Abstract
Thermally aggregated, endogenous proteins in Escherichia coli cells form the S fraction, which is separable by sucrose density gradient centrifugation. To date, relatively little is known about the mechanisms of elimination of the heat-aggregated proteins from E coli cells and the composition of the S fraction. We have identified several proteins of the S fraction using 2D-gel electrophoresis and microsequencing. A thermostable II class fructose-1,6-bisphosphate aldolase (Fda protein) appeared to be one of numerous proteins of the S fraction. Fda was purified from E coli overproducer strain and used as a model substrate for investigation of the role of Hsps in prevention and repair of thermal denaturation of proteins both in vivo and in vitro. We found that the heat inactivation of Fda was reversible and that its reactivation in vivo and in vitro required mainly the assistance of the DnaK/DnaJ chaperone system. The dnaK756 and dnaJ259 mutations had a negative effect on the reactivation of thermally inactivated Fda. Moreover, we showed that the reactivation process in vitro was enhanced when GroEL/GroES were added together with DnaK/DnaJ. GroEL/GroES alone were inefficient in the resolubilization or reactivation of the heat-aggregated Fda. It is supposed that the denaturation of the thermostable Fda in vivo results rather from a temporary and transient deficit of Hsps than from the direct heat effect.
INTRODUCTION
Heat shock–promoted denaturation of cellular proteins leads to abnormal interactions between these proteins and consequently to aggregation in both prokaryotic and eukaryotic cells. In drastic cases, and frequently at high- level production of some heterologous proteins in E coli, inclusion bodies are formed. They consist mainly of the protein of interest and account for a major fraction of the total synthesized proteins in E coli (Kane and Hartley 1988). Kucharczyk et al (1991) showed that there is a unique type of insoluble protein aggregates called the S fraction, which can be separated by sucrose density gradient ultracentrifugation.
This technique opened the possibility of observation of the heat shock effects in vivo in the entirely natural system. In the wild type (wt), the S fraction is detectable for only 15 minutes after the temperature change from 30°C to 45°C, and it disappears during the 10 minutes following incubation at 37°C. The rapid disappearance of the S fraction depends on heat shock response. In the rpoH165 mutant, the S fraction was found to be stable (Kucharczyk et al 1991).
The protein content in the S fraction in wt strains corresponded to 4% to 5% of the total cellular protein and 13% to 19% of the insoluble proteins (Kędzierska et al 1999). The S fraction, in comparison with inclusion bodies, has lower density during centrifugation in sucrose density gradient (unpublished result of Laskowska) and contains, besides sHsps, IbpA and IbpB and also major Hsps, DnaK, DnaJ (Kucharczyk et al 1991), and ClpB (unpublished result of Matuszewska). The association suggested that these Hsps might be involved in preserving the proteins in state of competence for renaturation or proteolysis.
Protein denaturation and aggregation is a reversible process because of the activity of molecular chaperones. They are known to play fundamental roles in the folding of proteins under physiological and stress conditions by prevention of protein aggregation and promoting refolding and reactivation of denatured proteins (Langer et al 1992; Hartl 1996). The mechanism of resolubilization and reactivation of proteins aggregated by heat shock is extensively investigated by various research groups. Our studies on the removal of the S fraction from E coli mutants defective in heat shock genes indicate that the disappearance of aggregated proteins results from renaturation by molecular chaperones (DnaK/DnaJ, GroEL/ GroES, ClpB) or degradation by heat shock proteases such as Lon, Clp, and HtrA (Laskowska et al 1996b; Kędzierska et al 1999). For example, it was found that the dnak756, dnaJ259 mutations stabilized the S, and the groEL44 and groES619 mutations caused its incomplete removal (Kędzierska et al 1999). On the other hand, the about 4-fold excess of DnaK/DnaJ or GroEL/GroES completely prevented the transient appearance of the S fraction in the wt strains (Kędzierska et al 1999). These data are in agreement with the results of Gragerov et al (1992), who showed that overproduction of either DnaK and DnaJ or GroEL and GroES in rpoH mutants prevented aggregation of newly synthesized proteins. Zolkiewski (1999), Goloubinoff et al (1999), and Mogk et al (1999) demonstrated that ClpB together with DnaK/DnaJ/GrpE form a multichaperone system that can rescue previously aggregated proteins and refold them into active forms, analogous to the yeast Hsp104/Ssa1/Ydj1 system (Glover and Lindquist 1998). An order of addition of chaperones suggests that an action of ClpB precedes that of Dnak/ DnaJ/GrpE system in the mechanism of protein disaggregation and reactivation (Goloubinoff et al 1999). DnaK chaperone system alone has been proposed to reintroduce proteins trapped in small aggregates (60–400 kDa), back into the chaperone network for refolding or into the protease network for degradation (Diamant et al 2000). Earlier, the ability of DnaK alone to disaggregate heat- inactivated proteins has been described by Skowyra et al (1990) based on the experiments with heat-inactivated and aggregated RNA polymerase.
