Abstract
It has recently been reported that αB-crystallin, a low-molecular-weight heat shock protein, may be released from cells by mechanical stretch. We investigated a physiological role of αB-crystallin in platelet function. αB-crystallin inhibited platelet aggregation induced by thrombin or botrocetin in hamsters and humans. These platelets had specific binding sites for αB-crystallin. Moreover, αB-crystallin significantly reduced thrombin-induced Ca2+ influx and phosphoinositide hydrolysis by phospholipase C in human platelets. Additionally, plasma levels of αB-crystallin were markedly elevated in cardiomyopathic hamsters. Levels of αB-crystallin in vessel walls after endothelial injury were markedly reduced. Therefore, our results suggest that αB-crystallin, which is discharged from vessel walls in response to endothelial injury, acts intercellularly as a regulator of platelet function.
INTRODUCTION
When cells are exposed to biological stresses such as heat, they produce heat shock proteins (HSPs) (Nover 1991; Nover and Scharf 1991). HSPs are classified into high- molecular-weight HSPs and low-molecular-weight HSPs according to their apparent molecular sizes. High-molecular-weight HSPs such as Hsp90 and Hsp70 are well known to function as molecular chaperones implicated in protein folding, oligomerization, and translocation (Ellis and van der vies 1991; Gething and Sambrook 1992). Low-molecular-weight HSPs such as Hsp27 and a αB- crystallin have significant similarities in terms of amino acid sequences (Hickey et al 1986; Ingolia et al 1982). Although their function is less well understood than that of high-molecular-weight HSPs, it is recognized that low- molecular-weight HSPs may act as chaperones as well (Benndorf et al 1994; Groenen et al 1994).
αB-crystallin is well known as a major structural protein of the vertebrate lens. It is presently recognized that αB-crystallin is present in various tissues and cells, including heart, brain, and skeletal muscle, where its cellular function is unknown (Kato et al 1991, 1993; Inaguma et al 1993). It has recently been reported that the levels of αB-crystallin in trabecular meshwork cells are markedly decreased after 1 hour in response to mechanical stretch (Mitton et al 1997). This finding suggested to us that αB-crystallin might be released from cells by stress such as mechanical stretch and act intercellularly. However, the exact mechanism of the decrease of αB-crystallin has not yet been clarified. In the present study, we investigated the behavior of αB-crystallin in injured vascular walls after endothelial mechanical stress in vivo using an experimental model of stenosis. Herein, we show that αB- crystallin binds to platelets and inhibits platelet aggregation.
MATERIALS AND METHODS
Materials
Thrombin and Bothorops jararaca (snake toxin) were obtained from Sigma Chemical (St Louis, MO, USA). Botrocetin was purified from Bothorops jararaca by the method of Fujimura (Fujimura et al 1991). Fura 2-AM was obtained from Dojindo (Kumanoto, Japan). Myo-[3H]inositol (81.3 Ci/mmol) was obtained from Amersham Japan (Tokyo, Japan). Other materials and chemicals were obtained from commercial sources.
Animals
Normal hamsters were purchased from SLC (Shizuoka, Japan). Cardiomyopathic hamsters (Bio 14.6 strain) and their wild type were obtained from Charles River Japan Inc (Tokyo, Japan). Male hamsters weighing 100 to 120 g were selected and fed a standard chow (RC4, Oriental Yeast Co, Ltd, Japan). All animal experiments were performed in according with institutional guidelines.
