Abstract
This minireview provides insight into feedback regulation of heme-dependent metabolism as a defensive cellular response against stress. Interactions among heme-, iron-, porphyrin-, and CO/NO-dependent metabolic pathways during the stress-induced response are emphasized in the context of feedback regulation. The hypothetical model of the latter interactions is presented as tightly controlled feedback cycles.
INTRODUCTION
The acquisition of stress tolerance is an important aspect of survival of living organisms. Virtually all organisms respond to an environmental stress by redirecting their protein synthetic machinery to produce a small set of proteins that are frequently termed heat shock proteins (HSPs). Most of the HSPs are named by their molecular weight (eg, HSP27, HSP32, and HSP70). These proteins contribute either to protecting and repairing cells after exposure to stress (transient response) or to the adaptation to prolonged stress (sustained response). The latter preventive adaptation represents an important ability of cells to raise their protective potential through intracellular activation of negative feedback control mechanisms that retard stress-induced injury. This protection is associated with coinduction of mRNA for both heme oxygenase (HO) and iron-free apoferritin (Eisenstein et al 1991; Balla et al 1992) as the chief intracellular reservoir for iron storage in a bioavailable nontoxic form. Three distinct isozymes of HO have been identified and cloned: an inducible form, HO-1, and constitutive forms, HO-2, and a related species, HO-3 (Maines 1988, 1997; McCoubrey et al 1997). Microsomal HO-1, identical with the HSP32 (Keyse and Tyrrell 1989; Yamaguchi et al 1993; Choi and Alan 1996), catabolizes heme to bilirubin, carbon monoxide (CO), and ferrous iron (Fe2+); the latter collects rapidly into ferritin and apoferritin, synthesis of which increases under stress. The temporal storage of iron in ferritin can restrict ferrous iron from participation in the Fenton reaction, thereby reducing the oxidant burden of the cells. This defense effect is enhanced by bilirubin, which is a very efficient peroxyl radical scavenger, especially when it acts together with tocopherol at its concentration in plasma of healthy adults (Neuzil and Stocker 1994). In contrast, hyperbilirubinemia may lead to the development of neurologic encephelopathy despite reduced oxidative injury (Ryter and Tyrrel 2000), as in neonatal Gunn rats exposed to hyperoxia (Dennery et al 1995).
Why should heme be a target for an adaptive stress response?
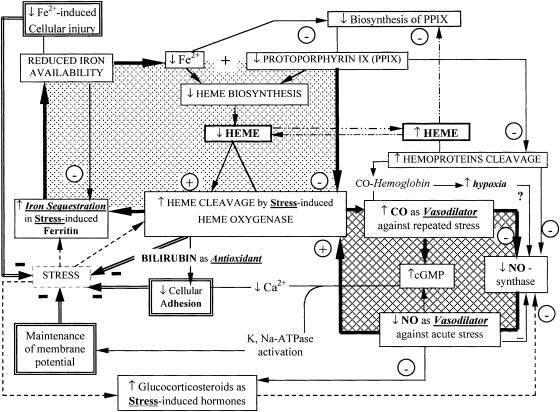
Heme, a ubiquitous iron-containing compound, a complex of iron with protoporphyrin IX (PPIX), is essential for the activity of all aerobic cells. Heme serves as the prosthetic group of numerous hemoproteins, including hemoglobin, myoglobin, cytochromes, guanylate cyclase, and nitric oxide (NO) synthase. However, heme can be inherently dangerous, particularly when released from proteins. In response to stresses, the intracellular level of heme is strictly regulated by repression of 5-aminolevulinic acid (ALA) synthase, the key enzyme of porphyrin synthesis, and enhanced HO-1 expression (Hentze and Kuhn 1996; Ponka 1999), although some other regulatory systems can also operate. The heme catabolism provided by HO-1 leads to the formation of low-molecular-mass redox-active iron, which is a more versatile catalyst of oxidative damage than heme (Lamb et al 1999). Because of this, the protective effects ascribed to HO-1 might actually reside in the adequate induction of ferritin (Eisenstein et al 1991; Balla et al 1992) rather than in HO-1 itself (Lamb et al 1999). The latter conclusion is confirmed with several models of injury. While acute heme exposure exacerbates oxidative injury, coordinated expression of HO- 1 and apoferritin in the endothelium protects from subsequent exposure to hydrogen peroxide, activated neutrophils, or oxidized low-density lipoprotein (Balla et al 1992; Juckett et al 1998). Together, HO-1 and ferritin allow rapid shifting of iron from heme into the ferritin core, where it is less available for different metabolic pathways. These changes are attended by a rise in cytoprotection against iron-mediated free radical injury; cell growth arrest via the