Abstract
Exposure of cells to arsenicals activates multiple stress pathways resulting in the induction of specific genes whose identity and role in the adaptation to arsenical-induced cellular stress are poorly understood. We report here the identification of a novel gene encoding an arsenite-inducible, cysteine- and histidine-rich RNA-associated protein, AIRAP, that is conserved among mammals, Drosophila and C elegans. Immunochemistry and cell fractionation experiments indicate that, when induced, AIRAP is present in both the nucleus and the cytoplasm, and cross-linking experiments indicate that it associates with RNA in vivo. The expression of a C elegans homologue of AIRAP, aip-1, is also induced by exposure to arsenite, and expression of an aip-1::gfp transgene is most pronounced in hypodermal cells. RNA-mediated interference (RNAi) of aip-1 lowers the resistance of nematodes to arsenite yet does not appear to affect viability under standard growth conditions. These experiments suggest a role for AIRAP/AIP-1 in protecting cells from the toxic effects of arsenite.
INTRODUCTION
Arsenicals are important human toxins by virtue of their ubiquity in the natural environment and the workplace. Acute toxicity is thought to result from an inhibition in protein function caused by the reaction of arsenicals with free sulfhydryl groups (Thompson 1993) However, from a clinical standpoint the chronic effects of arsenite exposure, such as carcinogenicity and neurological and renal system dysfunction, are more significant than acute toxicity (Snow 1992). As the biological basis for chronic arsenite toxicity is poorly understood, a considerable effort has been directed toward analyzing the cellular response to arsenic exposure.
Exposure to arsenite, the active trivalent form of the atom, elicits several cellular responses. Arsenite treatment of cells induces cytosolic chaperone expression and reduces protein translation. These responses are thought to mitigate arsenite's damaging effects by promoting the refolding or degradation of altered proteins and by limiting the synthesis of new proteins that may malfold when modified by arsenite (Brostrom and Brostrom 1998). The expression of heme oxygenase (Elbirt and Bonkovsky 1999) and γ-glutamylcysteine synthetase (Ochi 1997), which can combat oxidative stress and perhaps limit the reactivity of arsenite by modifying the cellular chemical environment, are also upregulated. Lastly, arsenite exposure induces the expression of metallothioneins that possibly act in the detoxification of arsenite and other transition metals (Palmiter 1998).
Arsenite treatment has been found to activate several signaling pathways. These include the activation of the heat shock transcription factor (Koizumi et al 1993; Mosser et al 1993), stress-activated protein kinase signaling cascades (Cavigelli et al 1996; Liu et al 1996), NFκB (Barchowsky et al 1996), and other less well defined pathways that lead to the phosphorylation of eIF2α (Brostrom and Brostrom 1998), induce the expression of CHOP/ GADD153 gene (Fawcett et al 1996; Guyton et al 1996), or activate metallothionein gene expression (Kreppel et al 1993). However, none of these known pathways activated by arsenite treatment are specific, as they are also induced by other unrelated forms of cellular stress. Here we report the identification of a novel gene selectively inducible by arsenite exposure and not other toxic stimuli. The product of this gene, AIRAP, defines a new class of arsenite-inducible proteins.
MATERIALS AND METHODS
Cell culture, treatment, fractionation, and staining
Primary cultures of murine proximal tubule epithelium (MPTE) from kidney were generated by following established procedures (Elliget and Trump 1991). MPTE cells, wild-type and Mtf-1-/- fibroblasts (Gunes et al 1998), and NIH3T3 and 293T cells were cultured in DMEM + 10% fetal bovine serum. Sodium arsenite, tunicamycin, ZnCl2, CuCl2, H2O2, and cycloheximide were purchased from Sigma. Sodium arsenite (30μM, 6.5 hours)-treated or -untreated NIH3T3 cells were pelleted and resuspended in hypotonic buffer, SI (50 mM Tris pH 7.9, 10 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulphonyl fluoride, 4 mg/mL Aprotonin, and 2 mg/mL Pepstatin A, at 100 mL SI/100-mm dish of cells). The suspension was placed on ice for 5 minutes, vigorously homogenized with a Dounce homogenizer for 2–3 minutes, and centrifuged to collect the nuclear pellet and cytoplasmic supernatant fractions. The cytoplasmic supernatant was further fractionated by centrifugation at 100 000 × g for 30 minutes (Beckman TLA-100.2 rotor), and the pellet and supernatant were collected. All samples were solubilized in loading buffer (final concentration of 25 mM Tris pH 6.8, 1% SDS, 20 mM DTT, 7.5% glycerol, 0.05% Bromophenol blue), boiled for 5 minutes, and analyzed by SDS- PAGE. For immunocytochemical detection of AIRAP, NIH3T3 cells were cultured on gelatinized glass coverslips, treated with arsenite at the indicated concentration for 6 hours, and fixed in 4% paraformaldehyde.
cDNA synthesis, Representational Difference Analysis, and full-length cDNA cloning
MPTE cells at ∼75% confluence were treated with sodium arsenite (50 μM, 4 hours). Poly(A)+ RNA was prepared and double-stranded cDNA synthesized using Stratagene's ZAP-cDNA synthesis kit with the modification that the first-strand primer contained a Bgl II site and 5-methyl-dCTP was omitted from the first-strand synthesis step. Representational Difference Analysis (RDA) was performed as described (Hubank and Schatz 1994). The first, second, and third round of subtractive hybridization were performed at “tester” to “driver” ratio of 1:100, 1: 800, and 1:40 000, respectively. After the 3 cycles of subtraction and amplification, the Dpn II digested differential products were fractionated on a 2% agarose gel, and identifiable bands were ligated into the BamH I site of pBS vector. The cloned cDNA fragments were then used as probes for Northern blot analysis on MOPS formaldehyde gels and to isolate full-length cDNA clones.