Immunoassay of αB-crystallin
The arteries were washed twice with 1 mL of phosphate- buffered saline (PBS) and frozen at −80°C for a few days before analysis. The frozen tissues from each group were homogenized and suspended in 0.3 mL of PBS, and each suspension was sonicated and centrifuged at 125 000 × g for 20 minutes at 4°C. The supernatant was used for the specific immunoassay of αB-crystallin, as described previously (Kaida et al 1999). In brief, we used an enzyme immunoassay system that employs polystyrene balls (3.2 mm in diameter, Immuno Chemicals, Okayama, Japan) carrying immobilized F(ab′)2 fragments of antibody and the same Fab′ fragments labeled with β-D-galactosidase from Escherichia coli. A polystyrene ball carrying antibodies was incubated with the purified standard for αB-crystallin or with an aliquot of the samples. This incubation was carried out at 30°C for 5 hours in a final volume of 0.5 mL of 10 mM sodium phosphate buffer, pH 7.0, containing 0.3 M NaCl, 0.5% hydrolyzed gelatin, 0.1% bovine serum albumin (BSA), 1 mM MgCl2, and 0.1% NaN3. After washing, each ball was incubated at 4°C overnight with 1.5 milliunits of galactosidase-labeled antibodies in a volume of 0.2 mL with 10 mM sodium phosphate buffer, pH 7.0, containing 0.l M NaCl, 1 mM MgCl2, 0.1% BSA, and 0.1% NaN3. The galactosidase activity bound to the ball was assayed using a fluorogenic substrate, 4- methylumbelliferyl-β-D-galactoside.
Platelet aggregation in vitro
The effect of αB-crystallin on platelet aggregation was investigated in either platelet rich plasma (PRP) or washed platelets. Human blood was donated from young healthy male volunteers who had not taken any medications for the preceding 7 days. The blood was collected into 3.8% sodium citrate and centrifuged at 155 × g for 12 minutes at room temperature to obtain PRP. Washed platelets for studies with thrombin were prepared as described previously (Matsuno et al 1997). Platelets were counted and adjusted to 4 × 108 cells/mL (final concentration). The concentration of 3.3 μg/mL botrocetin or 0.3 U/mL thrombin was chosen to induce about 60% of aggregation. αB-crystallin was preincubated for 20 minutes with PRP or washed platelets. Platelet aggregation was followed in an aggregometer (Aggrecorder II; DA-3220, Kyotodaiichi- Chemical, Kyoto, Japan) at 37°C with a stirring speed of 800 rpm. Aggregation is expressed as a percentage of the maximum light transmission obtained in the absence of αB-crystallin.
Pharmacokinetics of αB-crystallin and platelet aggregation ex vivo
Normal hamsters were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg), and αB-crystallin (30, 100, and 300 μg/kg) was injected as a bolus (300 μL) using a catheter via the left jugular vein. Blood samples (0.4 mL each) were taken at 5, l5, 30, and 60 minutes and 6 hours after the bolus injection for the measurement of plasma concentrations of αB-crystallin. In a separate experiment, αB-crystallin (30, 100, and 300 μg/kg) was also injected as a bolus, and blood samples (0.4 mL each) were then taken by heart puncture 5 minutes after the bolus injection. Platelet aggregation induced by botrocetin (3.3 μg/mL) using PRP was then investigated as described previously.
Radioiodination of αB-crystallin
125I-labeled αB-crystallin was prepared by chloramine-T- mediated iodination (Hunter and Greenwood 1962). αB- crystalline (20 μg) was mixed with 1 mCi Na[l25I] in 100 μL of 0.5 M sodium phosphate, pH 7.4, containing 0.5 M NaCl, then 100 μL of l mg/mL chroramine T in 50 mM sodium phosphate, pH 7.4, containing 0.5 M NaCl were added to the mixture, which was incubated for 15 seconds at room temperature. The reaction was terminated by adding 100 μL of 2.5 mg/mL sodium metabisulfate in 50 mM sodium phosphate, pH 7.4, containing 0.5 M NaCl, and the labeled protein was separated from free iodine by gel filtration on a Sephadex G-50 column equilibrated with Eagle's minimum essential medium, 25 mM Hepes/NaOH, pH 7.4, 0.5% BSA containing 5 mM Kl.
[l25I] αB-crystallin binding
The αB-crystallin binding assay was performed as previously described (Kodama et al 1992). In brief, platelets (1 × 108) were incubated in a binding assay medium for 10 minutes at 37°C, then mixed with 125I-αB-crystallin in the presence or absence of a 200-fold excess of unlabeled αB-crystallin in a final volume of 300 μL of an assay buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.4 mM NaH2PO4, 0.8 mM MgCl2, 5 mM glucose). After the incubation, the reaction mixture was layered onto 200 μL of phthalate oil (dibutylphthalate/ bis[2-ethylhexyl]–phthalate [3:2]) and centrifuged at 10 000 × g for 5 minutes. The sample was frozen and cut just above the cell pellet, and the cell-associated radioactivity was then determined.