lowering of the iron-dependent activity of ribonucleotide reductase, an essential enzyme for DNA- synthesis (Sussman 1989); and suppression of synthesis of heme prosthetic groups. As a result, a decline in pools of heme (as substrate for HO-1) and a rise in the level of PPIX (as inhibitor of HO-1 enzyme) occur. This imbalance completes the formation of a negative feedback cycle retarding the further activation of HO-1 under stress (Fig 1, left compartment). Moreover, the inhibitory effect of low iron on the synthesis of ALA-synthase can be viewed as an additional way to monitor the stress-induced production of protoporphyrin. This negative regulation acts as an intermediate feedback controller of HO-1 activity because the PP1X is the inhibitor of HO-1, which may limit heme catabolism (Hentze and Kuhn 1996). The adaptive cytoprotection requires the cooperative feedback control between coordinated expression of HO-1, ALA- synthase, apoferritin, and its reciprocal regulation. In view of the fact that the feedback restricts excessive HO- 1 activity and reduces hemoprotein content, this process must be tightly controlled. Excessive heme cleavage can be dangerous, as in Parkinson's disease (Schipper et al 1998) and endotoxin-induced hyperbilirubinemia and cholestasis (Camhi et al 1995; Goda et al 1998). The maintenance of the heme biosynthesis/degradation balance appears to be crucial in genetically modified HO-1 deficit cells (Poss and Tonegawa 1997).
Fig 1. Two-compartment model of self-cytoprotection against stress as a result of the feedback control interactions among heme-, iron-, porphyrin-, and NO/CO-dependent pathways. The latter interactions are presented in the form of 2 self-regulated feedback loops where coordinated regulation of heme-dependent metabolism can defend against negative stress-induced consequences, among them enhanced cellular oxidant injury, vasospasm, and local inflammation. The left compartment shows how the feedback cycle is capable of suppressing the stress-induced activation of heme oxygenase-1 (HO-1 or HSP32). The right compartment shows how stress-induced activation of the HO-1-CO-cGMP pathway is capable of increasing intracellular cGMP as a protective response against vasospasm, whereas the NO-synthase- NO-cGMP pathway is suppressed by glucocorticosteroid hormones
Crucial role of heme oxygenase–1/CO pathway in the regulation of blood pressure under stress
Heme is known to exert vasodepressive action, which is sensitive to inhibition of HO-1, attenuated by iron chelators and mimicked by exogenous CO but not by bilirubin (Motterlini et al 1998). These observations suggest that during stress the HO-heme-CO system may be involved in the regulation of blood pressure because stress-induced HO-1 and constitutively expressed HO-2 (as in spleen, brain, and testes) are the sole physiological sources of CO production (Maines 1988). Marks et al (1991) drew attention to the chemical similarities between CO and NO and proposed that CO may be a physiological regulator akin to NO. CO, like NO, can stimulate guanylyl cyclase activity by binding to heme at the active site of the enzyme that converts guanosine triphosphate to guanosine 3,5-cyclic monophosphate (cGMP) (Kharitonov et al 1995). Molecular targets for cGMP include protein kinase, phospho-diesterases, and ion channels (Maines 1992; Lincoln and Cornwell 1993). cGMP levels function as a sensor for intracellular events such as Ca2+ movements, heat shock, and redox status (Schmidt 1992). An important aspect of cGMP-signaling system suggests links among HO-1, NO-synthase, and guanyl cyclase that are controlled via NO as HO-1 inducer (Maines et al 1993) and CO as inhibitor of NO-synthase and HO-1 mRNA expression (Yee et al 1996). Both soluble guanylate cyclase and NO-synthase are hemoproteins, and the heme prosthetic group of these enzymes likely is a substrate for HO- 1. Thus, the HO-1 can play a role as a direct regulator of both these hemoproteins as well as regulating their turnover (Morita et al 1995). Although stress causes a remarkable reduction in NO-producing capability, the tissue level of cGMP is not significantly reduced (Maines et al 1995; Morita et al 1995). The latter is achieved by the compensatory induction of HO-1 under stress (Fig 1, right compartment).
