Abstract
We previously showed that the aggregated form of Hsp27 in cultured cells becomes dissociated as a result of phosphorylation with various types of stress. In order to clarify the signal transduction cascade involved, the effects of various inhibitors of protein kinases and dithiothreitol on the dissociation of Hsp27 were here examined by means of an immunoassay after fractionation of cell extracts by sucrose density gradient centrifugation. The dissociation of Hsp27 induced by exposure of U251 MG human glioma cells to metals (NaAsO2 and CdCl2), hypertonic stress (sorbitol and NaCl), or anisomycin, an activator of p38 mitogen–activated protein (MAP) kinase, was completely suppressed by the presence of SB 203580 or PD 169316, inhibitors of p38 MAP kinase, but not by PD 98059 and Uo 126, inhibitors of MAP kinase kinase (MEK), nor by staurosporine, Go 6983, and bisindolylmaleimide I, inhibitors of protein kinase C. Phorbol ester (PMA)–induced dissociation of Hsp27 was completely suppressed by staurosporine, Go 6983, or bisindolylmaleimide I and partially suppressed by SB 203580, or PD 169316 but not by PD 98059 or Uo 126, indicating mediation by 2 cascades. The presence of 1 mM dithiothreitol in the culture medium during exposure to chemicals suppressed the dissociation of Hsp27 induced by arsenite and CdCl2 but not by other chemicals. These results suggest that the phosphorylation of Hsp27 is catalyzed by 2 protein kinases, p38 MAP kinase–activated protein (MAPKAP) kinase- 2/3 and protein kinase C. In addition, metal-induced signals are sensitive to reducing power.
INTRODUCTION
Mammalian Hsp27 is a member of the α-crystallin small Hsp family and is a stress-inducible protein like αB-crystallin. Hsp27 is known to be phosphorylated at 2 (rodent) or 3 (human) serine residues in response to various types of stress (Landry et al 1991, 1992), this being catalyzed by MAP kinase–activated protein (KAP) kinase-2/-3, which itself can be phosphorylated and activated by p38 MAP kinase (Freshney et al 1994; Lee et al 1994). The delta isoform of protein kinase C (PKC-δ) also phosphorylates the same serine residues in Hsp27 (Maizels et al 1998), but the physiological significance of this has not been well elucidated.
Mammalian Hsp27 in cells is present in 2 forms: an aggregated large form with a molecular mass of about 500 kDa and a dissociated form of <100 kDa (Arrigo and Welch 1987). The aggregated form in muscles is a heteropolymer composed at least of Hsp27, αB-crystallin, and p20 (Kato et al 1992, 1994a, 1994b), these coprecipitating with antibodies to each protein and being copurified in the same fraction until separated in the presence of 7 M urea (Kato et al 1992, 1994a, 1994b). We reported previously that the aggregated form of Hsp27 is dissociated as a result of phosphorylation induced by various stressful conditions with concomitant loss of protection against heat stress (Kato et al 1994b). Recently, Rogalla et al (1999) confirmed our findings by expressing a mutant Hsp27 in which the serine phosphorylation sites were replaced by aspartic acid residues.
In order to clarify the signal transduction cascade for the phosphorylation of Hsp27, we have examined the effects of various inhibitors of protein kinases and dithiothreitol on its dissociation.
MATERIALS AND METHODS
Reagents
Phenylmethylsulfonyl fluoride, anisomycin, and trypsin inhibitor were obtained from Sigma Chemical Co (Tokyo, Japan); SB 203580, PD 169316, PD 98059, Go 6983, and bisindolylmaleimide I from Calbiochem-Novabiochem (La Jolla, CA, USA); Uo 126 from Promega (Madison, WI, USA); staurosporine, phorbol 12-myristate 13-acetate (PMA), okadaic acid, and calyculin A from Wako Pure Chemicals (Osaka, Japan); and Pefabloc SC from Boehringer Mannheim GmbH (Mannheim, Germany). Pepstatin A was obtained from Peptide Institute Inc (Osaka, Japan).