Measurement of cytoplasmic free calcium concentration
Intracellular free calcium concentration was determined after loading human platelets with a fluorescent dye, fura 2-AM. PRP was incubated with 5 μM fura 2-AM at 37°C for 30 minutes and then suspended at 2 × 108/mL in the assay buffer containing l mM CaCl2. The platelets were preincubated with αB-crystallin for 20 minutes and then stimulated by thrombin. Measurement of fura 2 was performed in a CAF-100 spectrofluorometer (JASCO, Japan). Fluorescence from fura 2 in platelets was excited with 2 excitation wavelengths of 340 and 380 nm, and the relative intensities of fluorescence were measured at 510 nm. Maximum fluorescence was achieved by lysing the platelets with 0.2% Triton X-100, and the minimum fluorescence was recorded in the presence of 2 mM EGTA. Cytoplasmic free calcium concentration was calculated by the Grynkiewicz equation (Grynkiewicz et al 1985).
Formation of inositol phosphates
Platelets were labeled with myo- [3H]inositol (100 μCi/ mL) for 3 hours. The labeled cells were preincubated with 10 mM LiCl for 10 minutes at 37°C in 1 mL of the assay buffer containing 1 mM CaCl2. The cells were pretreated with various doses of αB-crystallin for 20 minutes and then stimulated by thrombin at 37°C. The reaction was terminated by adding 1 mL of trichloroacetic acid. The acidic supernatant was treated with diethyl ether to remove the acid and neutralized with 0.1 N NaOH. The supernatant was applied to an anion exchange column containing 1 mL Dowex AG1-X8 (100–200 mesh formate, form; Bio-Rad Laboratories, Hercules, CA, USA). The radioactive inositol phosphates were eluted with 8 mL of 0.1 M formic acid containing 1 M ammonium formate as previously described (Berridge et al 1983; Kondo et al 1989).
Detection of αB-crystallin in vivo
The experimental procedure used to induce endothelial injury in the hamster carotid artery was performed as previously described (Matsuno et al 1995). The right common carotid artery of hamsters was subjected to mechanical stress by use of a catheter that denuded the endothelium and induced highly reproducible intimal proliferation of vascular smooth muscle cells over the entire length of the affected blood vessel. A 2FG catheter with a roughened tip was inserted through the external carotid artery and advanced into the thoratic aorta. The catheter was left in position for 30 seconds and rotated completely 3 times. By these means, endothelial cells were completely denuded, and several parts of the elastic lamina were ruptured. Platelet-rich thrombi were observed in injury areas immediately after the initiation of injury. Animals were divided into 6 groups. The injured carotid artery was removed 5, 30, and 60 minutes and 6 hours after the catheterization. Segments were homogenized in phosphate-buffer, and αB-crystallin levels were measured by the immunoassay.
The plasma levels of αB-crystallin in cardiomyopathic hamsters and their control hamsters were determined. Blood samples were taken via jugular vein at 6 weeks after they were born.
Determination of radioactivity
The radioactivity of 3H samples was determined using a Beckman LS6500IC liquid scintillation spectrometer (Fullerton, CA, USA). The radioactivity of l25I samples was determined using a Wallac 1480 WIZARD 3′′ automatic gamma counter (Turk, Finland).
Other methods
Protein concentrations in soluble extracts were determined using a protein assay kit (Bio-Rad) with BSA as the standard protein. Rat αB-crystallin, which was used as the standard for the immunoassay, was purified from skeletal muscle (Kato et al 1991).
Statistical analysis
The data were analyzed by ANOVA followed by Bonferroni method for multiple comparison between pairs, and a P < 0.05 was considered significant. All data are presented as the mean ± SEM of triplicate determinations.
