all POEM patients experienced an increase in DI of at least 3 mm2/mmHg as a result of surgery.
To examine the effect of final DI on postoperative iatrogenic GER, patients (both LHM and POEM) were divided into thirds based on their final DI, shown in Table 4. No patients in the group with the lowest final DI (< 6 mm2/mmHg) had symptoms suggestive of GER (i.e. a GerdQ score > 7), whereas two (20%) patients in the middle third (DI: 6 – 9 mm2/mmHg) and 4 (36%) patients in the highest third (DI > 9 mm2/mmHg) had GER symptoms.
Table 4.
The 32 patients with symptomatic follow-up were divided into thirds based on their final intraoperative EGJ distensibility. No patients in the third with the lowest distensibility had symptoms suggestive of gastroesophageal reflux (i.e., a GerdQ score > 7), as compared with 20% of patients in the middle third and 36% in the highest third.
| Final EGJ distensibility | Number (%) with reflux symptoms (i.e. GerdQ score > 7) |
|---|---|
| < 6 mm2/mmHg (n = 11) | 0 |
| 6 – 9 mm2/mmHg (n = 10) | 2 (20%) |
| > 9 mm2/mmHg (n = 11) | 4 (36%) |
Based on the above findings that too low a DI appeared to result in persistent achalasia symptoms, whereas a high final value predisposed towards postoperative GER, a sensitivity analysis was performed to determine the ideal range for final intraoperative DI. Patients with Eckardt scores ≤ 1 and GerdQ scores ≤ 7 (i.e. both minimal achalasia and GER symptoms) were classified as having “optimal” outcomes. Table 5 shows the results of this analysis. Patients who had a final DI between 4.5 and 8.5 mm2/mmHg had optimal symptomatic outcomes in 88% of cases, whereas those with a final DI < 4.5 or > 8.5 mm2/mmHg had optimal outcomes in only 47% of cases (p < .05). Therefore, an ideal DI range of 4.5 - 8.5 mm2/mmHg had a sensitivity of 68% and a specificity of 80% in predicting optimal symptomatic results.
Table 5.
An “ideal” range for final EGJ distensibility was determined to be 4.5 – 8.5 mm2/mmHg. 88% of patients within this range had optimal symptomatic outcomes at greater than 6 months follow-up, as compared with 47% of patients with a final distensibility either above or below the range.
| Symptomatic outcome | |||
|---|---|---|---|
| Final EGJ distensibility |
Optimal (Eckardt ≤ 1 and GerdQ ≤ 7) |
Suboptimal (Eckardt > 1 or GerdQ > 7) |
Percent optimal |
| 4.5 – 8.5 mm2/mmHg | 15 | 2 | 88% |
| < 4.5 or > 8.5 mm2/mmHg | 7 | 8 | 47% |
| p < .05 | |||
Discussion
This study demonstrates that EGJ distensibility, as measured by intraoperative FLIP, is predictive of postoperative symptomatic outcomes in achalasia. This appeared to apply to both patients in whom the EGJ was too tight, resulting in postoperative dysphagia, and those with the highest final distensibilities, who were predisposed towards developing iatrogenic GER. Patients within a “sweet spot” of final EGJ distensibility (4.5 – 8.5 mm2/mmHg) were almost twice as likely (88% vs. 47%) to have optimal symptomatic outcomes as those outside that window. The validity of this ideal range is supported by two studies that showed healthy controls to have a mean distensibility ranging from 5 to 8 mm2/mmHg9,10. Achalasia patients with a post-intervention distensibility values of less than 2.9 mm2/mmHg measured during follow-up endoscopy have been shown to have worse dysphagia symptoms10. Additionally, patients with primary gastroesophageal reflux disease have been shown to have elevated baseline distensibility when compared with healthy controls19.
The findings of the current study provide initial evidence to support the use of intraoperative FLIP as a “quality control” tool to ensure an adequate myotomy during operations for achalasia. During LHM and POEM, surgeons typically rely on intraoperative upper endoscopy to qualitatively assess resistance to endoscope passage through the EGJ, as a surrogate for myotomy efficacy. However, there is little objective evidence to suggest that this common practice is effective in assessing myotomy completeness. Along these lines, FLIP could be used as a quantitative adjunct measure to ensure a minimum distensibility threshold had been met. Additionally, FLIP could be used during LHM to calibrate the partial fundoplication, to avoid overly constricting the EGJ while at the same time creating an effective anti-reflux barrier. A small number of prior studies have suggested that intraoperative manometry can be used to evaluate myotomy adequacy during LHM20–22; however, performing and interpreting such studies in real-time is cumbersome. More importantly, distensibility is likely a better marker of EGJ pathophysiology under conditions of general anesthesia, as achalasia patients typically have elevated EGJ relaxation pressures after swallowing, but most often have normal resting pressures.
