Abstract
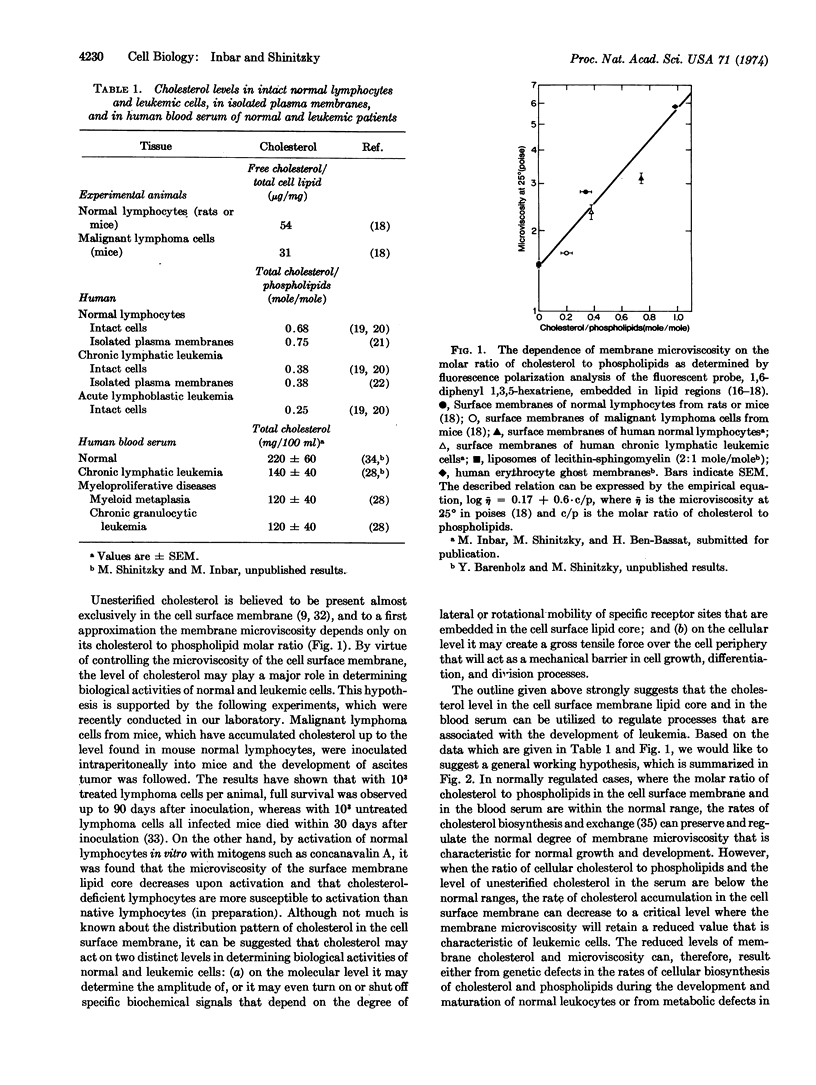
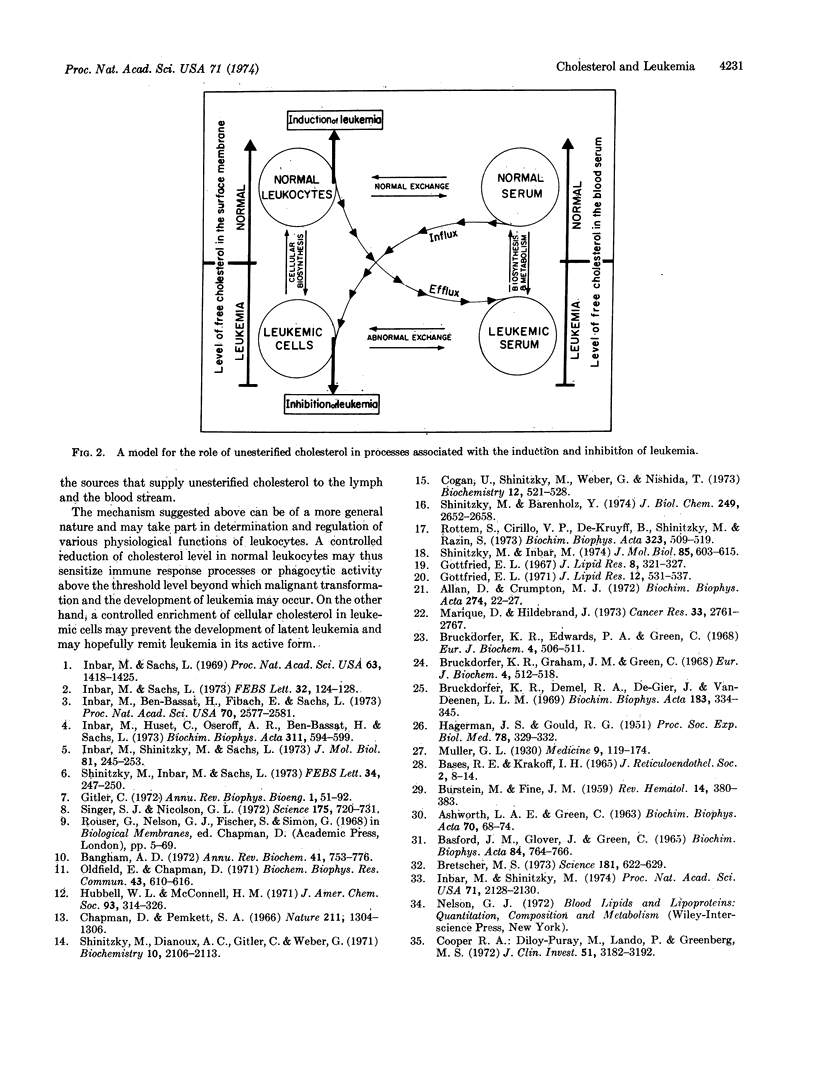
Leukemia in mice and humans is accompanied by a marked deficiency of unesterified cholesterol in the surface membrane of leukemic cells as compared to normal leukocytes. This deficiency induces a significant reduction in their membrane microviscosity. Since cholesterol in the cell surface membrane is exchangeable with cholesterol in the serum lipoproteins, concomitant to the cellular deficiency of cholesterol, the average level of cholesterol in the blood serum of leukemic patients is substantially below the average normal level. Based on these observations and the effect of membrane microviscosity on biological functions, a working hypothesis that describes the role of cholesterol in the development and inhibition of leukemia is suggested. This hypothesis can also account for the effect of cholesterol and membrane microviscosity on various other cellular activities of leukocytes.