RESULTS
Effect of αB-crystallin on platelet aggregation in vitro
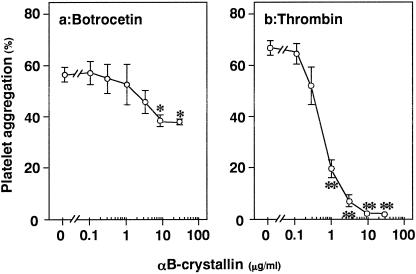
We first examined the effect of αB-crystallin on platelet aggregation induced by botrocetin or thrombin using human PRP. αB-crystallin significantly inhibited the platelet aggregation induced by botrocetin (Fig 1a) or thrombin (Fig 1b). The inhibitory effect of αB-crystallin was dose dependent in the range between 0.1 and 30 μg/mL. αB- crystallin (10 μg/mL) almost completely suppressed the platelet aggregation induced by thrombin while reducing the botrocetin-induced platelet aggregation by about 40%.
Fig 1. Effect of αB-crystallin on platelet aggregation in vitro using human platelets. The aggregation of platelets stimulated by botrocetin (3.3 μg/mL) using PRP (a) or thrombin (0.3 U/mL) using washed platelets (b) was performed after preincubation for 20 minutes with αB-crystallin or vehicle. Each value represents the mean ± SEM of triplicate determinations. * P < 0.05 vs the value of botrocetin alone, ** P < 0.05 vs the value of thrombin alone
Plasma levels of αB-crystallin and effect of αB- crystallin on platelet aggregation ex vivo
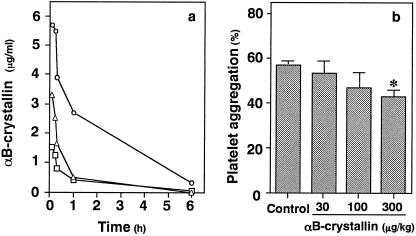
Plasma levels of αB-crystallin after an intravenous bolus injection at doses of 30, 100, or 300 μg/kg in hamsters are shown in Figure 2a. In addition, we investigated the effect of αB-crystallin on platelet aggregation ex vivo using hamsters. Platelet aggregation induced by botrocetin was inhibited by about 30% when αB-crystallin at a dose of 300 μg/kg was administered intravenously (Fig 2b).
Fig 2. The levels of plasma αB-crystallin after an intravenous injection (a) and effect of αB-crystallin on platelet aggregation ex vivo using normal hamster PRP (b). αB-crystallin was injected intravenously as a bolus at doses of 30 (circle), 100 (triangle), and 300 (square) μg/ kg. The aggregation of platelets stimulated by botrocetin (3.3 μg/mL) was performed after preincubation for 20 minutes with αB-crystallin. Each value represents the mean ± SEM of triplicate determinations. * P < 0.05 versus the value of botrocetin without αB-crystallin.
Characterization of binding of 125I-αB-crystallin to platelets
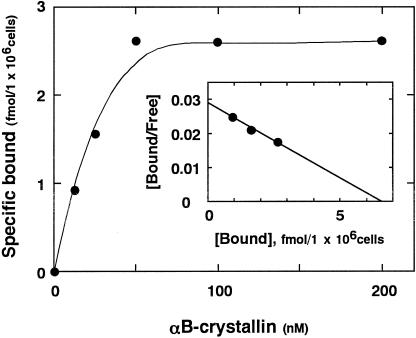
125I-αB-crystallin binding to human platelets at 37°C reached equilibrium after 30 minutes. Thus, further binding experiments were performed at 37°C for 30 minutes. Incubation of increasing concentrations of 125I-αB-crystallin with human platelets showed that the specific binding consisted of a saturable component (Fig 3). The levels of nonspecific binding was linearly dependent on the concentration αB-crystallin and was about 30% of the total binding. Scatchard plot analysis of the binding data revealed that there were affinity sites for αB-crystallin on human platelets (Fig 3). The Kd values of the affinity sites were 30 nM, and the average numbers were 3900 per cell.