It is known that stress is a potent stimulator of adrenal glucocorticoid release that is responsible for lowering transcription of the NO-synthase gene (Hatlor et al 1997). In contrast, steroid treatment showed no discernible effect on HO-1 mRNA (Maines et al 1995). For this reason, the HO-1 dependent upregulation of CO/cGMP is able to prevent the dangerous development of vasospasm under stress. Pretreatment of animals with a HO-1 inhibitor (as the zinc deuteroporphyrin 2,4-bis glycol) markedly decreased aortic CO and cGMP levels and completely restored the stress-promoted vasoconstriction (Johnson et al 1997). This finding supports a crucial role of the CO/ cGMP pathway in the regulation of blood pressure under stress (Maines 1993). Note that free hemoglobin, acting as a CO-trapping reagent, eliminates CO in situ and thereby evokes sinusoidal or vascular spasm (Sano et al 1997). The CO generation may also be reduced by acute NADPH consumption in other microsomal enzyme systems such as cytochrome P450 because NADPH and molecular oxygen are essential to CO formation by heme cleavage (Maines 1997). In other words, CO generation at submicromolar concentration serves as an endogenous factor that actively lowers vascular and sinusoidal tone, without inhibiting oxygen consumption or lowering tissue ATP levels, but only when stress-induced injury is not followed by acute hemolysis and/or NADPH tissue deficiency. Based on the previously mentioned metabolic interactions, I propose a working 2-compartment model (Fig 1) of positive and negative regulation of the main heme-dependent processes as a cascade linked by sequential changes/steps, which permits a greater stress resistance to oxidative injury and vasospasm.
The anti-inflammatory properties of HO-1 and its end products
The common consequence(s) of the stress effect is associated with cellular injury involved in local and/or systemic inflammation, the early sign of which is the infiltration of leukocytes into the damaged tissues via enhanced adhesion to vascular endothelium. Glucocorticosteroids suppress this inflammation by inhibiting endothelial expression of adhesion molecules for circulating leukocytes (Cronstein et al 1992). HO-1 may also inhibit inflammation and protect endothelium and smooth muscle cells from oxidant stress (Platt and Nath 1998), primarily via inhibition of leukocyte adhesion elicited by pro-oxidant stimuli (Hayashi et al 1999). The decrease in the oxidant-elicited leukocyte adhesive responses under HO-1-inducing conditions were restored by perfusion with Zinc-PP1X (ZnPPIX), as an HO-1 inhibitor, but not with copper-PPIX, which did not inhibit the enzyme (Hayashi et al 1999). Furthermore, the effect of ZnPP1X was repressed by syperfusion with bilirubin and biliverdin at the micromolar level but not by the same concentration of CO (Hayashi et al 1999). HO-1 may be one of the protective factors in atherogenesis (Siow et al 1999) by inhibiting the proinflammatory effects of low- density-lipoprotein (LDL) oxidation in the artery wall. This HO-1-mediated effect is achieved by the reduction of monocyte chemotaxis and transmigration in response to LDL oxidation (Ishikawa et al 1997). Conversely inhibition of HO-1 by its PP1X inhibitor enhanced chemotaxis. In contrast, pretreatment with bilirubin or biliverdin at the micromolar level reduced this chemotaxis (Ishikawa et al 1997). The anti-inflammatory properties of HO- 1 were found also in an experimental model of acute pleurisy, where increased HO-1 expression closely correlated with decreased numbers of inflammatory cells in the pleural cavity and reduced exudate volume. Pretreatment with tin protoporphyrin (an HO-1 inhibitor) abolished the effect (Willis et al 1996). It was found that HO- 1 may also inhibit inflammatory processes through blocking leukocytes activation (Willis et al 1996) and inhibiting platelet aggregation (Brune and Ullrich 1987). The HO-1- induced inhibition of platelet