In addition to evaluating the prognostic capabilities of intraoperative measurements with FLIP, we sought to assess and compare the impact of LHM and POEM on EGJ distensibility. While both procedures resulted in a dramatic increase in distensibility, this increase was significantly larger in patients undergoing POEM. We have previously shown that performing a partial fundoplication during LHM results in a decrease in distensibility11; however, this decrease (1.3 mm2/mmHg) may not be large enough to account for the difference in overall distensibility change between POEM and LHM (7 vs. 5.1 mm2/mmHg) seen in the current study. In the prior study we did not observe such a difference between the two procedures, and the increase in distensibility change during POEM seen in the current cohort may be the result of improved technique as our experience with the procedure has increased. While POEM patients experienced a larger increase in distensibility, they ended with a mean final value (8.4 mm2/mmHg) at the upper limit of our “ideal” range (4.5 – 8.5 mm2/mmHg). This could mean that while POEM results in an effective myotomy, patients may be at higher risk for the development of iatrogenic GER. Thus far, only a single non-randomized study has compared the results of pH-monitoring studies between LHM and POEM patients postoperatively, finding similar rates of pathologic reflux in both groups (32% vs. 39%, respectively)8. Certainly, further studies are needed to define the incidence of pathologic GER after POEM, and in relation to LHM.
We also examined the relationship between baseline EGJ distensibility and other markers of achalasia disease state and esophageal physiology. Patients with higher EGJ pressures were found to have lower distensibility, as were those with more severe symptoms preoperatively. These findings add the validity evidence regarding the use of distensibility as a marker of EGJ dysfunction in patients with achalasia. In the future, baseline FLIP measurements may serve as an important prognostic factor, as patients with higher EGJ pressures preoperatively have been shown to experience superior outcomes23.
The current study is limited by the fact that patients were not randomized to treatment group and, although baseline characteristics and distensibility were similar, there may have been unmeasured differences between the patients undergoing LHM and POEM. Additionally, in assessing the prognostic capability of intraoperative FLIP measurements, we relied solely on patient symptoms in a relatively small cohort. These results will need to be augmented with other markers of postoperative esophageal physiology, such as HRM, TBE, and 24-hour pH monitoring studies. While we currently recommend that both LHM and POEM patients return for each of these studies as part of routine follow-up at one year postoperatively, the number available in this cohort is currently insufficient to meaningfully compare their results with intraoperative distensibility measurements.
In conclusion, in patients who underwent LHM and POEM for treatment of achalasia, intraoperative EGJ distensibility measurements with FLIP were predictive of postoperative symptomatic outcomes. Patients within an ideal final distensibility range were almost twice as likely to have optimal outcomes in terms of achalasia and GER symptoms as those with either lower or higher final values. While both operations resulted in a large improvement in distensibility, this increase was larger in patients undergoing POEM. These results provide initial evidence that FLIP has the potential to act as a useful tool for myotomy calibration during operations for achalasia.
Acknowledgements
The authors would like to acknowledge Rowena Martinez, RN, and Colleen Krantz, RN, for their help coordinating the clinical aspects of this study. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-079902 (J.E. Pandolfino).
Footnotes
Presented at SAGES: Oral presentation number S156 – Resident/Fellow Scientific Session. April 4th, 2014.
Disclosures
Nathaniel Soper is on the scientific advisory boards of TransEnterix and Miret Surgical, which are unrelated to this study. Ezra Teitelbaum, John Pandolfino, Peter Kahrilas, Lubomyr Boris, Frederic Nicodeme, Zhiyue Lin, and Eric Hungness have no conflicts of interest or financial ties to disclose.