Fig 3. Plot of 125I-αB-crystallin. Binding to human platelets. Binding experiments were performed as described under “Materials and Methods.” The insert shows Scatchard plot analysis
Effect of αB-crystallin on thrombin-induced Ca2+ mobilization in human platelets
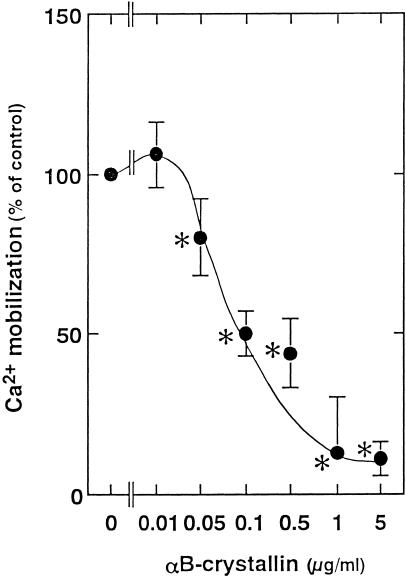
Thrombin induced a rapid and sharp increase in intracellular calcium concentration, followed by a slow and phasic increase, which then gradually decreased. αB-crystallin, which alone did not affect the basal levels of intracellular free Ca2+ (data not shown), reduced the slow and phasic increase in cytoplasmic free Ca2+ (Fig 4). The effect of αB-crystallin was dose dependent in the range between 0.05 and 5 μg/mL.
Fig 4. Effect of αB-crystallin on the thrombin (0.3 U/mL)-induced Ca2+ influx in human platelets. Each value represents the mean ± SEM of triplicate determinations in a single experiments (representative of 3 experiments in all). The value of control was 345.4 ± 40.0 nM. * P < 0.05 versus the value of thrombin alone
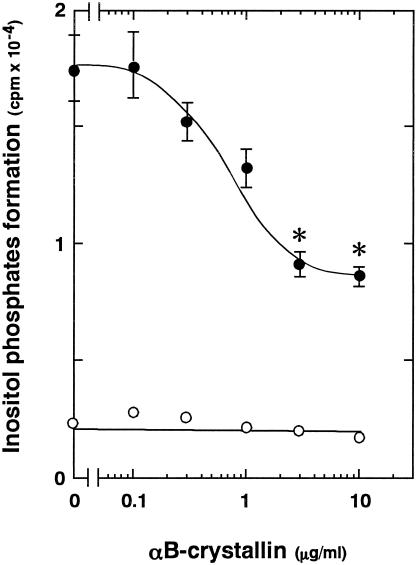
Effect of αB-crystallin on thrombin-induced inositol phosphates formation in human platelets
It has been shown that thrombin stimulates phosphoinositide hydrolysis by phospholipase C in platelets (Grand et al 1996). Phosphoinositide hydrolysis by phospholipase C is well recognized to form inositol phosphates and diacylglycerol (Berridge 1993). Thus, we next examined the effect of αB-crystallin of the thrombin-induced formation of inositol phosphates. The thrombin-stimulated formation of inositol phosphates was significantly reduced by αB-crystallin (Fig 5). The inhibitory effect on the formation of inositol phosphates was dose dependent in the range between 0.1 and 10 μg/mL.
Fig 5. Effect of αB-crystallin on thrombin-induced formation of inositol phosphates in human platelets. The labeled cells were pretreated with various doses of αB-crystallin for 20 minutes and then stimulated by thrombin or vehicle for 10 minutes. The formation of inositol phosphates was then determined. Each value represents the mean ± SEM of triplicate determinations in a single experiments (representative of 3 experiments in all). * P < 0.05 vs the value of thrombin alone
Levels of αB-crystallin in plasma of cardiomyopathic hamsters
The Bio 14.6 strain of hamsters shows a predominant hypertrophic stage (Gertz 1992), and they are most widely used for investigation. Plasma levels of αB-crystallin in these cardiomyopathic hamsters were 2.045 ng/mL (n = 3). On the other hand, those in control hamsters were 0.143 ng/ml (n = 3).