Keywords: malignant transformation, membrane microviscosity, lipid layers, normal leukocytes, leukemic cells, blood serum
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH L. A., GREEN C. The uptake of lipids by human alpha-lipoprotein. Biochim Biophys Acta. 1963 Feb 19;70:68–74. doi: 10.1016/0006-3002(63)90719-4. [DOI] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Isolation and composition of human thymocyte plasma membrane. Biochim Biophys Acta. 1972 Jul 3;274(1):22–27. doi: 10.1016/0005-2736(72)90276-3. [DOI] [PubMed] [Google Scholar]
- BASES R. E., KRAKOFF I. H. STUDIES OF SERUM CHOLESTEROL LEVELS IN LEUKEMIA. J Reticuloendothel Soc. 1965 May;2:8–14. [PubMed] [Google Scholar]
- BASFORD J. M., GLOVER J., GREEN C. EXCHANGE OF CHOLESTEROL BETWEEN HUMAN BETA-LIPOPROTEINS AND ERYTHROCYTES. Biochim Biophys Acta. 1964 Dec 2;84:764–766. doi: 10.1016/0926-6542(64)90039-3. [DOI] [PubMed] [Google Scholar]
- BURSTEIN M., FINE J. M. [On the level of beta-lipoproteins in myelomas and in Waldenstroem's macroglobulinemia]. Rev Hematol. 1959 Oct-Nov;14:380–383. [PubMed] [Google Scholar]
- Bangham A. D. Lipid bilayers and biomembranes. Annu Rev Biochem. 1972;41:753–776. doi: 10.1146/annurev.bi.41.070172.003541. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Edwards P. A., Green C. Properties of aqueous dispersions of phospholipid and cholesterol. Eur J Biochem. 1968 May;4(4):506–511. doi: 10.1111/j.1432-1033.1968.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Graham J. M., Green C. The incorporation of steroid molecules into lecithin sols, beta-lipoproteins and cellular membranes. Eur J Biochem. 1968 May;4(4):512–518. doi: 10.1111/j.1432-1033.1968.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Diloy Puray M., Lando P., Greenverg M. S. An analysis of lipoproteins, bile acids, and red cell membranes associated with target cells and spur cells in patients with liver disease. J Clin Invest. 1972 Dec;51(12):3182–3192. doi: 10.1172/JCI107145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler C. Plasticity of biological membranes. Annu Rev Biophys Bioeng. 1972;1:51–92. doi: 10.1146/annurev.bb.01.060172.000411. [DOI] [PubMed] [Google Scholar]
- Gottfried E. L. Lipid patterns in human leukocytes maintained in long-term culture. J Lipid Res. 1971 Sep;12(5):531–537. [PubMed] [Google Scholar]
- Gottfried E. L. Lipids of human leukocytes: relation to celltype. J Lipid Res. 1967 Jul;8(4):321–327. [PubMed] [Google Scholar]
- HAGERMAN J. S., GOULD R. G. The in vitro interchange of cholesterol between plasma and red cells. Proc Soc Exp Biol Med. 1951 Oct;78(1):329–332. doi: 10.3181/00379727-78-19064. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Fibach E., Sachs L. Mobility of carbohydrate-containing structures on the surface membrane and the normal differentiation of myeloid leukemic cells to macrophages and granulocytes. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2577–2581. doi: 10.1073/pnas.70.9.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Huet C., Oseroff A. R., Ben-Bassat H., Sachs L. Inhibition of lectin agglutinability by fixation of the cell surface membrane. Biochim Biophys Acta. 1973 Jul 18;311(4):594–599. doi: 10.1016/0005-2736(73)90132-6. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Mobility of carbohydrate containing sites on the surface membrane in relation to the control of cell growth. FEBS Lett. 1973 May 15;32(1):124–128. doi: 10.1016/0014-5793(73)80753-7. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M. Increase of cholesterol level in the surface membrane of lymphoma cells and its inhibitory effect on ascites tumor development. Proc Natl Acad Sci U S A. 1974 May;71(5):2128–2130. doi: 10.1073/pnas.71.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Rotational relaxation time of concanavalin A bound to the surface membrane of normal and malignant transformed cells. J Mol Biol. 1973 Dec 5;81(2):245–253. doi: 10.1016/0022-2836(73)90192-7. [DOI] [PubMed] [Google Scholar]
- Marique D., Hildebrand J. Isolation and characterization of plasma membranes from human leukemic lymphocytes. Cancer Res. 1973 Nov;33(11):2761–2767. [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Effects of cholesterol and cholesterol derivatives on hydrocarbon chain mobility in lipids. Biochem Biophys Res Commun. 1971 May 7;43(3):610–616. doi: 10.1016/0006-291x(71)90658-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M., Sachs L. Rotational diffusion of lectins bound to the surface membrane of normal lymphocytes. FEBS Lett. 1973 Aug 15;34(2):247–250. doi: 10.1016/0014-5793(73)80804-x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]