activation by CO compares with well-known NO-induced inhibition of platelet aggregation (Naseem and Bruckdorfer 1997). Both in vivo and in vitro, CO at low concentrations differentially and selectively inhibited the expression of lipopolysaccharide (LPS)-induced proinflammatory cytokines as TNF-α, interleukin-1, and macrophage inflammatory protein-1 and increased the LPS-induced expression of the anti-inflammatory cytokine interleukin-10 (Otterbein et al 2000). In a like manner, rats exposed to low CO concentrations in the air exhibited a marked attenuation of hyperoxia-induced inflammation via reduced neutrophil infiltration into the airway (Otterbein et al 1999). Both mice and humans deficient in HO-1 expression have a phenotype of an increased inflammatory state (Poss and Tonegawa 1997). These data indicate the possibility that low CO may have an important protective function in inflammatory diseases states and thus has potential therapeutic uses.
The biochemical mechanism(s) responsible for this CO- mediated protection is not clearly understood, but it is thought to be associated with the ability of endogenous CO to suppress the production of proinflammatory cytokines, such as platelet-derived growth factor, endothelin and/or interleukin-1, and TNF-α (Platt and Nath 1998; Otterbein et al 2000), and to activate the Na+,K+-ATPase for the maintenance of membrane potential (Nathanson et al 1995). Because of this, the anti-inflammatory potential of HO-1 expression is attractive for practical use, for example, in therapy for rheumatoid arthritis (Caltabiano et al 1986) and transplantation where rapid expression of HO-1 in cardiac xenografts can be essential to ensure long-term xenograft survival (Soares et al 1998). Although the end products of HO-1 may act as antistress agents, it may also be toxic to the lungs and other oxygen-sensitive tissues at elevated concentration (Forbes 1970).
CONCLUDING REMARKS
The adaptive phenomenon of self-cytoprotection reflects a coordinated complex of molecular feedback mechanisms, which can save living organisms and their cells from irreversible damage through the temporary acquisition of stress tolerance. This resistance phenotype is manifested at 3 levels of heme-dependent organization: (1) physiological: increased resistance to vasospasm and inflammation; (2) cellular: enhanced oxidative resistance to cytotoxic environments; and (3) biochemical-molecular: the presence of a biochemical products that diminishes or counteracts damaging effect of stress. These products may also be cytotoxic at elevated concentration and in relation to the cell-type origin. The antistress signaling response consists of complementary transcriptional regulation: the coordinated induction of apoferritin, HO-1, ALA-synthase, and NO-synthase (see Fig 1), which is carried out by a complex of regulatory elements (Abraham et al 1996; Elbirt and Bonkoxsky 1999) and pathways (Ryter and Tyrrel 2000). Moreover, type of nutrition and some drugs are capable of modulating this cytoprotective response. Examples are found in the cardiovascular action of aspirin, which, in low concentration, can induce apoferritin synthesis in endothelial cells (Oberle et al 1998) and by this pathway can contribute to vessel protection against stress-induced injury. An endogenous decline in this defense occurs frequently under stressful conditions, like rapid aging and the paraneoplastic disorders, the progression of which coincided with ferritin inactivation by oxygen-derived free radicals in vivo (Schwartsburd 1995, 1998).
The 2-compartments model (Fig 1) presented here is consistent with a large body of experimental observations and is important in understanding the value of the delicate balance in feedback regulation among iron-, heme-, porphyrin-, and CO/NO-dependent metabolic processes that are favorable for cytoprotection. This model provides an essential scheme for future scientific and medical application.