Levels of αB-crystallin in hamster injured arteries
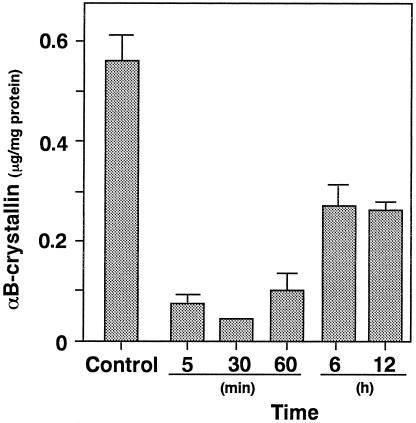
The levels of αB-crystallin in uninjured artery were 0.57 ± 0.09 μg/mg protein. The levels were immediately reduced as compared with those of intact carotid arteries (Fig 6). The minimum levels of αB-crystallin (0.08 ± 0.02 μg/mg protein) were observed at 30 minutes after the injury, and the levels were gradually increased.
Fig 6. Levels of αB-crystallin in the vessel wall after endothelial injury. Animals were divided into a noninjured control group (n = 8) and injured group (n = 8). The injured carotid arteries were removed 5, 30, and 60 minutes and 6 and 12 hours after the initiation of injury. Each value represents the mean ± SEM
Immunohistological observation of αB-crystallin
Morphological examination was performed in order to further clarify the effect of endothelial injury on the levels of αB-crystallin in hamster vessel wall. αB-crystallin in uninjured vascular wall was clearly present at high levels (Fig 7a,c). On the contrary, the levels were markedly reduced by endothelial injury (Fig 7b,d).
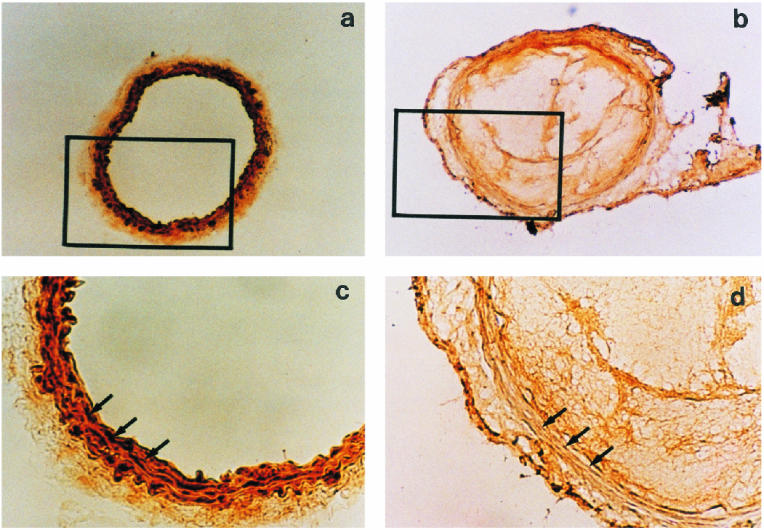
Fig 7. Histochemical analysis of αB-crystallin in hamster carotid artery after endothelial injury. Representative cross section from the carotid artery of a noninjured hamster (a and c, magnification 100× and 400×, respectively) and from a hamster 60 minutes after injury (b and d, magnification 100× and 400×, respectively) are shown after immunostaining and staining with hematoxylin. Arrows indicate the medial area
DISCUSSION
HSPs are well recognized to act as molecular chaperones and protect cells from hazardous conditions. However, the details of their physiological roles are not precisely clarified. It has recently been reported that mechanical stretch causes 26% reduction in the levels of αB-crystallin, one of low-molecular-weight HSPs, in trabecular meshwork cells within 2 minutes, and the decrease is 90% after 1 hour (Mitton et al 1997). These findings led us to speculate that some stress might release HSPs such as αB- crystallin from cells. In the present study, we investigated the behavior of αB-crystallin in detail and its physiological roles in platelet functions.
We showed that the levels of αB-crystallin in injured arteries were markedly lower than those of noninjured arteries in vivo. Our findings are consistent with the previous report (Mitton et al 1997). These results were clearly supported by the data of immunohistochemical observations. Thus, it is probable that αB-crystallin is released from vascular wall after endothelial injury. These findings led us to speculate that αB-crystallin in normal blood vessel walls immediately responds to mechanical stress, such as endothelial injury in this case, and is probably released from the injured arterial wall into the circulation. Furthermore, we demonstrated that the αB-crystallin levels in plasma of cardiomyopathic hamsters were much higher than those of control hamsters. Thus, this observation strongly suggests that αB-crystallin actually functions in pathological conditions.