The purpose of this work was to extend the studies on the mechanism of formation and elimination of thermally aggregated proteins from cells. The use of a natural system in vivo and its protein components in vitro was considered to be important because we have noticed previously that reactions with exogenous substrates, in some cases, gave results different from those obtained with the native ones (Laskowska et al 1996b). For example, the HtrA, heat shock protease (Lipińska et al 1989; Strauch et al 1989), endowed with chaperone properties (Spiess et al 1999), recognized as its substrates the endogenous S fraction proteins and the exogenous casein. However, in the first case, Mg2+ ions were stimulatory, and quite surprisingly, DnaJ (but not DnaK) added in excess inhibited the proteolysis of the S fraction by the HtrA protease, which was interpreted as a protection of the denatured proteins from extensive proteolysis. None of these observations concerned the proteolysis of casein, which was not stimulated by Mg2+ and not inhibited by DnaJ (Laskowska et al 1996b).
Therefore, identification of several proteins of the S fraction was undertaken. We searched for an enzyme with easily determinable activity to follow its denaturation/renaturation in vivo and in vitro. Thermostable, class II fructose-1,6-bisphosphate aldolase (Fda protein) was identified as the S fraction component and used as a model substrate in the experiments described here. Our results show that DnaK/DnaJ were essential for its resolubilization and reactivation. Thus, the disaggregation activity of DnaK chaperone system described before by others (Skowyra et al 1990; Diamant et al 2000) was confirmed. The action of DnaK/DnaJ was slightly enhanced by GroEL/GroES, while GroEL/ES alone were inefficient in the resolubilization or reactivation of thermally aggregated Fda. Apparently, the mechanism of the action of Hsps may be different for particular proteins. We infer that the denaturation of the thermostable Fda in vivo resulted from a temporary limitation of DnaK/DnaJ supply immediately after heat shock rather than of the direct action of heat.
MATERIALS AND METHODS
E coli strains and growth conditions
B178 (W3101 galE, sup+) was obtained from B. Lipińska (Gdańsk, Poland) (Lipińska et al 1989), and B178 dnaK756 (Georgopoulos and Herskovitz 1971; Georgopoulos 1977), B178 dnaJ (Yochem et al 1978), B178 groEL44 (tetR) (Zeilstra-Ryalls et al 1993), and B178 groES619 (tetR) (Zeilstra- Ryalls et al 1993) were obtained via M. Żylicz (Gdańsk). AJW101 (TG1: supE, hsdD5, thi(lac-proAB) [F′ traD36, proAB+, lacIq, 2M15],) ibpA/B::kan was supplied by P. Lund (Birmingham, England). XL1-Blue (recA1, endA1, gyrA96, thi-1, hsdR17, supE44 [F′ proAB+, lacIq, ZM15, Tn10 (tetR)], relA1, lac, [pKEN8 (fda+, ampR]) strain overproducing the Zn2+-dependent E coli fructose-1,6-bisphosphate aldolase (Henderson et al 1994) was purchased from the American Type Culture Collection (ATCC 77472). W3350 (galE, galK, supo) originated from A. Campbell (Stanford, CA, USA) (Campbell 1961).