Expression plasmids and antisera production
A MYC (9E10) epitope-tagged AIRAP mammalian expression vector was constructed by overlapping PCR of AIRAP cDNA with oligos 5′ GAC AGG ATC CAT AAT GGA GTT TCC TGA CT 3′, 5′ AGA GAT CAG CTT CTG CTC CTT CAC TGT GCG CCT GAG 3′ and 5′ GGG GCT CGA GTC ACA GAT CCT CCT CAG AGA TCA GCT TCT GCT C 3′ and expressed from a pCDNA3 mammalian expression plasmid (Invitrogen). A bacterial expression plasmid encoding a full-length fusion of AIRAP and GST was constructed in pGEXKG (Pharmacia). The purified fusion protein was cleaved with thrombin, and the liberated intact AIRAP was used to immunize rabbits. The antisera was used at a dilution of 1:5000 for Western blot and 1:500 for indirect immunofluorescent detection of the endogenous protein.
UV cross-linking of AIRAP bound to RNA in vivo
The procedure for in vivo UV cross-linking of proteins to RNA has been described (Zinszer et al 1997). To detect the endogenous AIRAP-RNA complex, NIH3T3 cells were treated with 50 μM sodium arsenite for 6 hours. For transient overexpression of AIRAP, 293T cells were transfected with MYC epitope-tagged AIRAP and subjected to UV cross-linking, 48 hours after transfection with or without a preceding 1-hour exposure to 100 mM sodium arsenite. In both cases, cells grown in 100-mm dishes were irradiated with 850 mJ/cm2 in a UV Stratalinker 2400 (Stratagene). Cell extracts were prepared in HEPES- Triton X-100 buffer (20 mM HEPES pH 7.5, 150 mM NaCl2, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulphonyl fluoride, 4mg/mL Aprotonin, and 2 mg/mL Pepstatin A) at a volume of 150 mL/100-mm dish of cells, clarified at 16 000 × g for 10 minutes to remove insoluble material. RNase A1 was added to the soluble lysate (1 mg/150 mL lysate) and incubated at room temperature for 45 minutes. SDS was added to 1% w/v, and the lysate was heated to 90°C for 5 minutes. The lysate was further clarified by spinning at 200 000 × g for 30 minutes. The supernatant was diluted 1:10 in the same buffer containing no SDS, precleared with Protein A Sepharose beads, and immunoprecipitated with the indicated antibodies overnight at 4°C. Radioactive labeling of the immunoprecipitated protein was preformed as described (Zinszer et al 1997).
The nature of the labeled polynucleotide species cross- linked in vivo to AIRAP was examined by excising the PAGE-SDS gel fragment containing the labeled band and treating it with proteinase K to degrade the protein component of the covalent complex as described (Zinszer et al 1997). The labeled species was allowed to diffuse out of the gel and was recovered by ethanol precipitation. The recovered labeled material was subjected to digestion with RNase A or Dnase I and loaded onto an 8-M urea 15% acrylamide gel run in Tris-Borate buffer and exposed to autoradiography. RNase substantially degraded the labeled species, while DNase treatment had no effect (Fig 4C). To reduce the background in the in vitro T4 kinase labeling step, we found it necessary to treat the crude cellular lysate with RNase and clarify it before immunopurifying and labeling the protein-RNA complex (Zinszer et al 1997). Failure to treat the crude lysate with RNase leads to loss of AIRAP-RNA complex during clarification (Fig 4A, lane 5). The small size of the RNA fragment recovered from the immunopurified AIRAP-RNA complex (Fig 4C) is likely to be a consequence of this preceding RNase digestion step that leaves intact only that portion of the RNA that is “protected” by its interaction with the protein.
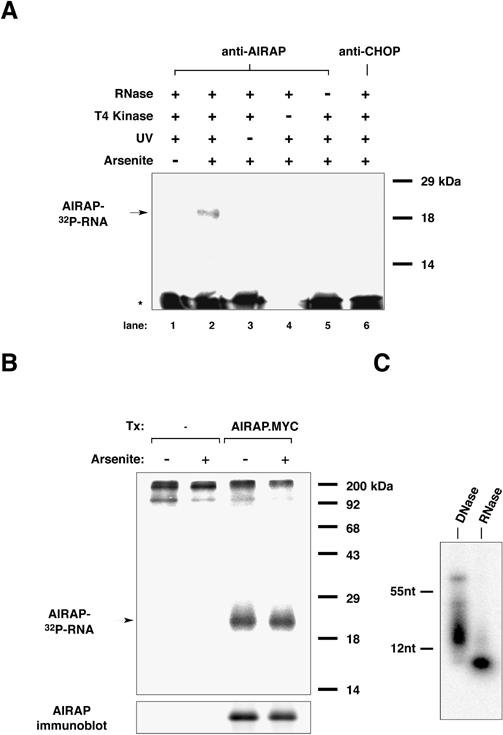
Fig 4. In vivo cross-linking of AIRAP to RNA. (A) Autoradiogram of the immunopurified covalent complex formed in NIH3T3 cells between endogenous AIRAP and an associated polynucleotide species, resolved by 12% SDS-PAGE. The immunopurified complex had been labeled in vitro with 32P γ-ATP using T4 polynucleotide kinase. Note that the labeled complex (horizontal arrow, AIRAP- [32P]RNA) was dependent on arsenite treatment of the cells, anti- AIRAP immunoprecipitation, UV cross-linking, and the addition of T4 kinase. It was absent from the control anti-CHOP immunoprecipitation (lane 6) or from lane 5, in which pretreatment of the lysate with RNase A had been omitted (see “Materials and Methods” for details). The intensely labeled bands at the bottom of the gel (asterisk) are presumed to represent small, soluble fragments of contaminating polynucleotides that were labeled by T4 kinase. (B) Autoradiogram of a gel prepared as in “A” but using lysates from untransfected or 293T cells transfected with an expression plasmid encoding MYC epitope-tagged AIRAP protein. Where indicated, the cells had been treated with 100 μM sodium arsenite for 1 hour. (C) Sensitivity of the labeled polynucleotide fragment recovered from the immunoprecipitated and gel-purified AIRAP-RNA complex gel to digestion with RNase. Shown is an autoradiogram of an 8-M urea 15% acrylamide gel with mobility markers indicated to the right.