In the present study, we found that αB-crystallin specifically bound to human platelets with the Kd values of 30 nM during the course of investigating the effect of αB- crystallin on platelets since platelets are recognized to play crucial roles in thrombus and neointima formation after vascular injury (Ferns et al 1991; Ross 1993). These findings suggest that platelets have specific binding sites for αB-crystallin. Therefore, we suspected that αB-crystallin might have important roles in platelets.
In order to investigate the physiological role of the αB- crystallin that is released from injured vessel walls, we examined the effect of αB-crystallin on the aggregation of platelets in vitro using PRP. We showed that αB-crystallin inhibited the aggregation of human platelets induced by botrocetin and thrombin. Botrocetin stimulates platelet aggregation through inducing the binding of von Willebrand factor (vWF) to platelet glycoprotein (GP) 1b (Kawasaki et al 1996). Thus, our findings suggest that αB- crystallin might interact the vWF-GPlb axis. In addition, we demonstrated that thrombin-induced platelet aggregation was significantly suppressed by αB-crystallin. We previously showed that Hsp20, a low-molecular-weight HSP, has inhibitory roles in platelet aggregation, but Hsp27 is unable to inhibit (Matsuno et al 1998). It is recognized that the 3 low-molecular-weight HSPs—αB-crystallin, Hsp20, and Hsp27—are associated in cells as previously described (Kato et al 1994). Taking these findings into account, it is possible that when vascular smooth muscle cells are exposed to some stress such as mechanical stress, the 3 HSPs in these cells are dissociated in response to the stress, and then αB-crystallin and Hsp20 among them intercellularly modulate platelet functions. In addition, our findings of pharmacokinetics of αB-crystallin in hamsters indicate that αB-crystallin could play an inhibitory role of platelet activation in vivo after a bolus intravenous injection.
We next examined the effect of αB-crystallin on the intracellular signaling system of thrombin in human platelets. αB-crystallin significantly suppressed the Ca2+ mobilization and phosphoinositide-hydrolyzing phospholipase C activity induced by thrombin. It is recognized that both intracellular Ca2+ mobilization and the activation of protein kinase C have crucial roles in signal transduction initiated by thrombin receptor activation resulting in regulating platelet functions (Berridge 1993; Grand et al 1996). Therefore, based on these findings, it is possible that αB-crystallin inhibits platelet aggregation through the suppression of these intracellular tranducing events. Further investigations would be necessary to clarify the detailed mechanism of αB-crystallin in modulating platelet function.
In conclusion, these results strongly suggest that αB- crystallin, which is discharged from vessel walls in response to endothelial injury, acts intercellularly as a regulator of platelet function. Moreover, these findings could be a part of new concept of antiplatelet therapy in the treatment of cardiovascular diseases.
Acknowledgments
We are grateful to Dr Keiji Miyazawa for invaluable technical assistance.