For isolation of the S fraction, the bacteria were grown in 100 mL of LB medium with aeration to A600 = 0.25, then transferred to 45°C for 15 minutes (heat shock).
Plasmid
Plasmid pKEN8 (ampR) bearing the fda gene under the control of the tac and T7 promoters was used. Expression of the fda gene was induced by addition of IPTG (1 mM) and M13/T7 phage (10–40 pfu/cell; only in the case of E coli XL1-Blue strain). M13/T7 phage was a gift from T. Kaczorowski (Gdańsk, Poland). DNA plasmid preparation and transformation of E coli strains listed previously were done according to Sambrook et al (1989).
Proteins
Overexpression of the fda gene in E coli XL1-Blue pKEN8 and preparation of cell lysate were according to Henderson et al (1994). The enzyme purification was carried out as described by Berry and Marshall (1993). Briefly, the cell extract was applied to DE52 column (Whatman, Maidstone, UK) and, after washing it with 80 mM NaCl in 50 mM Tris-HCl, pH 8.0, the enzyme was eluted with 120 mM NaCl in the same buffer and purified by sizing chromatography on a Superdex 200 column (Sigma, St. Louis, MO, USA). The enzyme was homogenous in 0.1%SDS-12%PAGE, and specific activity corresponded to 34 U/mg.
Protein concentration was estimated by the Bradford method (Bradford 1976) with BSA as a standard.
Fda activity determination in vivo
For the Fda activity determination in vivo, bacterial strains were grown in 50 mL of SOB medium (Sambrook et al 1989) supplemented with ampicillin (100 μg/mL), at 30°C to A600 = 0.25, then IPTG to 1 mM final concentration was added for 2 hours. Subsequently, the bacteria were submitted to heat shock (45°C, 15 minutes; inactivation period) in the presence of kanamycin (100 μg/mL) or tetracycline (25 μg/mL), then transferred to 37°C (reactivation period). The antibiotics were added to prevent new protein synthesis after heat shock, which would obscure the results of Fda reactivation (Schröder et al 1993). Samples (7 mL) were collected at time 0 (immediately before the temperature shift to 45°C), 15 minutes after the transfer to 45°C, and further at 10-minute intervals during growth at 37°C. The cells were pelleted from the samples, resuspended in 100 mM Tris-HCl buffer, pH 7.5, (0.7 mL), and lysed in BioBlock Sonifier Vibra Cell (five 9- second pulses with 9-second intervals, amplitude 20%, on ice). Cell debris was sedimented at 1200 × g for 10 minutes. Samples of cell lysates (0.3 mL) were withdrawn, and the coupled enzyme assay for the fructose-1,6-bisphosphate cleavage activity of Fda was carried out as described by Sigma Quality Test Procedure (Bergmeyer 1984) at 25°C, and the decrease in A340nm was measured.
Thermal inactivation and reactivation of Fda in vitro
The purified Fda (170 nM, in 100 mM Tris-HCl pH 8.0 buffer containing 10 mM MgCl2 and 0.3 mM ZnCl2) was heat denatured during 10-minute incubation at 55°C (∼90% of the native Fda activity was lost). Subsequently, ATP (1 mM) and Hsps in the molar ratios (Fda)2:DnaK: DnaJ:(GroEL)14:(GroES)7 = 1:5:2.5:1:1 were added. Total volume of a reaction mixture was 200 μL. The Fda activity was measured after 60-minute incubation at room temperature as described previously.
When the ability of Hsps to protect Fda from thermal inactivation was tested, Hsps and ATP were added before incubation at 55°C. The solutions were then centrifuged at 14 000 × g for 15 minutes, and supernatants and precipitates were analyzed by 0.1%SDS-12%PAGE. Electrophoresed proteins were blotted to nitrocellulose membrane (Serva), and Fda was detected by Western blotting followed by scanning densitometry (see the following discussion).
Anti-Fda serum
The purified Fda was used as an immunogen for anti-Fda serum preparation (Harlow and Lane 1988). The serum (diluted 1:200) reacted with 0.1 μg of the purified Fda. Before use, unspecific antibodies were removed by affinity chromatography on 1-mL HiTrap NHS-activated Sepharose column (Amersham Pharmacia, Vienna, Austria) with the purified Fda as a ligand according to the manufacturer's recommendation (Amersham Pharmacia).