Regulation of aip-1 gene expression and arsenite tolerance in C elegans
The aip-1::gfp transgene was constructed using PCR to amplify a 3.5-Kb promoter region that was then subcloned in-frame with the GFP gene in the pPD95.75 vector. The PCR primers used were 5′ TCC GAG ATC TGG GAA CTC CGC CAT AGC TGA 3′ and: 5′ CGT CTC TAC AAA AGA TCA ATT GTC TGT 3′. The aip-1 cDNA was cloned by RT-PCR using RNA obtained from wild-type (N2) C elegans grown on media containing 3 mM arsenite for 4.5 hours. The oligonucleotides used for RT PCR were 5′ CCC CGG ATC CAT GGC GGA GTT CCC AAA TCT CGG A 3′ and 5′ CCC CTC ATG AGA CGG TGC AGT TGG AAT TGG 3′. The 623-bp amplified product was ligated into pGEM7Z (Promega) and used as a probe for Northern blot analysis or as a template for the in vitro synthesis of double-stranded RNA. Double-stranded RNA for RNAi of ire-1 was synthesized from the ire-1 cDNA (yk8e9a).
aip-1::gfp transgenic animals were generated by coinjection of the aip-1::gfp construct and the clone pSK1 containing the lin-15 gene into lin-15(n765)X animals (Clark et al 1994). Extrachromosomal array–containing animals were identified by rescue of the lin-15 multivulva (Muv) phenotype at 25°C. Three integrated lines (SJ4001: aip-1:: gfp[zcIs1]; lin-15[n765]X; SJ4002 aip-1::gfp[zcIs2]; lin- 15[n765]X; SJ4003 aip-1::gfp[zcIs3]; lin-15[n765]X) were established following trimethylpsoralen mutagenesis (Yandell et al 1994). Larval stage 4 (L4) aip-1::gfp; lin-15 animals were transferred to plates containing 0, 1.5, or 4.5 mM arsenite. As these concentrations of arsenite prevented Escherichia coli growth, we seeded the plates of both arsenite-treated and -untreated worms with a strain of OP50 (E coli) selected to grow on sodium arsenite. To study the sensitivity of worms lacking aip-1 function, we eliminated aip-1 activity by RNAi (Fire et al 1998). myo-3: :gfp animals (PD4251: ccIs4251 I; dpy-20[e1282] IV) were injected with a 1:1 mixture of gfp and ire-1 or gfp and aip- 1 dsRNAs. Successful RNA interference was determined by the loss of myo-3::gfp expression in muscle tissue of F1 progeny of injected animals. GFP negative animals were raised to the L4 stage and then transferred to plates prepared with the indicated concentration of arsenite for survival studies (30 worms/condition).
RESULTS
To discover new genes inducible by arsenite treatment, we applied the method of representational difference analysis (RDA) to pools of cDNA obtained from cultured primary mouse renal proximal tubular epithelial (MPTE) cells that were exposed to sodium arsenite or left untreated. MPTE cells were used because many of the physiologically relevant target cells of arsenite toxicity are epithelial and RDA can identify cDNA fragments that are present at different levels in 2 cDNAs pools. Four candidate cDNAs were isolated and cloned. By Northern blot analysis, these cDNAs hybridized to RNAs that were present at higher levels in arsenite-treated cells than in untreated cells, indicating that these cDNAs represent genes inducible by arsenite exposure (Fig 1).
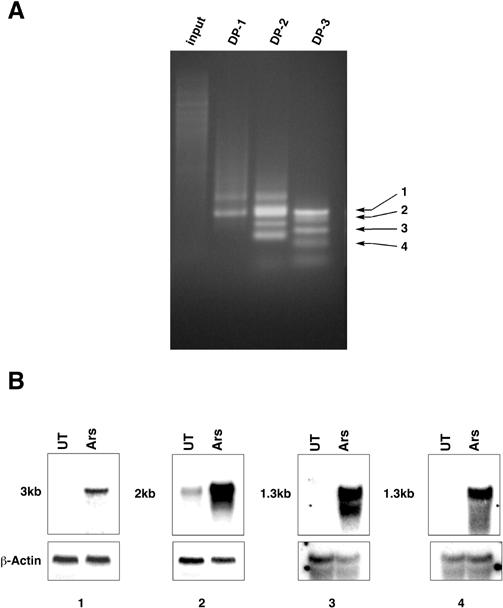
Fig 1. Identification of arsenite-inducible genes by representational difference analysis. (A) The input and “difference product” after each of 3 cycles of amplification were analyzed by gel electrophoresis and Ethidium Bromide staining (DP-1, DP-2, DP-3). The positions of the 4 (1–4) cDNAs isolated are indicated by arrows. (B) Northern blot analysis of RNA from untreated (UT) and arsenite-treated (Ars) MPTE cells probed with each of the 4 cDNAs. The size of the hybridizing RNA is indicated on the left. The filter was washed and subsequently hybridized with a β-actin cDNA as a control for RNA loading and integrity
Sequence analysis revealed that 2 cDNAs were derived from the Hsp70A gene, a gene known to be induced by arsenite treatment (Lee et al 1991). The third cDNA was derived from the p62 gene, which encodes a protein that interacts with the SH2 domain of p56lck (Joung et al 1996). The p62 gene is inducible by arsenite, cadmium, and oxidative stress agents, such as paraquat (Ishii et al 1996). More recently, the p62 protein has been shown to bind multiubiquitinated proteins and is believed to participate in their sequestration in specific cytosolic sites (Shin 1998). The fourth cDNA isolated in our screen represented a novel gene that we named AIRAP, as discussed in the following.