Culture and treatment of cells
U251 MG human glioma cells were grown at 37°C in Eagle's minimum essential medium (Nissui Pharmaceutical Co, Tokyo, Japan), supplemented with 10% fetal bovine serum (Equitech-Bio Inc, Ingram, TX, USA) in a humidified atmosphere of 95% air, 5% CO2. Cells were seeded on 60-mm dishes, and the medium was changed every 2 or 3 days. The cells at confluence were exposed to various chemicals, including 200 μM NaAsO2, 200 μM CdCl2, 0.15 M NaCl, 0.4 M sorbitol, 4 mM H2O2, 10 μg/mL anisomycin, and 1 μM PMA in the presence or absence of protein kinase inhibitors. After incubation at 37°C for 90 minutes in a CO2 incubator, cells in each dish were washed twice with 5 mL of phosphate-buffered saline (PBS, containing 8 g of NaCl, 0.2 g of KCl, 1.15 g of Na2HPO4, and 0.2 g of KH2PO4 in 1000 mL of H2O) and frozen at −80°C for a few days. The frozen cells on each dish were collected and suspended in 0.3 mL of 0.1 M Hepes-NaOH buffer, pH 7.5, containing 0.1 M NaF, 100 nM okadaic acid, 100 nM calyculin A, 0.3 mg/mL Pefabloc SC, 0.1 mg/mL trypsin inhibitor, 250 μg/mL Pepstatin A, and 1 mM phenylmethylsulfonyl fluoride. Each suspension was sonicated for 10 seconds and centrifuged at 125 000 × g for 20 minutes at 4°C. The supernatants were immediately subjected to centrifugation on sucrose density gradients.
Sucrose density gradient centrifugation of extracts
Each cell extract (0.2-mL aliquots) was layered on a 3.5- mL linear gradient of sucrose (10–40%) in 50 mM Tris- HCl, pH 7.5, containing 0.1 M NaF and 5 mM EDTA, and centrifuged at 4°C at 130 000 × g for 16 hours in a swinging bucket rotor (RPS56T; Hitachi, Tokyo, Japan). After centrifugation, each sample was fractionated into 15 test tubes, unless otherwise specified, into which 0.25 mL of 10 mM sodium phosphate buffer, pH 7.0, containing 0.1% (w/v) bovine serum albumin had been introduced.
Immunoassay and Western blot analysis of Hsp27
Concentrations of Hsp27 were estimated by a specific immunoassay as described previously (Kato et al 1992). Each fraction, collected without 10 mM phosphate buffer containing bovine serum albumin, was also subjected to SDS-PAGE (12.5% polyacrylamide) and subsequent Western blot analysis with antibodies against Hsp27, as described previously (Kato et al 1992).
Other methods
Concentrations of protein in extracts of cells were estimated with a protein assay kit (Bio-Rad) with bovine serum albumin as the standard. Human Hsp27 was purified from skeletal muscle, and affinity purified rabbit antibodies against human Hsp27 were prepared as detailed earlier (Kato et al 1992).