REFERENCES
- Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- Balla J, Balla G, Nath K, Vercellatti GM. Endothelial cell heme oxygenase and ferritin induction by heme proteins: a possible mechanism limiting shock damage. Clin Res. 1992;40:323A. [PubMed] [Google Scholar]
- Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by the activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- Caltabiano MM, Koestler TP, Poste G, Greig RG. Induction of mammalian stress protein by a triethylphosphine gold compound used in the therapy of rheumatoid arthritis. Biochem Biophys Res Commun. 1986;138:1074–1080. doi: 10.1016/s0006-291x(86)80391-6. [DOI] [PubMed] [Google Scholar]
- Camhi SL, Alam J, Otterbein L, Sylvester SL, Choi AM. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am J Respir Cell Mol Biol. 1995;13:387–398. doi: 10.1165/ajrcmb.13.4.7546768. [DOI] [PubMed] [Google Scholar]
- Choi AM, Alan J. Heme oxygenase-1: function, regulation and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kimmel SC, Levin RI, Martiniuk F. A mechanism for the anti-inflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennery PA, McDonagh AF, Spitz DR, Rodgers PA. Hyperbilirubinemia results in reduced oxidative injury in neonatal Gunn rats exposed to hyperoxia. Free Radic Biol Med. 1995;19:395– 404. doi: 10.1016/0891-5849(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Eisenstein RS, Garcia-Magol B, Petingell W, Munro MN. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different form of iron. Proc Natl Acad Sci USA. 1991;88:688– 692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbirt KK, Bonkoxsky HL. Heme oygenase: recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438–447. [PubMed] [Google Scholar]
- Forbes WH. Carbon monoxide uptake via the lungs. An NY Acad Sci. 1970;174:72–75. doi: 10.1111/j.1749-6632.1970.tb49773.x. [DOI] [PubMed] [Google Scholar]
- Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. Distribution of heme oxygenase isoforms in rat liver. J Clin Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatlor Y, Akimoto K, Nakaniski N, Kasai K. Glucocorticoid regulation of nitric oxide and tetrahydrobiopterin in rat model of endotoxic shock. Biochem Biophys Res Commun. 1997;240:298–303. doi: 10.1006/bbrc.1997.7653. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Takamiya R, Yamaguchi T, et al. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generation by the enzyme. Circ Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- Hentze MZ, Kuhn LC. Molecular control of vertebrate iron metabolism: m RNA-based regulatory circuits operated by iron, NO and oxidative stress. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Navab M, Leitinyer N, Fogelman AM, Lusis A. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Colombari E, Colombari DS, Lavesa M, Talman WT, Nasjletti A. Role of endogenous CO in central regulation of arterial pressure. Hypertension. 1997;30:962–967. doi: 10.1161/01.hyp.30.4.962. [DOI] [PubMed] [Google Scholar]
- Juckett M, Zheng Y, Yuan H, Pastor T, Antholine W, Weber M, Vercellotti G. Heme and the endothelium: effects of nitric oxide on catalytic iron and heme degradation by heme oxygenase. J Biol Chem. 1998;272:23388–23397. doi: 10.1074/jbc.273.36.23388. [DOI] [PubMed] [Google Scholar]
- Keyse SM, Tyrrell KM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov VG, Sharma VS, Pilz RB. Basis of guanylate cyclase activation by carbon monoxide. Proc Natl Acad Sci USA. 1995;92:2568– 2571. doi: 10.1073/pnas.92.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb NJ, Quinlan G, Mumby S, Evans TW, Gutterridge JMC. Haem oxygenase shows pro-oxidant activity in microsomal and cellular systems: implications for the release of low-molecular- mass iron. Biochem J. 1999;344:241–256. [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Intracellular cyclic cGMP receptor proteins. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: function multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Maines MD, Eke BC, Webe CM, Ewing YF. Corticosterone has a permissive effect on expression of heme oxygenase-1 in CAI- CA3 neurons of hippocampus in thermal-stressed rats. J Neurochem. 1995;64:1769–1779. doi: 10.1046/j.1471-4159.1995.64041769.x. [DOI] [PubMed] [Google Scholar]