Basal levels of AIRAP mRNA were barely detectable. However, in both MPTEs and rodent fibroblasts, AIRAP mRNA was induced more than 15-fold by arsenite treatment. Lower levels of induction (3–5-fold) were observed in cells exposed to zinc at a concentration that fully activated metallothionein gene expression. Heat shock, oxidative stress (H2O2), and copper or tunicamycin treatment (an agent that causes stress in the endoplasmic reticulum) did not increase AIRAP mRNA levels (Fig 2A). However, these treatments did induce a relevant stress response as evidenced by the upregulation of Hsp70, p62, metallothionein-II, and/or BiP mRNA levels. AIRAP mRNA induction by arsenite treatment was observed within 2–3 hours and occurred in the presence of the protein synthesis inhibitor cycloheximide (Fig 2B). Thus, the increase in AIRAP mRNA levels appeared to be a primary and proximal event in the response of cells to arsenite exposure.
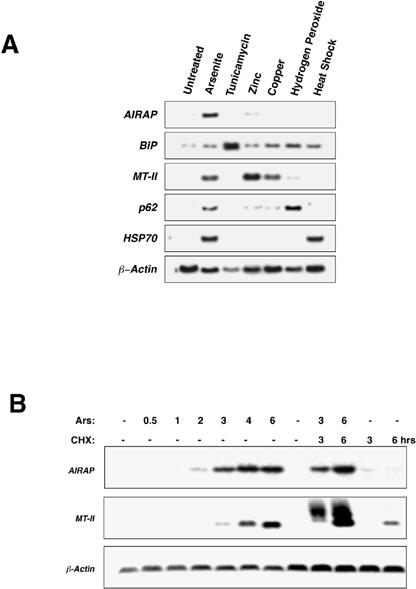
Fig 2. Analysis of AIRAP gene expression in stressed cells. (A) Northern blot analysis of RNA derived from untreated NIH3T3 cells and cells that had been treated with either sodium arsenite (50 μM), tunicamycin (2.5 μg/mL), zinc chloride (100 μM), copper chloride (250 μM), or hydrogen peroxide (100 μM) for 6 hours or for 1 hour of heat shock (44°C). The filter was hybridized sequentially with cDNA probes for the AIRAP, BiP, MT-II (metallothionein-II), p62, HSP70, and β-actin genes (see text for details). (B) Time course of AIRAP and MT-II mRNA induction by arsenite treatment (“Ars” 50 μM) in NIH3T3 cells with and without the protein synthesis inhibitor cyclohexamide (“CHX”, 25 μg/mL, added 30 minutes before arsenite exposure). β-actin was used as a control. (C) Examination of the effect of a brief (1-hour) pretreatment (Tx1) with arsenite (Ars), zinc (Zn), heat shock (HS), or no treatment (UT) on the regulation of AIRAP and MT-II mRNA by later treatment with arsenite (Tx2). A schematic of the time course of the treatment is shown. The intensity of the radioactive signal in each lane is provided. (D) Northern blot analysis of AIRAP and MT-II mRNA levels in untreated, arsenite- treated (Ars, 6 hours), and zinc chloride–treated (Zn, 6 hours) wild- type and mtf-1 mutant mouse embryonic fibroblasts
Treatment with a stress-causing agent activates distinct stress pathways and will attenuate the response of those pathways to a later stress stimulus (Parsell et al 1993; Brostrom and Brostrom 1998). To ascertain further the specificity of the AIRAP induction pathway, we measured the ability of a brief, 1-hour pretreatment with arsenite, zinc, or heat shock to modulate the arsenite-induced expression of AIRAP mRNA 16 hours later. Arsenite pretreatment reduced the induction of AIRAP mRNA by subsequent exposure to arsenite, whereas prior zinc exposure had only a modest effect and prior heat shock no effect (Fig 2C).
As exposure to high concentrations of zinc increased AIRAP mRNA levels, we investigated the involvement of the well-characterized metal-response pathway, mediated by the transcription factor MTF-1 (Gunes et al 1998), in AIRAP induction. We compared AIRAP and metallothionein-II mRNA levels in wild-type and Mtf-1 mutant mouse fibroblasts following treatment with arsenite and zinc. AIRAP mRNA levels were increased by arsenite treatment (and to a lesser extent zinc treatment) in both wild-type and Mtf-1 mutant cells, whereas metallothionein- II mRNA levels were only elevated in wild-type cells (Fig 2D). We conclude that the signaling pathway(s) needed to activate AIRAP gene expression is distinct from the Mtf-1-mediated metal-response pathway and is largely specific for arsenite.
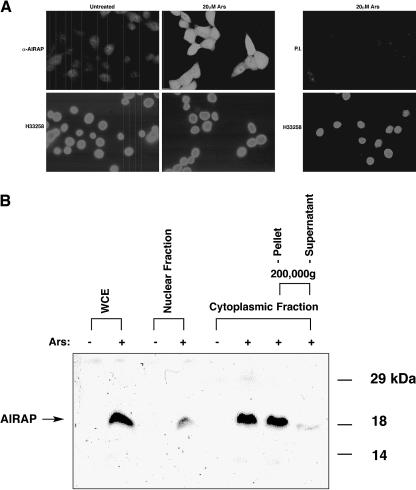
The full-length murine AIRAP cDNA (GenBank accession no. AF224494) was cloned from a cDNA library prepared from arsenite-treated MPTEs. To examine the cellular localization of the AIRAP gene product, we raised antisera to the 171 amino-acid protein encoded by the cDNA. Consistent with the low basal level of AIRAP mRNA, immunostaining of untreated cells with the AIRAP antiserum produced a very low signal, barely distinguishable from background. AIRAP staining was observed in both the nucleus and the cytoplasm following arsenite treatment of NIH3T3 cells (Fig 3A). The nuclear staining was diffusely nucleoplasmic and was occluded from the nucleolus. Localization to discrete subcytoplasmic or nuclear domains was not evident. An identical staining pattern was observed in arsenite-treated hamster CHO and human Hela cells (data not shown).