RESULTS
Effects of protein kinase inhibitors on dissociation of Hsp27 induced by various chemicals
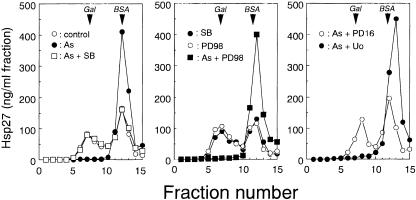
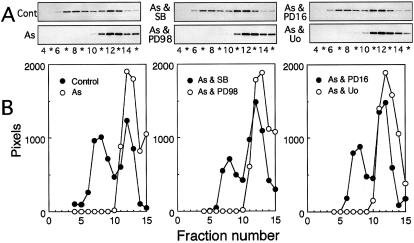
Extracts of confluent cultures of U251 MG human glioma cells contained about the same amounts of the aggregated large form and the dissociated small form of Hsp27, detected by the specific immunoassay of each fraction separated by sucrose density gradient centrifugation (Fig 1). Cells exposed at 37°C for 90 minutes to 200 μM sodium arsenite, 200 μM CdCl2, 10 μg/mL anisomycin, 0.15 M NaCl, 0.4 M sorbitol, 4 mM H2O2, or 0.1 μM PMA contained Hsp27 mostly in the dissociated form (Figs 1–3). When cells were exposed to the chemicals together with the inhibitor of p38 MAP kinase, SB 203580 (Lee et al 1994), or PD 169316 (Kummer et al 1997), the dissociation of Hsp27 inducible by arsenite, CdCl2, anisomycin, NaCl (not shown), sorbitol, or H2O2 was completely suppressed, and the sedimentation profiles of Hsp27 were almost identical to those obtained with untreated cells (Figs 1 and 2). However, the dissociation of Hsp27 induced by arsenite (Fig 1) and other chemicals described previously was barely suppressed by PD 98059 (Dudley et al 1995) or Uo 126 (Favata et al 1998), inhibitors of MAP kinase kinase (data not shown).
Fig 1. Arsenite-induced dissociation of Hsp27 is suppressed by inhibitors of p38 MAP kinase but not MAP kinase kinase (MEK). U251 MG cells were exposed to 200 μM sodium arsenite (As) at 37°C for 90 minutes with or without 10 μM each of SB 203580 (SB), PD 169316 (PD16), PD 98059 (PD98), or Uo 126 (Uo). The cells were also exposed to each inhibitor alone under the same conditions. Each cell extract was fractionated by centrifugation on a sucrose density gradient (10–40%). The specimen in each tube was withdrawn from the bottom as 15 fractions, and 10-μL aliquots of each fraction were subjected in duplicate to the specific immunoassay described in “Materials and Methods.” Arrows indicate the positions at which β-d-galactosidase from Escherichia coli (Gal 540 kDa) and bovine serum albumin (BSA, 67 kDa) sedimented. Control, untreated cells. The data are representative of 3 separate experiments
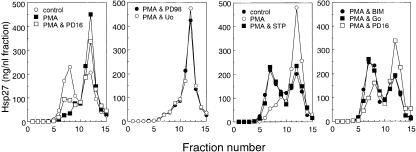
Fig 3. Phorbol 12-myristate 13-acetate (PMA)–induced dissociation of Hsp27 is suppressed partially by an inhibitor of p38 MAP kinase and completely by inhibitors of protein kinase C. Cells were exposed to 1 μM PMA at 37°C for 90 minutes with or without 10 μM PD 169316 (PD16), 10 μM PD 98059 (PD98), 10 μM Uo 126 (Uo), 100 nM staurosporine (STP), 5 μM bisindolylmaleimide I (BIM), or 5 μM Go 6983 (Go). Each cell extract was subjected to sucrose density gradient centrifugation, and the concentrations of Hsp27 were determined as described in the caption to Figure 1. Control, untreated cells. The data are representative of 2 separate experiments
Fig 2.
