Abstract
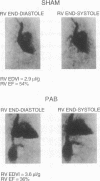
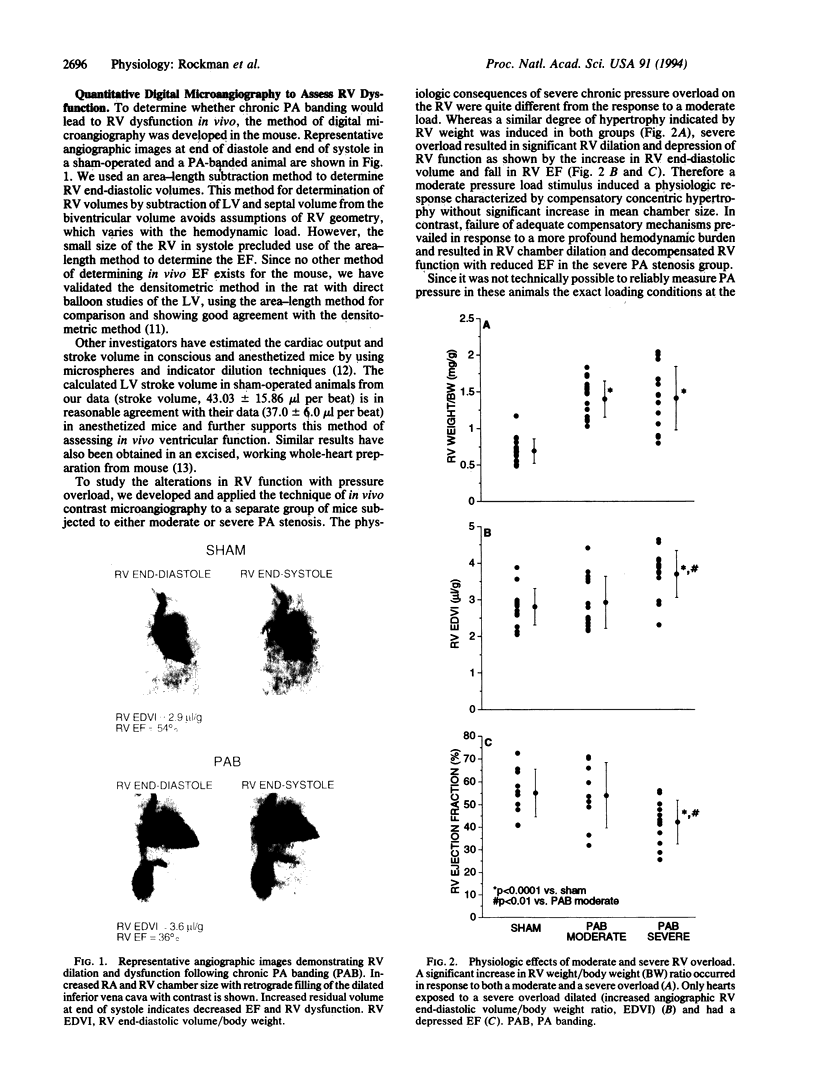
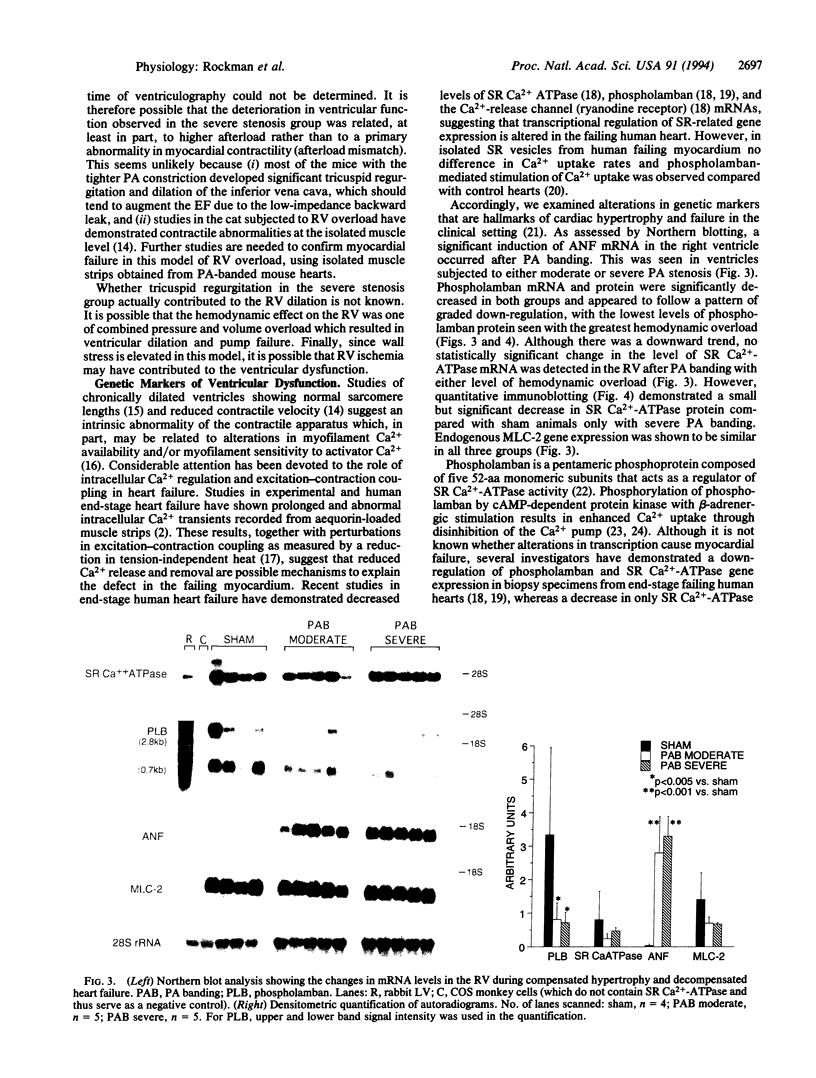
The present study reports the development and characterization of a murine model of right ventricular dysfunction following graded constriction in the pulmonary artery via microsurgical approaches. To analyze in vivo ventricular function, a technique of x-ray contrast microangiography was developed to allow the quantitative analysis of ventricular volumes and of ejection fraction in normal and pressure-overloaded right ventricle. Severe, chronic pulmonary arterial banding for 14 days resulted in right ventricular dilatation and dysfunction, associated with right atrial enlargement, and angiographic evidence of tricuspid regurgitation. These effects were dependent on the extent of hemodynamic overload, since more moderate pulmonary arterial constriction resulted in hypertrophy with maintenance of right ventricular function. With severe pulmonary artery constriction, the murine right ventricle displays a failing heart phenotype including chamber dilation with reduced function that resembles right ventricular dysfunction in man during chronic pulmonary arterial hypertension. Northern and immunoblot analyses demonstrate a marked down-regulation of phospholamban mRNA and its corresponding protein with both levels of constriction, while a less pronounced but significant depression of sarcoplasmic reticulum Ca(2+)-ATPase protein was observed with severe overload, suggesting that this pattern is an early genetic marker of ventricular dysfunction. By coupling mouse genetics with this murine model and the ability to assess cardiac function in vivo, one should be able to test the role of the down-regulation of phospholamban and other defined alterations in the cardiac muscle gene program in the onset of the failing heart phenotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert N. R., Mulieri L. A. Increased myothermal economy of isometric force generation in compensated cardiac hypertrophy induced by pulmonary artery constriction in the rabbit. A characterization of heat liberation in normal and hypertrophied right ventricular papillary muscles. Circ Res. 1982 Apr;50(4):491–500. doi: 10.1161/01.res.50.4.491. [DOI] [PubMed] [Google Scholar]