Fig 3. Detection of the endogenous arsenite-induced AIRAP protein in NIH3T3 cells. (A) Immunofluorescent localization of AIRAP in untreated cells and cells exposed to 20 μM sodium arsenite for 6 hours. Photomicrographs of fixed cells stained with rabbit anti-AIRAP serum (α-AIRAP) or preimmune serum (P.I.) and a secondary fluorescein-labeled goat anti-rabbit serum are shown. The karyophilic dye H33258 labels all the cells. (B) Immunoblot detection of AIRAP. Whole cell extracts (WCE), nuclear fraction, cytoplasmic fraction, and 200 000 × g ultracentrifugation supernatant and pellet from untreated (−) and arsenite-treated (30 μM, 6.5 hours) (+) cells were resolved by 12% SDS-PAGE and immunoblotted with the anti- AIRAP antisera. The arrow indicates the 19-kDa AIRAP protein
AIRAP was undetected by immunoblot in lysates from untreated cells. A strong reactive band of 19 kDa, consistent with the predicted size of the protein, was observed in lysates derived from arsenite-treated cells (Fig 3B). Most of the AIRAP immune-reactivity was recovered in the cytoplasmic fraction of cell lysates, while some reactivity was associated with the nuclear pellet. This nuclear reactivity is consistent with the immunostaining data shown in Figure 3A but may also be due to contamination of the nuclear fraction with cytoplasm. Further fractionation experiments showed that almost all of the cytoplasmic AIRAP was present in an insoluble fraction (Fig 3B). This insoluble AIRAP was resistant to extraction by either 1 M NaCl or 50% formamide yet was readily solubilized by boiling in SDS (data not shown; see also the following discussion). These results suggest that most AIRAP is incorporated into an insoluble complex in the cytoplasm.
As AIRAP was detected in both the nucleus and the cytoplasm and formed insoluble complexes, we examined its association with any polynucleotide species that might be present in both compartments (most likely an RNA). To identify any polynucleotides associated with AIRAP in vivo, arsenite-treated cells were exposed to UV light to cross-link AIRAP to its immediate contingents. AIRAP and any cross-linked molecules were purified from cell lysates by immunoprecipitation. The AIRAP-containing immune complex was treated with T4 polynucleotide kinase and 32P-labeled γ-ATP, disrupted by boiling in SDS, and then resolved by 12% SDS-PAGE. A labeled 19-kDa band, corresponding in size to the AIRAP protein, was present only in lysates derived from UV cross-linked arsenite-treated cells and precipitated by antiserum to AIRAP (Fig 4A). No signal was detected when either the UV cross-linking or the T4 kinase treatment was omitted, arguing that the labeled species was a polynucleotide fragment covalently bound to AIRAP and not the result of AIRAP phosphorylation by a kinase activity contaminating the immunoprecipitation. Furthermore, no signal was detected using antisera to CHOP, a DNA-binding protein that is induced by arsenite treatment (Fawcett et al 1996; Guyton et al 1996).
To determine whether arsenite treatment affected the association of AIRAP with the polynucleotide species, we uncoupled AIRAP protein expression from arsenite treatment. MYC epitope-tagged AIRAP was expressed in 293T cells under the CMV promoter, and the amount of radiolabeled material bound to the MYC-tagged AIRAP, as determined using the cross-linking immunoprecipitation assay, was compared in arsenite-treated and -untreated cells. The MYC-tagged AIRAP signal was detected only in the transfected cells, and its intensity was not affected by arsenite treatment (Fig 4B), suggesting that arsenite treatment does not alter AIRAP's interaction with the polynucleotide. The associated labeled polynucleotide was found to be sensitive to RNase A treatment and resistant to DNase I treatment, suggesting that AIRAP was associated with RNA in vivo (Fig 4C; see “Materials and Methods” for details).
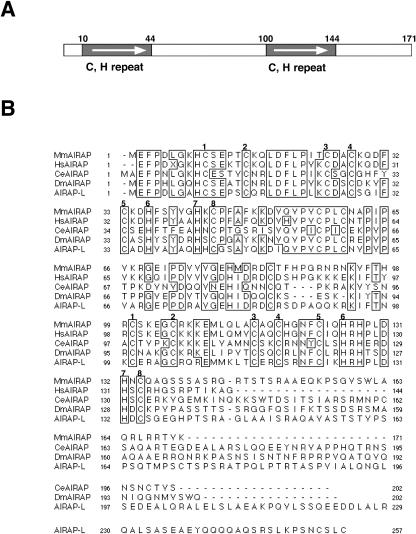
A search of the GenBank databases using the BLAST algorithm (Altschul et al 1990) identified a C elegans gene predicted to encode a similar protein (F58E10.4, accession no. Z81555, 41% identity). A similar search of the EST databases revealed the existence of AIRAP-related proteins in D melanogaster (ESTs AI134276 and AI238366, 49% identity) as well as an additional related mammalian gene (EST AA449622, 61% identity over the conserved 134 N-terminal residues). The mRNA levels of this putative AIRAP-like gene were not affected by arsenite treatment of MPTEs or murine fibroblasts (data not shown). Alignment of the predicted protein sequences of these related genes revealed the presence of a highly conserved 34 residue motif consisting of 8 invariant cysteines and histidines repeated twice in the protein (Fig 5). This arrangement of cysteine and histidine residues is a configuration similar to those known to form a metal coordination complex, in particular the RING-finger type (Saurin et al 1996).