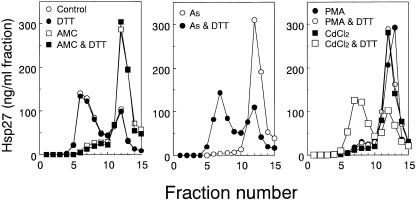
Dissociation of Hsp27 induced by sorbitol, CdCl2, anisomycin, or H2O2 is suppressed by PD 169316, an inhibitor of p38 MAP kinase. Cells were exposed to 0.4 M sorbitol, 200 μM CdCl2, 10 μg/ mL anisomycin (AMC), or 4 mM H2O2 at 37°C for 90 minutes with or without 10 μM PD 169316 (PD16). Each cell extract was fractionated by centrifugation, and the concentrations of Hsp27 were determined as described in the caption to Figure 1. Control, untreated cells. The data are representative of 2 separate experiments
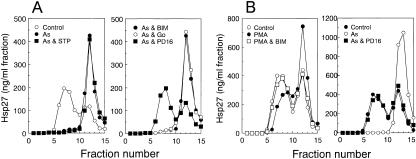
PMA-induced dissociation of Hsp27 was also only partially inhibited by SB 203580 (data not shown) or PD 169316 (Fig 3) and was not affected by PD 98059 or Uo 126 (Fig 3). However, it was completely suppressed in the presence of staurosporine (Tamaoki et al 1986), Go 6983 (Gschwendt et al 1996), or bisindolylmaleimide I (Toullec et al 1991), inhibitors of protein kinase C (Fig 3), which did not inhibit the dissociation of Hsp27 induced by arsenite under conditions in which PD 169316 exerted effective suppression (Fig 4). The exposure of cells to each inhibitor alone barely affected the sedimentation profile of Hsp27 (data not shown). Suppression of arsenite-induced dissociation of Hsp27 by inhibitors of p38 MAP kinase and of PMA-induced dissociation by inhibitors for protein kinase C was also observed in HeLa cells (Fig 4). Western blot analyses of each fraction after sucrose density gradient centrifugation confirmed the immunoassay results (Fig 5).
Fig 4.
(A) Inhibitors of protein kinase C do not suppress the arsenite-induced dissociation of Hsp27. U251 MG cells were exposed to 200 μM sodium arsenite at 37°C for 90 minutes with or without 100 nM staurosporine, 5 μM bisindolylmaleimide I (BIM), 5 μM Go 6983 (Go), or 10 μM PD 169316 (PD16). (B) Effects of protein kinase inhibitors on the dissociation of Hsp27 in HeLa cells. HeLa cells were exposed at 37°C for 90 minutes to 1 μM PMA with or without 5 μM bisindolylmaleimide I (BIM) or to 200 μM sodium arsenite (As) with or without 10 μM PD 169316 (PD16). Each cell extract was subjected to sucrose density gradient centrifugation, and the concentrations of Hsp27 were determined as described in the caption to Figure 1. Control, untreated cells. The data are representative of 2 separate experiments
Fig 5. Western blot analysis of Hsp27 in fractions separated by centrifugation. U251 MG cells were exposed to sodium arsenite with or without protein kinase inhibitors, and extracts were fractionated by centrifugation on a sucrose density gradient as described in the caption to Figure 1. The 7.5-μL aliquots of each fraction were subjected to SDS-PAGE and subsequent Western blot analysis with antibodies against human Hsp27 and peroxidase-labeled antibodies against rabbit IgG as second antibodies as described in “Materials and Methods.” (A) Peroxidase activity on nitrocellulose sheets was visualized on X-ray film. (B) The relative density of each band in each treatment shown in (A) was estimated by the NIH Image program. Control, untreated control cells. The data are representative of 2 separate experiments
These results suggest that the signal transduction pathway for phosphorylation of Hsp27 induced by most chemicals, except PMA, is mediated by p38 MAP kinase, while PMA-induced dissociation of Hsp27 is mainly due to phosphorylation by protein kinase C.
Effects of dithiothreitol on the dissociation of Hsp27
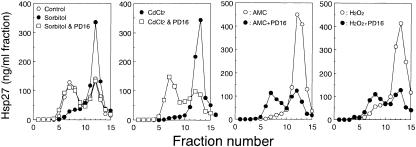
We previously observed arsenite-induced expression of Hsp27 to be suppressed by a high concentration (>1 mM) of dithiothreitol (Kato et al 1997). Phosphorylation of αB- crystallin in response to arsenite was also decreased in the presence of 2 mM dithiothreitol (Ito et al 1997). To test the effects of the reducing agent on dissociation of Hsp27, cells were exposed to various chemicals with or without 2 mM dithiothreitol, and extracts were subjected to sucrose density gradient centrifugation. As shown in Figure 6, dithiothreitol suppressed completely the dissociation of Hsp27 induced by arsenite and CdCl2 but not that due to PMA, anisomycin (Fig 6), Sorbitol, NaCl, or H2O2 (data not shown). Exposure of cells to dithiothreitol alone did not affect the Hsp27 sedimentation profile (Fig 6). These results suggest that the phosphorylation of Hsp27 induced by arsenite or CdCl2 is mediated by a process that is sensitive to reducing power.
