Abstract
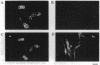
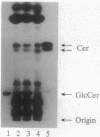
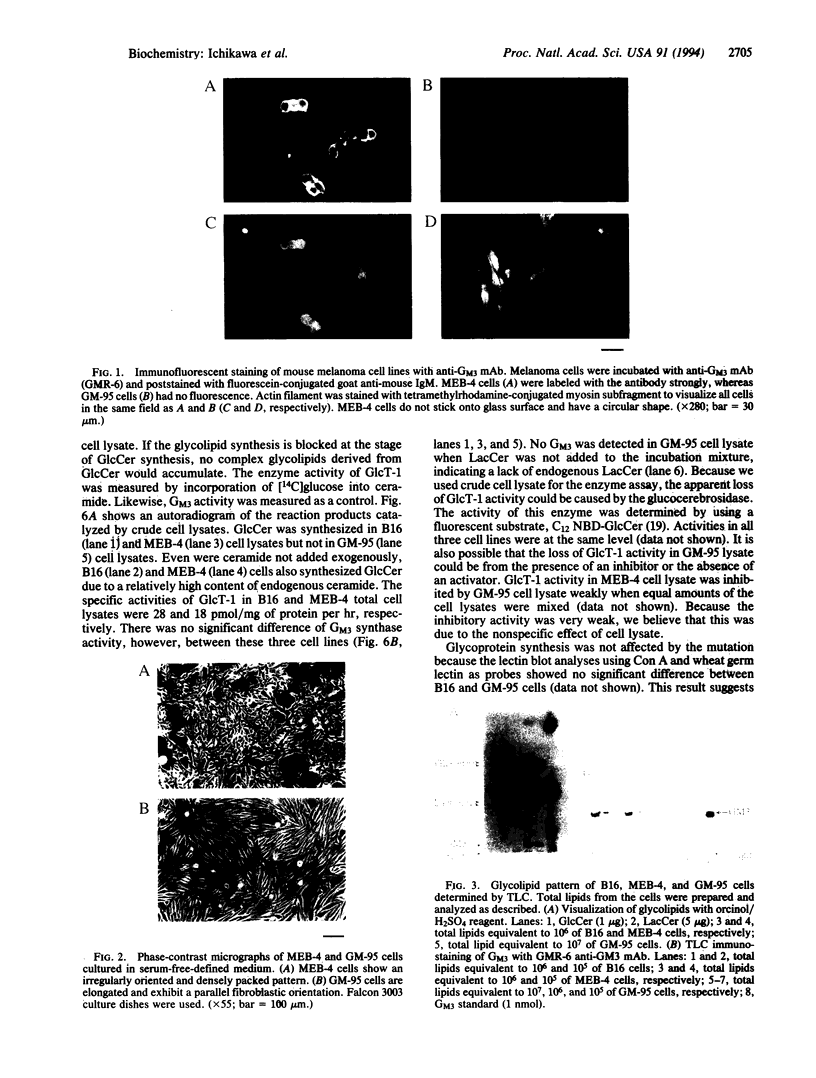
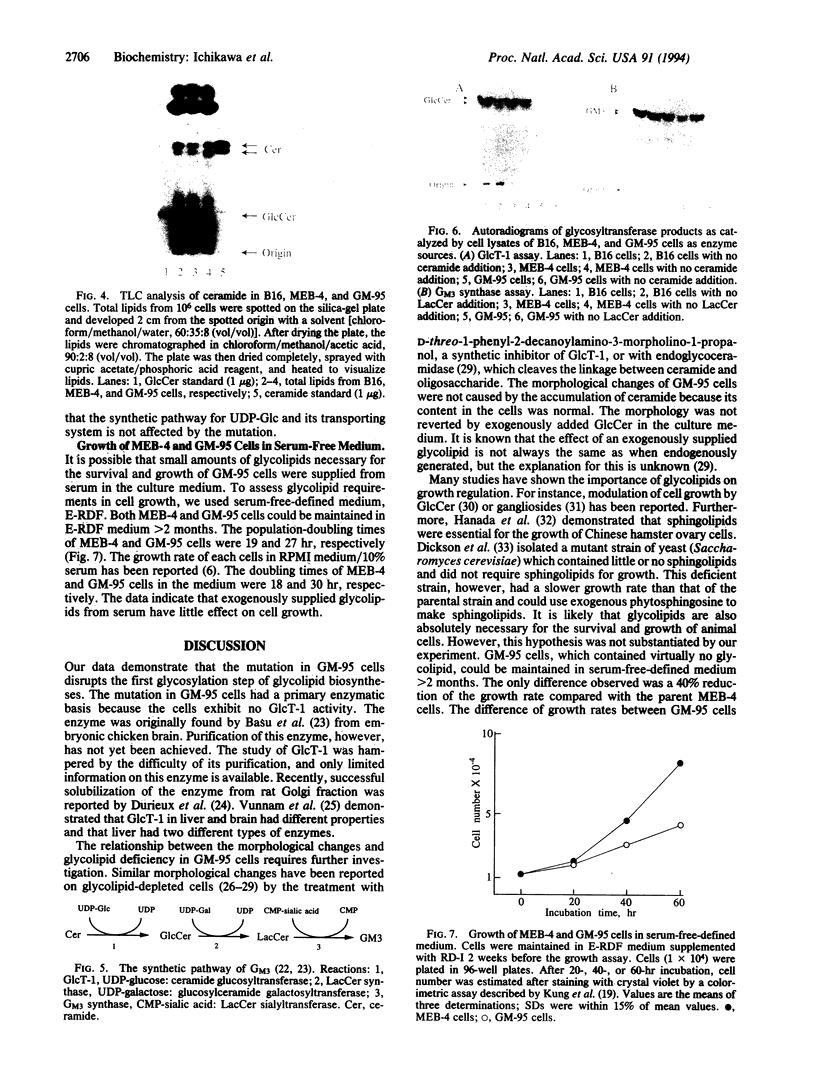
Mouse B16 melanoma cell line, GM-95 (formerly designated as MEC-4), deficient in sialyllactosylceramide was examined for its primary defect. Glycolipids from the mutant cells were analyzed by high-performance TLC. No glycolipid was detected in GM-95 cells, even when total lipid from 10(7) cells was analyzed. In contrast, the content of ceramide, a precursor lipid molecule of glycolipids, was normal. Thus, the deficiency of glycolipids was attributed to the first glucosylation step of ceramide. The ceramide glucosyltransferase (EC 2.4.1.80) activity was not detected in GM-95 cells. There was no significant difference of sialyllactosylceramide synthase activity, however, between GM-95 and the parental cells. The deficiency of glycolipids in GM-95 cells was associated with changes of the cellular morphology and growth rate. The parental cells showed irregular shapes and tended to overlap each other. On the other hand, GM-95 cells exhibited an elongated fibroblastic morphology and parallel arrangement. The population-doubling times of GM-95 and the parental cells in serum-free medium were 28 hr and 19 hr, respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce A., Maccioni H. F., Caputto R. Enzymic binding of sialyl groups to ganglioside derivatives by preparations from the brain of young rat. Arch Biochem Biophys. 1966 Sep 26;116(1):52–58. doi: 10.1016/0003-9861(66)90011-7. [DOI] [PubMed] [Google Scholar]
- Barbour S., Edidin M., Felding-Habermann B., Taylor-Norton J., Radin N. S., Fenderson B. A. Glycolipid depletion using a ceramide analogue (PDMP) alters growth, adhesion, and membrane lipid organization in human A431 cells. J Cell Physiol. 1992 Mar;150(3):610–619. doi: 10.1002/jcp.1041500322. [DOI] [PubMed] [Google Scholar]
- Basu S., Kaufman B., Roseman S. Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain. J Biol Chem. 1973 Feb 25;248(4):1388–1394. [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Nuwayhid N., Stanley P., Briles E. I., Hirschberg C. B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984 Dec;39(2 Pt 1):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Wells G. B., Schmidt A., Lester R. L. Isolation of mutant Saccharomyces cerevisiae strains that survive without sphingolipids. Mol Cell Biol. 1990 May;10(5):2176–2181. doi: 10.1128/mcb.10.5.