Fig 5. Cross-species conservation of AIRAP amino acid sequences (A) Diagram of the primary structure of the predicted gene product of murine AIRAP. The two 34-residue repeats containing the invariant cysteines and histidines are indicated (“C, H repeat”). (B) Alignment (Higgins and Sharp 1988)of AIRAP sequences from Mus musculus (Mm), Homo sapiens (Hs, AI659863), C elegans (Ce, Z81555), Drosophila melanogaster (Dm, AI134276) and an AIRAP- like human gene (AIRAP-L, AA449622). Sequences for human and fly genes are from ESTs, while sequences for mouse and C elegans are from full-length cDNAs and genomic clones. The 8 invariant cysteine and histidine residues that form the internal repeat in the protein are indicated by numbers above the alignment. Residues identical in at least 4 of the 5 protein sequences are boxed
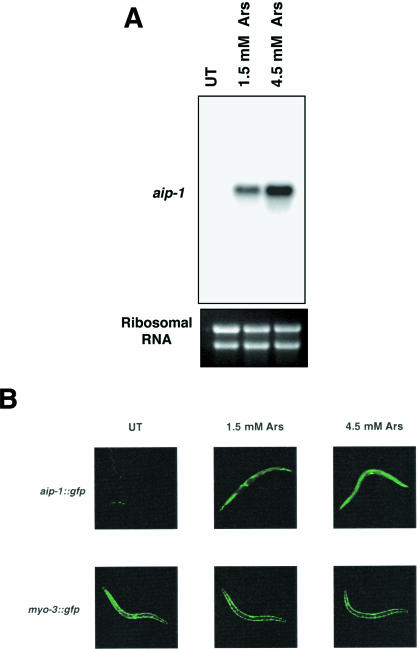
Given the high level of sequence similarity between the mouse AIRAP and the C elegans AIRAP-related gene, we investigated the possible conservation of function between these 2 genes. We first cloned the cDNA for the C elegans AIRAP homologue by RT-PCR from arsenite-treated animals. Northern blot analysis of RNA obtained from adult C elegans revealed that the levels of the AIRAP homologue mRNA are also increased by arsenite treatment, suggesting that the protein levels might be similarly elevated (Fig 6A). The C elegans gene was thus named aip-1 (arsenite-inducible protein-1).
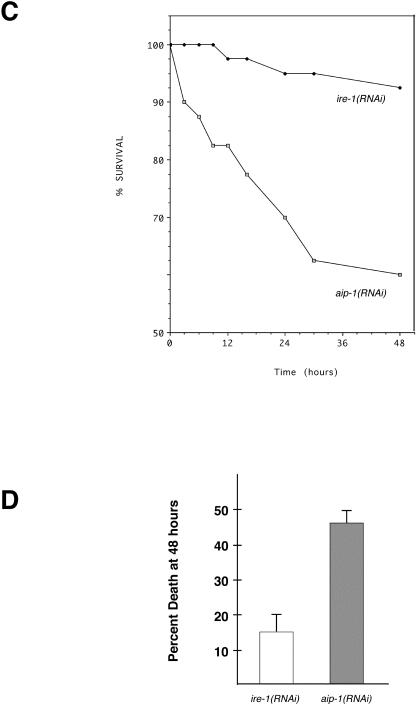
Fig 6. aip-1, a C elegans homologue of AIRAP, is induced by arsenite exposure and protects animals from arsenite toxicity. (A) Northern blot analysis of RNA recovered from untreated (UT) animals and animals treated with arsenite (Ars) for 4.5 hours in liquid media and probed with the aip-1 cDNA. Lower panel shows Ethidium Bromide staining of ribosomal RNAs, indicating equivalent quantities of RNA in all lanes. (B) aip-1::gfp expression is induced by arsenite treatment. aip-1::gfp animals were raised overnight on NGM plates without (UT) or with sodium arsenite (Ars). Arsenite treatment had no effect on the GFP expression pattern or intensity in the control myo-3::gfp strain. (C) Comparison of the survival over time in a cohort of 30 aip-1(RNAi) and control ire-1(RNAi) animals exposed to 3 mM sodium arsenite. (D) Comparison of survival of aip-1(RNAi) and control ire-1(RNAi) animals exposed to 3 mM arsenite for 48 hours. Shown is the mean (±SEM) survival in 3 experiments involving 30 animals in each experimental arm
To examine the expression pattern of aip-1, we generated a strain containing an aip-1::gfp transgene consisting of the aip-1 promoter fragment fused to the green fluorescent protein (GFP) coding sequences. When raised on standard media in the absence of arsenite (NGM), aip-1:: gfp animals exhibited a variable and low level of GFP expression that was confined primarily to the head and tail hypodermal (epidermal) regions. GFP expression was dramatically increased in aip-1::gfp animals within 4 hours following arsenite exposure and was most prominent in all hypodermal cells and to a lesser extent in intestinal cells (Fig 6B). By contrast, GFP expression was unaffected by arsenite treatment in myo-3::gfp animals, which express high levels of GFP in the muscle nuclei (Fire et al 1998), indicating that arsenite exposure does not have nonspecific effects on transgene expression or GFP intensity. Because the aip-1::gfp reporter construct contains only the first 5 amino acids of AIP-1, the regulation of aip-1 induction is likely transcriptional rather than posttranscriptional.
We used RNA-mediated interference (RNAi) methods (Fire et al 1998) to investigate the role of aip-1 in arsenite resistance in C elegans. myo-3::gfp animals were injected with a mixture of in vitro synthesized double-stranded (ds) aip-1 and gfp RNA or a control mixture of ire-1 and gfp dsRNA (ire-1 plays a role in stress signaling from the endoplasmic reticulum but is not active during arsenite toxicity). The F1 offspring of animals injected with either aip-1 and gfp or ire-1 and gfp dsRNA that lost GFP expression in body-wall muscle nuclei (indicating the inactivation of the myo-3::gfp GFP expression and presumably also aip-1 or ire-1 function by RNAi) were selected for further study (typically, over 90% of the progeny of injected animals lost the GFP signal).