Fig 6. Arsenite- or CdCl2-induced dissociation of Hsp27 is completely suppressed in the presence of dithiothreitol. U251 MG cells were exposed at 37°C for 90 minutes to 10 μg/mL anisomycin (AMC), 200 μM sodium arsenite (As), 200 μM CdCl2, or 1 μM PMA with or without 1 mM dithiothreitol (DTT). Each cell extract was fractionated by centrifugation on a sucrose density gradient, and the concentrations of Hsp27 were estimated by the immunoassay as described in the caption to Figure 1. Control, untreated cells. The data are representative of 2 separate experiments
DISCUSSION
Phosphorylation of serine residues is a common posttranslational modification of α-crystallin small Hsps, Hsp27, and αB-crystallin in cells being phosphorylated in response to various types of stress (Arrigo 1990; Landry et al 1992; Ito et al 1997). αA-crystallin in lenses is in part phosphorylated (Chiesa et al 1988), but it is not known whether this is stress related. Phosphorylation of Hsp20 in vascular smooth muscle is observed during cyclic nucleotide-dependent vasodilation induced by protein kinase A (Beal et al 1997).
Phosphorylation of αB-crystallin at 3 serine residues is known to be catalyzed by 3 different protein kinases. Ser- 45 and Ser-59 seem to be phosphorylated by p44/42 MAP kinase and MAPKAP kinase-2, respectively (Kato et al 1998), whereas the enzyme acting on Ser-19 has not been identified. Phosphorylation of the 2 (rodent Hsp25) or 3 (human Hsp27) serine residues in Hsp27 is reported to be catalyzed by MAPKAP kinase-2 (Stokoe et al 1992), MAPKAP kinase-3 (McLaughlin et al 1996), and the δ isoform of protein kinase C (Maizels et al 1998). Selectivity of phosphorylation sites in Hsp27 or Hsp25 for these 3 enzymes has been reported to be apparently absent (Stokoe et al 1992; McLaughlin et al 1996; Maizels et al 1998).
The present results indicate that dissociation due to phosphorylation of Hsp27 is sensitive to protein kinase inhibition, depending on the inducing agent. The dissociation of Hsp27 in U251 MG cells induced by most chemicals, except for PMA, was completely suppressed when SB 203580 or PD 169316, inhibitors of p38 MAP kinase, was present during exposure. In contrast, the dissociation of Hsp27 induced by PMA was completely suppressed in the presence of Go 6983, bisindolylmaleimide I, or staurosporine, known to be inhibitors of protein kinase C. These inhibitors of protein kinase C did not influence the dissociation induced by other chemicals, and inhibitors of MAP kinase kinase were without effect in either case. These results indicate that 2 signal transduction cascades are responsible for phosphorylation of Hsp27: the p38 MAP kinase cascade, probably catalyzed by MAPKAP kinase-2/3, and that involving protein kinase C, as indicated by Maizels et al (1998). Since inhibitors of p38 MAP kinase also partially suppressed the dissociation of Hsp27 induced by PMA, there is clearly some cross talk in this case.
Interestingly, exposure of cells to the chemicals used in this study also induces phosphorylation of αB-crystallin, another member of the α-crystallin small Hsp family, and most, except for PMA, enhance the phosphorylation of its Ser-59 selectively effected by MAPKAP kinase-2 in vitro, by a process sensitive to inhibition of p38 MAP kinase in vivo (Ito et al 1997; Kato et al 1998). These observations indicate that Hsp27 and αB-crystallin can be phosphorylated by the same protein kinase, MAPKAP kinase-2/- 3. In contrast, PMA-induced phosphorylation of Ser-45 in αB-crystallin, known to be targeted by p44/42 MAP kinase, was found to be suppressed by staurosporine (Ito et al 1997), Go 6983, and bisindoylmaleimide I (unpublished data) as described previously for the Hsp27-PMA case. However, inhibition of αB-crystallin phosphorylation was also apparent in the presence of PD 98059, an inhibitor of MAP kinase kinase in vivo (Ito et al 1997; Kato et al 1998). These results indicate that PMA-induced phosphorylation of serine residues in Hsp27 and Ser-45 in αB-crystallin is catalyzed by the different protein kinases through different signaling cascades.