- Maines MD, Mark J, Ewing J. Heme oxygenase, a likely regulator of cGMP production in the brain: induction in vivo of HO- 1 compensates for depression in NO-synthase activity. Mol Cell Neurosci. 1993;4:398–405. doi: 10.1006/mcne.1993.1050. [DOI] [PubMed] [Google Scholar]
- Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon- monoxide have a physiological function? Trends Pharmacol Sci. 1991;12:185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- Morita T, Perrella MA, Lee ME, Kourembanes S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Gonzales A, Foresti R, Clark J, Gree CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive response in vivo. Circ Res. 1998;83:568–574. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- Naseem KM, Bruckdorfer KR. Inhibition of platelet activation by CO comparison with inhibition of platelet aggregation via NO. Biochem Soc Trans. 1997;25:3965S. doi: 10.1042/bst025396s. [DOI] [PubMed] [Google Scholar]
- Nathanson JA, Scavone C, Scanlon C. The cellular Na+ pump as a site of action for carbon monoxide and cellular activity. Neuron. 1995;14:781–794. doi: 10.1016/0896-6273(95)90222-8. [DOI] [PubMed] [Google Scholar]
- Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for α-tocopherol: inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- Oberle S, Polte T, Abate A, Podhaisky HP, Schroder H. Aspirin increases ferritin synthesis in endothelial cells: a novel antioxidant pathway. Circ Res. 1998;82:1021–1022. doi: 10.1161/01.res.82.9.1016. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Adam J, et al. Carbon monoxide has anti-inflammatory effect involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- Platt JL, Nath KA. Heme oxygenase protective gene or Trojan horse. Nat Med. 1998;4:1364–1365. doi: 10.1038/3947. [DOI] [PubMed] [Google Scholar]
- Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Free Radic Biol Med. 2000;8:289– 309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Sano T, Shiomi M, Wakabayshi Y, et al. Endogenous carbon monoxide suppression stimulates bile acid-dependent biliary transport in perfused rat liver. Am J Physiol. 1997;272:G1268–G1275. doi: 10.1152/ajpgi.1997.272.5.G1268. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Liberman EG, Stopa EG. Neural heme oxygenase-1 expression in idiopatic Parkinson's disease. Exp Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Schmidt HHW. NO, CO and ˙OH endogenous soluble guanylyl cyclase-activating factor. FEBS Lett. 1992;307:102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Soares MP, Anrather J, Lin Y, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;32:497–504. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- Schwartsburd PM. On the origin of heterogeneity of lipofuscin fluorophores and their possible interrelations. Gerontology. 1995;41:29–37. doi: 10.1159/000213723. [DOI] [PubMed] [Google Scholar]
- Schwartsburd PM. An integrated view on the genesis of irreversible anemia and endotoxemia acceleration by tumor preservation. Front Perspect. 1998;7:33–40. [Google Scholar]
- Siow RCM, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atheiogenic actions of Bilirubin and carbon monoxide? Cardiovascul. Res. 1999;41:385–394. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- Sussman HH 1989 Iron and tumor cell growth. In: Iron in Immunity, Cancer & Inflammation, ed De Sousa M, Brock JH. John Wiley & Sons, New York, 261–282. [Google Scholar]
- Willis D, Moore AF, Frederick R, Willouguby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Sato H, Bannai S. Induction of stress proteins in mouse peritoneal macrophages by oxidized low-density lipoproteins. Biochem Biophys Res Commun. 1993;193:1198–1201. doi: 10.1006/bbrc.1993.1752. [DOI] [PubMed] [Google Scholar]
- Yee EL, Pitt BR, Billiar RR, Kim YM. Effect of nitric oxide on heme metabolism in pulmonary endothelial cells. Am J Physiol. 1996;271:L512–L518. doi: 10.1152/ajplung.1996.271.4.L512. [DOI] [PubMed] [Google Scholar]