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinur T., Grabowski G. A., Desnick R. J., Gatt S. Synthesis of a fluorescent derivative of glucosyl ceramide for the sensitive determination of glucocerebrosidase activity. Anal Biochem. 1984 Jan;136(1):223–234. doi: 10.1016/0003-2697(84)90329-4. [DOI] [PubMed] [Google Scholar]
- Durieux I., Martel M. B., Got R. Solubilization of UDPglucose-ceramide glucosyltransferase from the Golgi apparatus. Biochim Biophys Acta. 1990 May 24;1024(2):263–266. doi: 10.1016/0005-2736(90)90352-o. [DOI] [PubMed] [Google Scholar]
- Fenderson B. A., Ostrander G. K., Hausken Z., Radin N. S., Hakomori S. A ceramide analogue (PDMP) inhibits glycolipid synthesis in fish embryos. Exp Cell Res. 1992 Feb;198(2):362–366. doi: 10.1016/0014-4827(92)90392-l. [DOI] [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hanada K., Nishijima M., Kiso M., Hasegawa A., Fujita S., Ogawa T., Akamatsu Y. Sphingolipids are essential for the growth of Chinese hamster ovary cells. Restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. J Biol Chem. 1992 Nov 25;267(33):23527–23533. [PubMed] [Google Scholar]
- Higashi H., Fukui Y., Ueda S., Kato S., Hirabayashi Y., Matsumoto M., Naiki M. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem. 1984 May;95(5):1517–1520. doi: 10.1093/oxfordjournals.jbchem.a134760. [DOI] [PubMed] [Google Scholar]
- Inokuchi J., Momosaki K., Shimeno H., Nagamatsu A., Radin N. S. Effects of D-threo-PDMP, an inhibitor of glucosylceramide synthetase, on expression of cell surface glycolipid antigen and binding to adhesive proteins by B16 melanoma cells. J Cell Physiol. 1989 Dec;141(3):573–583. doi: 10.1002/jcp.1041410316. [DOI] [PubMed] [Google Scholar]
- Inokuchi J., Radin N. S. Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol, inhibitor of murine glucocerebroside synthetase. J Lipid Res. 1987 May;28(5):565–571. [PubMed] [Google Scholar]
- Kaufman B., Basu S., Roseman S. Enzymatic synthesis of disialogangliosides from monosialogangliosides by sialyltransferases from embryonic chicken brain. J Biol Chem. 1968 Nov 10;243(21):5804–5807. [PubMed] [Google Scholar]
- Kijimoto-Ochiai S., Katagiri Y. U., Ochiai H. Analysis of N-linked oligosaccharide chains of glycoproteins on nitrocellulose sheets using lectin-peroxidase reagents. Anal Biochem. 1985 May 15;147(1):222–229. doi: 10.1016/0003-2697(85)90031-4. [DOI] [PubMed] [Google Scholar]