Under standard growth conditions, the aip-1(RNAi) and ire-1(RNAi) animals appeared normal, suggesting that these genes are not required for viability. However, when transferred to agar plates containing 3 mM arsenite, aip- 1(RNAi) animals exhibited a much higher mortality rate than that of ire-1(RNAi) animals (Fig 6C,D) or that of wild-type and gfp(RNAi) animals (data not shown). The toxic effects of arsenite exposure were quantified by determining the number of dead animals 48 hours after transfer to the arsenite-containing plates. The average mortality of the ire-1(RNAi) “control” animals was 15 ± 5% (mean ± SEM, n = 90, 3 experiments), whereas the mortality of the aip-1(RNAi) animals averaged 46.7 ± 5.8% (mean ± SEM, n = 90, 3 experiments). The probability of these differences being due to chance are less than 0.0022 (2-tailed t-test). These results support a role for aip-1 in protecting against the toxic effects of arsenite.
DISCUSSION
AIRAP is unique among known arsenite-induced genes based on the regulation of its expression and the potential activities of its gene product. Neither the heat shock response pathway, presumed to be triggered by malfolded proteins in the cytoplasmic compartment, nor the metal- responsive pathway mediated by MTF-1 regulates AIRAP expression. Although arsenite is an oxidant and a transition metal, AIRAP expression is not upregulated in response to other oxidants and is only modestly induced by exposure to other metals, such as zinc. Both arsenite and endoplasmic reticulum stress caused by tunicamycin treatment lead to the phosphorylation of eIF2α on serine 51 (Brostrom and Brostrom 1998). Both agents activate a common target gene, CHOP, whose induction depends on the activity of eIF2α kinases (our unpublished observations). However, tunicamycin treatment has no effect on AIRAP expression (Fig 2A), arguing that signaling pathways activated by phosphorylated eIF2α alone are not sufficient to trigger AIRAP expression. Furthermore, while stress-activated MAP kinases (JNKs, p38-MAP kinases) are known to be activated by arsenite exposure (Cavigelli et al 1996; Liu et al 1996), other noxious stimuli that activate the JNK pathway, such as hydrogen peroxide and tunicamycin, fail to induce AIRAP expression. Together, these observations suggest that AIRAP gene expression is induced either by the simultaneous, combinatorial activation of several signaling pathways triggered by arsenite exposure or by a novel arsenite-responsive signaling pathway.
AIRAP was detected in both the nucleus and cytoplasm. The presence of a conserved arrangement of cysteine and histidine residues, spaced in a manner consistent with the ability to form a metal-coordination complex, and the ability of AIRAP to be cross-linked to RNA in vivo suggest that it functions in association with RNA. The AIRAP nuclear staining was not speckled in appearance, nor was AIRAP detected in the nucleolus, indicating that AIRAP is not likely associated with a ribosomal or splicosomal RNA. Although the RNA species associated with AIRAP is unknown, these data are most consistent with its being an mRNA or a pre-mRNA.
The cysteine- and histidine-rich sequences present in AIRAP are reminiscent of the zinc-coordinating RING- finger motif, although the arrangement of residues does not conform to the strict RING or RING-H2 consensus (Saurin et al 1996). RING-H2 motifs have been found in proteins that serve as ubiquitin ligases, suggesting a link between this special domain and the regulation of protein degradation (Lorick et al 1999; Tyers and Jorgensen 2000). Exposure to arsenite promotes a heat shock response that is also associated with activation of ubiquitin-mediated proteolysis (Bond et al 1988; Mathew and Morimoto 1998). The presence of RING-finger-like domains in AIRAP conjures the possibility that it might participate in ubiquitin-mediated protein degradation. It has recently been suggested that the ubiquitin-proteosome machine may also play a role in degradation of certain mRNAs (Laroia et al 1999). Thus, the ability of AIRAP to associate with RNA and its hypothesized role as a component of the ubiquitin-proteosome pathway might be linked mechanistically.
Our data indicate that AIRAP mRNA and protein levels are both raised in response to arsenite treatment, suggesting that AIRAP gene activity is regulated predominantly by the level of the mRNA. Analysis of aip-1::gfp transgenic nematodes indicates that induction of aip-1 likely occurs at the transcriptional level, and experiments performed with the murine AIRAP promoter point to similar regulation of the mammalian gene (data not shown). Although arsenite exposure might also directly or indirectly lead to posttranslational modifications to AIRAP, we have not detected any. For example, the many cysteine residues in AIRAP are potential targets of arsenite modification, and such alterations might influence its RNA-binding properties. We found that arsenite treatment did not change the amount of RNA associated with AIRAP when expressed under a heterologous promoter in 293T cells (Fig 4), nor did arsenite treatment alter the reactivity of the AIRAP cysteines with the thiol-reactive agent 4-acetamido-4′-maleimidylstilbene-2-2′′-disulphonic acid (AMS, data not shown). Furthermore, AIRAP derived from arsenite-treated and -untreated cells had the same apparent size by SDS-PAGE, suggesting that no significant covalent modification or processing of AIRAP occurs in the presence of arsenite.
The high level of sequence similarity between the mouse AIRAP and C elegans AIP-1 and the fact that both are upregulated in response to arsenite exposure strongly suggest that these proteins share a conserved function. Furthermore, heat shock, tunicamycin, and H2O2 treatment failed to induce AIRAP or aip-1 (Fig 2A; data not shown), indicating that the specificity of the arsenite-response pathway is similarly conserved. Given the RNAi evidence that aip-1 is needed to promote survival of C elegans exposed to arsenite, AIRAP likely also protects mammalian cells from the toxic effects of arsenite.