- Arai M., Alpert N. R., MacLennan D. H., Barton P., Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993 Feb;72(2):463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- Barbee R. W., Perry B. D., Ré R. N., Murgo J. P. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am J Physiol. 1992 Sep;263(3 Pt 2):R728–R733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- Chien K. R. Molecular advances in cardiovascular biology. Science. 1993 May 14;260(5110):916–917. doi: 10.1126/science.8493528. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Zhu H., Knowlton K. U., Miller-Hance W., van-Bilsen M., O'Brien T. X., Evans S. M. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- Cooper G., 4th, Tomanek R. J., Ehrhardt J. C., Marcus M. L. Chronic progressive pressure overload of the cat right ventricle. Circ Res. 1981 Apr;48(4):488–497. doi: 10.1161/01.res.48.4.488. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Origins of DNA replication in metazoan chromosomes. J Biol Chem. 1993 Jan 5;268(1):1–4. [PubMed] [Google Scholar]
- Feldman A. M., Ray P. E., Silan C. M., Mercer J. A., Minobe W., Bristow M. R. Selective gene expression in failing human heart. Quantification of steady-state levels of messenger RNA in endomyocardial biopsies using the polymerase chain reaction. Circulation. 1991 Jun;83(6):1866–1872. doi: 10.1161/01.cir.83.6.1866. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Weinberg E. O., Ray P. E., Lorell B. H. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ Res. 1993 Jul;73(1):184–192. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., Mulieri L. A., Leavitt B. J., Allen P. D., Haeberle J. R., Alpert N. R. Alteration of contractile function and excitation-contraction coupling in dilated cardiomyopathy. Circ Res. 1992 Jun;70(6):1225–1232. doi: 10.1161/01.res.70.6.1225. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med. 1990 Jan 11;322(2):100–110. doi: 10.1056/NEJM199001113220206. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Mahony L., Jones L. R. Developmental changes in cardiac sarcoplasmic reticulum in sheep. J Biol Chem. 1986 Nov 15;261(32):15257–15265. [PubMed] [Google Scholar]