REFERENCES
- Benndorf R, Hayes K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supermolecular organization of murine small heat shock protein HSP25 abolish its actin polymelization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dawson RMC, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, van der Vies SM. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;252:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Titani K, Usami Y, et al. Isolation and chemical characterization of two structurally and functionally distinct forms of botrocetin, the platelet coagglutinin isolated from the venom of bothrops jararaca. Biochemistry. 1991;30:1957–1964. doi: 10.1021/bi00221a032. [DOI] [PubMed] [Google Scholar]
- Gertz EW. Cardiomyopathic syrian hamster: a possible model of human disease. Prog Exp Tumor Res. 1972;16:242–260. doi: 10.1159/000393374. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–44. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grand RJA, Turnell AS, Grabham PW. Cellular consequences of thrombin-recetor activation. Biochem J. 1996;313:353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen PJTA, Merck KB, DeJong WW, Bloemendal H. Structure and modifications of the junior chaperone-crystallin: from lens transparency to molecular pathology. Eur J Biochem. 1994;225:1– 19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hickey E, Brandon SE, Potter R, Stein J, Weber LA. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986;14:4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WM, Greenwood FC. Preparation of iodine-131 labeled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Inaguma Y, Goto S, Shinohara H, Hasegawa K, Ohshima K, Kato K. Physiological and pathological changes in levels of two small stress proteins, hsp27 and αB-crystallin in rat hindlimb muscles. J Biochem. 1993;114:378–384. doi: 10.1093/oxfordjournals.jbchem.a124184. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida T, Kozawa O, Ito T, et al. Vasopressin stimulates the induction of heat shock protein 27 and αB-crystallin via protein kinase C activation in vascular smooth muscle cells. Exp Cell Res. 1999;246:327–337. doi: 10.1006/excr.1998.4277. [DOI] [PubMed] [Google Scholar]
- Kato K, Goto S, Hasegawa K, Inaguma Y. Coinduction of two low-molecular-weight stress proteins, αB-crystallin and HSP28, by heat or arsenite stress in human glioma cells. J Biochem. 1993;114:640–647. doi: 10.1093/oxfordjournals.jbchem.a124230. [DOI] [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T. Purification and characterization of a 20-kDa protein that is highly homologous to B-crystallin. J Biol Chem. 1994;269:15302– 15309. [PubMed] [Google Scholar]
- Kato K, Shinohara H, Kurobe N, Inaguma Y, Shimizu K, Oshima K. Tissue distribution and developmental profiles of immunoreactive αB-crystallin in the rat determined with a sensitive immunoassay system. Biochem Biophys Acta. 1991;1074:201–208. doi: 10.1016/0304-4165(91)90062-l. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Fujimura Y, Usami Y, et al. Complete amino acid sequence and identification of the platelet glycoprotein lb-binding site of Jararaca GPlb-BP′ a snake venom protein isolated from Bothrops jararaca. J Biol Chem. 1996;271:10635–10639. doi: 10.1074/jbc.271.18.10635. [DOI] [PubMed] [Google Scholar]
- Kodama M, Miyazawa K, Ishii T, Kitamura N. Characterization of hepatocyte-growth-factor receptors on Meth A cells. Eur J Biochem. 1992;204:857–864. doi: 10.1111/j.1432-1033.1992.tb16705.x. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kozawa O, Takatsuki K, Oiso Y. Ca2+ influx stimulated by vasopressin is mediated by phosphoinositide hydrolysis in rat smooth muscle cells. Biochem Biophys Res Commun. 1989;161:677–682. doi: 10.1016/0006-291x(89)92652-1. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Kozawa O, Niwa M, Uematsu T. Inhibition of von Willebrand factor binding to platelet GP lb by a fractionated aurintricarboxylic acid prevents restenosis after vascular injury in hamster carotid artery. Circulation. 1997;96:1299–1304. doi: 10.1161/01.cir.96.4.1299. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Kozawa O, Niwa M, Usui A, Ito H, Uematsu T, Kato K. A heat shock-related protein, p20, plays an inhibitory role in platelet activation. FEBS Lett. 1998;429:327–329. doi: 10.1016/s0014-5793(98)00626-7. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Stassen JM, Hoylaerts MF, Vermylen J, Deckmyn H. Fast and reproducible vascular neointima formation in the hamster carotid artery: effects of trapidil and captopril. Thromb Haemostasis. 1995;74:1591–1596. [PubMed] [Google Scholar]
- Mitton KP, Tumminia SJ, Arora J, Zelenka P, Epstein DL, Russell P. Transient loss of αB-crystallin: an early cellular response to mechanical stretch. Biochem Biophys Res Commun. 1997;235:69–73. doi: 10.1006/bbrc.1997.6737. [DOI] [PubMed] [Google Scholar]
- Nover L 1991 Inducers of hsp synthesis: heat shock and chemical stressors. In: Heat Shock Response, ed Nover L. CRC Press, Boca Raton, FL, 5–40. [Google Scholar]
- Nover L, Scharf K-D 1991 Heat shock proteins. In: Heat Shock Response, ed Nover L. CRC Press, Boca Raton, FL, 41–128. [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]