- Kueng W., Silber E., Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989 Oct;182(1):16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nozue M., Sakiyama H., Tsuchiya K., Hirabayashi Y., Taniguchi M. Melanoma antigen expression and metastatic ability of mutant B16 melanoma clones. Int J Cancer. 1988 Nov 15;42(5):734–738. doi: 10.1002/ijc.2910420518. [DOI] [PubMed] [Google Scholar]
- Okada Y., Radin N. S., Hakomori S. Phenotypic changes in 3T3 cells associated with the change of sphingolipid synthesis by a ceramide analog, 2-decanoylamino-3-morpholino-1-phenylpropanol (compound RV538). FEBS Lett. 1988 Aug 1;235(1-2):25–29. doi: 10.1016/0014-5793(88)81227-4. [DOI] [PubMed] [Google Scholar]
- Sakiyama H., Matsushita E., Kuwabara I., Nozue M., Takahashi T., Taniguchi M. Characterization of a melanoma antigen with a mouse-specific epitope recognized by a monoclonal antibody with antimetastatic ability. Cancer Res. 1988 Dec 15;48(24 Pt 1):7173–7178. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam R., Radin N. S. Quantitation of lipids by charring on thin-layer plates and scintillation quenching: application to ceramide determination. Anal Biochem. 1981 Apr;112(2):338–345. doi: 10.1016/0003-2697(81)90302-x. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Deshmukh G. D., Mahdiyoun S., Thomas T. P., Wu D., Barcelon F. S., Radin N. S. Modulation of renal epithelial cell growth by glucosylceramide. Association with protein kinase C, sphingosine, and diacylglycerol. J Biol Chem. 1991 Dec 5;266(34):22968–22974. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka T., Tsuji T., Nojiri H., Holmes E. H., Hakomori S. Selection of a mutant cell line based on differential expression of glycosphingolipid, utilizing anti-lactosylceramide antibody and complement. J Biol Chem. 1993 Jan 25;268(3):2211–2216. [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunnam R. R., Radin N. S. Short chain ceramides as substrates for glucocerebroside synthetase. Differences between liver and brain enzymes. Biochim Biophys Acta. 1979 Apr 27;573(1):73–82. doi: 10.1016/0005-2760(79)90174-7. [DOI] [PubMed] [Google Scholar]
- Weis F. M., Davis R. J. Regulation of epidermal growth factor receptor signal transduction. Role of gangliosides. J Biol Chem. 1990 Jul 15;265(20):12059–12066. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]