References
- 1.Triadafilopoulos G, Boeckxstaens GE, Gullo R, Patti MG, Pandolfino JE, Kahrilas PJ, Duranceau A, Jamieson G, Zaninotto G. The Kagoshima consensus on esophageal achalasia. Dis Esophagus. 2012;25:337–348. doi: 10.1111/j.1442-2050.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- 2.Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, Smout AJ, Tack J, Zwinderman AH, Zaninotto G, Busch OR, European Achalasia Trial I. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 3.Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstatter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 4.Yaghoobi M, Mayrand S, Martel M, Roshan-Afshar I, Bijarchi R, Barkun A. Laparoscopic Heller’s myotomy versus pneumatic dilation in the treatment of idiopathic achalasia: a meta-analysis of randomized, controlled trials. Gastrointest Endosc. 2013;78:468–475. doi: 10.1016/j.gie.2013.03.1335. [DOI] [PubMed] [Google Scholar]
- 5.Novais PA, Lemme EM. 24-h pH monitoring patterns and clinical response after achalasia treatment with pneumatic dilation or laparoscopic Heller myotomy. Aliment Pharmacol Ther. 2010;32:1257–1265. doi: 10.1111/j.1365-2036.2010.04461.x. [DOI] [PubMed] [Google Scholar]
- 6.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 7.Hungness ES, Teitelbaum EN, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, Soper NJ. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17:228–235. doi: 10.1007/s11605-012-2030-3. [DOI] [PubMed] [Google Scholar]
- 8.Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A Comparative Study on Comprehensive, Objective Outcomes of Laparoscopic Heller Myotomy With Per-Oral Endoscopic Myotomy (POEM) for Achalasia. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 9.Pandolfino JE, de Ruigh A, Nicodeme F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013;25:496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of Treatment for Patients With Achalasia Depends on the Distensibility of the Esophagogastric Junction. Gastroenterology. 2012;143:328–335. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum EN, Boris L, Arafat FO, Nicodeme F, Lin ZY, Kahrilas PJ, Pandolfino JE, Soper NJ, Hungness ES. Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surgical Endoscopy and Other Interventional Techniques. 2013;27:4547–4555. doi: 10.1007/s00464-013-3121-2. [DOI] [PubMed] [Google Scholar]
- 12.Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am. 2001;11:281–292. vi. [PubMed] [Google Scholar]
- 13.Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 14.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ International High Resolution Manometry Working G. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaziri K, Soper NJ. Laparoscopic Heller myotomy: technical aspects and operative pitfalls. J Gastrointest Surg. 2008;12:1586–1591. doi: 10.1007/s11605-008-0475-1. [DOI] [PubMed] [Google Scholar]
- 16.McMahon BP, Frokjaer JB, Kunwald P, Liao D, Funch-Jensen P, Drewes AM, Gregersen H. The functional lumen imaging probe (FLIP) for evaluation of the esophagogastric junction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G377–G384. doi: 10.1152/ajpgi.00311.2006. [DOI] [PubMed] [Google Scholar]
- 17.Perretta S, McAnena O, Botha A, Nathanson L, Swanstrom L, Soper NJ, Inoue H, Ponsky J, Jobe B, Marescaux J, Dallemagne B. Acta from the EndoFLIP(R) Symposium. Surg Innov. 2013 doi: 10.1177/1553350613513515. [DOI] [PubMed] [Google Scholar]
- 18.Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, Lind T. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatek MA, Pandolfino JE, Hirano I, Kahrilas PJ. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointest Endosc. 2010;72:272–278. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattioli S, Ruffato A, Lugaresi M, Pilotti V, Aramini B, D'Ovidio F. Long-term results of the Heller-Dor operation with intraoperative manometry for the treatment of esophageal achalasia. J Thorac Cardiovasc Surg. 2010;140:962–969. doi: 10.1016/j.jtcvs.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 21.Endo S, Nakajima K, Nishikawa K, Takahashi T, Souma Y, Taniguchi E, Ito T, Nishida T. Laparoscopic Heller-Dor surgery for esophageal achalasia: impact of intraoperative real-time manometric feedback on postoperative outcomes. Dig Surg. 2009;26:342–348. doi: 10.1159/000244512. [DOI] [PubMed] [Google Scholar]
- 22.Chapman JR, Joehl RJ, Murayama KM, Tatum RP, Shi G, Hirano I, Jones MP, Pandolfino JE, Kahrilas PJ. Achalasia treatment: improved outcome of laparoscopic myotomy with operative manometry. Arch Surg. 2004;139:508–513. doi: 10.1001/archsurg.139.5.508. discussion 13. [DOI] [PubMed] [Google Scholar]
- 23.Kilic A, Schuchert MJ, Pennathur A, Gilbert S, Landreneau RJ, Luketich JD. Long-term outcomes of laparoscopic Heller myotomy for achalasia. Surgery. 2009;146:826–831. doi: 10.1016/j.surg.2009.06.049. discussion 31-3. [DOI] [PubMed] [Google Scholar]