- Miura T., Miyazaki S., Guth B. D., Kambayashi M., Ross J., Jr Influence of the force-frequency relation on left ventricular function during exercise in conscious dogs. Circulation. 1992 Aug;86(2):563–571. doi: 10.1161/01.cir.86.2.563. [DOI] [PubMed] [Google Scholar]
- Morgan J. P. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med. 1991 Aug 29;325(9):625–632. doi: 10.1056/NEJM199108293250906. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Colyer J., Wang J. H., Krall J. Phospholamban-mediated stimulation of Ca2+ uptake in sarcoplasmic reticulum from normal and failing hearts. J Clin Invest. 1990 May;85(5):1698–1702. doi: 10.1172/JCI114623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. A., Grupp I. L., Subramaniam A., Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res. 1991 Jun;68(6):1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- Rockman H. A., Ross R. S., Harris A. N., Knowlton K. U., Steinhelper M. E., Field L. J., Ross J., Jr, Chien K. R. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Jr, Sonnenblick E. H., Taylor R. R., Spotnitz H. M., Covell J. W. Diastolic geometry and sarcomere lengths in the chronically dilated canine left ventricle. Circ Res. 1971 Jan;28(1):49–61. doi: 10.1161/01.res.28.1.49. [DOI] [PubMed] [Google Scholar]
- Simmerman H. K., Collins J. H., Theibert J. L., Wegener A. D., Jones L. R. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986 Oct 5;261(28):13333–13341. [PubMed] [Google Scholar]
- Spann J. F., Jr, Covell J. W., Eckberg D. L., Sonnenblick E. H., Ross J., Jr, Braunwald E. Contractile performance of the hypertrophied and chronically failing cat ventricle. Am J Physiol. 1972 Nov;223(5):1150–1157. doi: 10.1152/ajplegacy.1972.223.5.1150. [DOI] [PubMed] [Google Scholar]
- Wegener A. D., Simmerman H. K., Lindemann J. P., Jones L. R. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989 Jul 5;264(19):11468–11474. [PubMed] [Google Scholar]