Conversion of nonphosphorylated and aggregated form of Hsp27 to the phosphorylated and dissociated form results in decreased tolerance of heat stress (Kato et al 1994b). One nonphosphorylatable mutant of Hsp27 expressed in cells formed a large polymer, as did wild-type Hsp27, and was similarly protective against stress, and cells blocked phosphorylation of Hsp27 with SB 203580 did not abolish the protective activity of Hsp27 against tumor necrosis factor α cytotoxicity (Mehlen et al 1997; Preville et al 1998). However, a mutant Hsp27, in which the phosphorylatable serine residues were replaced by aspartic acid, did not form polymers and showed no protective effects (Rogalla et al 1999). These results suggest that the cytoprotective activity of Hsp27 is associated with the large aggregate and that phosphorylation of serine residues in Hsp27 is not required for the protective function of Hsp27 against cell insult. However, the physiological significance of phosphorylation and dissociation of Hsp27 in response to stress remains to be clarified.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan for scientific research on priority areas.
REFERENCES
- Arrigo A-P. Tumor necrosis factor induces the rapid phosphorylation of the mammalian heat shock protein Hsp28. Mol Cell Biol. 1990;10:1276–1280. doi: 10.1128/mcb.10.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A-P, Welch WJ. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987;262:15359–15369. [PubMed] [Google Scholar]
- Beal AC, Kato K, Goldenring JR, Rasmussen H, Brophy CM. Cyclic nucleotide-dependent vasorelaxation is associated with the phosphorylation of a small heat shock-related protein. J Biol Chem. 1997;272:11283–11287. doi: 10.1074/jbc.272.17.11283. [DOI] [PubMed] [Google Scholar]
- Chiesa R, Gawinowicz-Kolks MA, Kleiman NJ, Spector A. Definition and comparison of the phosphorylation sites of the A and B chains of bovine α-crystallin. Exp Eye Res. 1988;46:199–208. doi: 10.1016/s0014-4835(88)80077-0. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors: differentiation from protein kinase C isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of αB-crystallin in response to various types of stress. J Biol Chem. 1997;272:29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T. Purification and characterization of a 20-kDa protein that is highly homologous to αB-crystallin. J Biol Chem. 1994a;269:15302– 15309. [PubMed] [Google Scholar]
- Kato K, Hasegawa K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, Hsp27. J Biol Chem. 1994b;269:11274–11278. [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. Phosphorylation of αB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 1998;273:28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Okamoto K. Modulation of the arsenite-induced expression of stress proteins by reducing agents. Cell Stress Chaperones. 1997;2:199–209. doi: 10.1379/1466-1268(1997)002<0199:motaie>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Shinohara H, Goto S, Inaguma Y, Morishita R, Asano T. Copurification of small heat shock protein with αB crystallin from human skeletal muscle. J Biol Chem. 1992;267:7718–7725. [PubMed] [Google Scholar]
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- Landry J, Chretien P, Laszlo A, Lambert H. Phosphorylation of Hsp27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991;147:93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human Hsp27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Maizels ET, Peters CA, Kline M, Cutler REJ, Shanmugam M, Hunzicker-Dunn M. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem J. 1998;332:703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin MM, Kumar S, McDonnell PC, Van Horn S, Lee JC, Livi GP, Young PR. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber LA, Arrigo A-P. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Preville X, Schultz H, Knauf U, Gaestel M, Arrigo A-P. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFα- and hydrogen peroxide-induced cell death. J Cell Biochem. 1998;69:436–452. [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Commun. 1986;135:403–410. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]