Fig. 2. Continued
Fig 6. Continued
Acknowledgments
We thank Matthieu Schapira and Jon Grossman for useful discussions; Yuji Kohara for the gift of the C elegans ire-1 EST, yk8e9a; Andy Fire for the pPD95.75 plasmid; and the Caenorhabditis Genetics Center for some of the nematode strains used in this study. Supported by NIEHS grant ES08681. J.S. and M.C. are partially supported by an MSTP training grant to the New York University School of Medicine. D.R. is a Stephen Birnbaum Scholar of the Leukemia Society of America.
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- Bond U, Agell N, Haas AL, Redman K, Schlesinger MJ. Ubiquitin in stressed chicken embryo fibroblasts. J Biol Chem. 1988;263:2384–2388. [PubMed] [Google Scholar]
- Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- Clark SG, Lu X, Horvitz HR. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics. 1994;137:987–997. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbirt KK, Bonkovsky HL. Heme oxygenase: recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438–447. [PubMed] [Google Scholar]
- Elliget KA, Trump BF. Primary cultures of normal rat kidney proximal tubule epithelial cells for studies of renal cell injury. In Vitro Cell Dev Biol. 1991;27A:739–748. doi: 10.1007/BF02633220. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Eastman HB, Martindale JL, Holbrook NJ. Physical and functional association between GADD153 and CCAAT/ enhancer-binding protein beta during cellular stress. J Biol Chem. 1996;271:14285–14289. doi: 10.1074/jbc.271.24.14285. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in. Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Gunes C, Heuchel R, Georgiev O, et al. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 1998;17:2846–2854. doi: 10.1093/emboj/17.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton K, Xu Q, Holbrook N. Induction of the mammalian stress response gene GADD153 by oxidative stress: role of the AP-1 element. Biochem J. 1996;314:547–554. doi: 10.1042/bj3140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DG, Sharp PM. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237– 244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hubank M, Schatz D. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 1994;22:5640–5648. doi: 10.1093/nar/22.25.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Yanagawa T, Kawane T, Yuki K, Seita J, Yoshida H, Bannai S. Murine peritoneal macrophages induce a novel 60-kDa protein with structural similarity to a tyrosine kinase p56lck- associated protein in response to oxidative stress. Biochem Biophys Res Commun. 1996;226:456–460. doi: 10.1006/bbrc.1996.1377. [DOI] [PubMed] [Google Scholar]
- Joung I, Strominger JL, Shin J. Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain. Proc Natl Acad Sci USA. 1996;93:5991–5995. doi: 10.1073/pnas.93.12.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi T, Negishi M, Ichikawa A. Activation of heat shock transcription factors by delta 12-prostaglandin J2 and its inhibition by intracellular glutathione. Biochem Pharmacol. 1993;45:2457– 2464. doi: 10.1016/0006-2952(93)90227-n. [DOI] [PubMed] [Google Scholar]
- Kreppel H, Bauman J, Liu J, McKim J, Klaassen C. Induction of metallothionein by arsenical in mice. Fundam Appl Toxicol. 1993;20:184–189. doi: 10.1006/faat.1993.1025. [DOI] [PubMed] [Google Scholar]
- Laroia G, Cuesta R, Brewer G, Schneider RJ. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science. 1999;284:499–502. doi: 10.1126/science.284.5413.499. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Curetty L, Corry PM. Differences in preferential synthesis and redistribution of HSP70 and HSP28 families by heat or sodium arsenite in Chinese hamster ovary cells. J Cell Physiol. 1991;149:77–87. doi: 10.1002/jcp.1041490111. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guyton KZ, Gorospe M, Xu Q, Lee JC, Holbrook NJ. Differential activation of ERK, JNK/SAPK and P38/CSBP/RK map kinase family members during the cellular response to arsenite. Free Radic Biol Med. 1996;21:771–781. doi: 10.1016/0891-5849(96)00176-1. [DOI] [PubMed] [Google Scholar]
- Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A, Morimoto RI. Role of the heat-shock response in the life and death of proteins. Ann NY Acad Sci. 1998;851:99–111. doi: 10.1111/j.1749-6632.1998.tb08982.x. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Duchaine J, Massie B. The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol Cell Biol. 1993;13:5427–5438. doi: 10.1128/mcb.13.9.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi T. Arsenic compound-induced increases in glutathione levels in cultured Chinese hamster V79 cells and mechanisms associated with changes in gamma-glutamylcysteine synthetase activity, cysteine uptake and utilization of cysteine. Arch Toxicol. 1997;71:730–740. doi: 10.1007/s002040050454. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci USA. 1998;95:8428–8430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Taulien J, Lindquist S. The role of heat-shock proteins in thermotolerance. Philos Trans R Soc Lond B Biol Sci. 1993;339:279–285. doi: 10.1098/rstb.1993.0026. [DOI] [PubMed] [Google Scholar]
- Saurin AJ, Borden KL, Boddy MN, Freemont PS. Does this have a familiar RING? Trends Biochem Sci. 1996;21:208–214. [PubMed] [Google Scholar]
- Shin J. P62 and the sequestosome, a novel mechanism for protein metabolism. Arch Pharm Res. 1998;21:629–633. doi: 10.1007/BF02976748. [DOI] [PubMed] [Google Scholar]
- Snow E. Metal carcinogenesis: mechanistic implications. Pharmacol Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Thompson DJ. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993;88:89–114. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- Tyers M, Jorgensen P. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr Opin Genet Dev. 2000;10:54–64. doi: 10.1016/s0959-437x(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Yandell MD, Edgar LG, Wood WB. Trimethylpsoralen induces small deletion mutations in. Caenorhabditis elegans. Proc Natl Acad Sci USA. 1994;91:1381–1385. doi: 10.1073/pnas.91.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Sok J, Immanuel D, Yin Y, Ron D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci. 1997;110:1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]