Cell fractionation and isolation of the S fraction
The S fraction containing proteins aggregated by heat shock was prepared from E coli W3350 (wt) for identification of several of its proteins and from E coli W3350[pKEN8] for estimation of the Fda levels after heat shock and after activity regenerating treatments. Sucrose density gradient ultracentrifugation as described by Kucharczyk et al (1991) and Kędzierska et al (1999) was applied. Briefly, the bacterial cultures (100 mL) were submitted to heat shock (see “E coli Strains and Growth Conditions”), then quickly chilled by pouring them onto 100 mL of frozen 10 mM Tris-HCl, pH 7.4. The cells were harvested by centrifugation by 10 minutes at 7000 × g and then suspended in 200 mM Tris-HCl, pH 7.4, so as to obtain A600 = 28 U/mL, and the spheroplasts were made according to Witholt et al (1976). The suspension was diluted with an equal volume of 1 mM sucrose in 200 mM Tris-HCl, pH 7.4, and then supplemented with egg-white lysozyme solution (12 mg/mL in 100 mM EDTA, pH 7.4) to a final concentration of 60 μg/mL. After 4 minutes on ice, an equal volume of ice-cold distilled water containing 2 mM PMSF and 1 mM DTT was added. After an additional 10 minutes on ice, the spheroplasts were subjected to sonication. Unbroken cells were removed by centrifugation. The supernatant was used for isolation of the S fraction. It was layered on 2-step sucrose-density gradient (SG0) composed of 1 mL 55% and 5 mL 17% (w/w) sucrose in 3 mM EDTA solution, pH 7.4, and centrifuged for 90 minutes in a Beckman SW41- Ti rotor at 240 000 × g. A 4-mL fraction was collected from the top of the gradient. It contained soluble cytoplasmic and periplasmic proteins. Opalescent fractions containing bacterial membranes and the heat-aggregated proteins were collected together (crude membrane fraction) and submitted to fractionation in a 6-step sucrose- density gradient (SG1) that consisted of 55% (1.4 mL); 50%, 45%, and 40% (2.3 mL of each); and 35% (1.4 mL) (w/w) sucrose in 3 mM EDTA solution, pH 7.4. After centrifugation in a Beckman SW41-Ti rotor at 240 000 × g for 16 hours, 30 subfractions were collected from the bottom (total volume was 12 mL). Aliquots (50 μL) were taken from SG1 subfractions for determination of the protein concentration (Bradford 1976). The subfractions (numbers 2–4; buoyant density, 1.26 g/mL) constituting the S fraction were pooled, diluted with water (to 5% of sucrose), and concentrated by centrifugation for 30 minutes at 120 000 × g in a Beckman SW41-Ti rotor. The sediment was suspended in water so as to obtain 1 μg/ μL of protein.
2D-gel electrophoresis and SDS-PAGE
2D-gel electrophoresis used for isolation of a few proteins from the S fraction was performed as described by O'Farrell (1975). Isoelectrofocusing in the first dimension (1.6%pH 6–8 and 0.4%pH 3.5–10 carrier ampholyte mixture; Pharmacia) and 0.1%SDS-15%PAGE (Laemmli 1970) in the second were performed. To decrease blocking of N-terminal amino acids, 0.1 mM sodium thioglycolate (Sigma) was added to electrophoresis buffer. The preelectrophoresis was run for 2 hours at 3 mA with buffer containing 0.05 mM glutathione (Sigma). Following the electrophoresis (30 mA, 5 hours, at 4°C), proteins were electrotransferred to PVDF membrane (Serva, Heidelberg, Germany) (Wilson and Yuan 1989). The proteins were visualized by staining with Ponceau-S (Sigma). Arbitrarily chosen protein spots were cut out from the membrane and used directly for microsequencing. The N-terminal amino acids sequence analysis was performed on a gas- phase sequencer (Perkin Elmer, Applied Biosystems, USA, Norwalk, CT) at BioCenter by P. Mak (Jagiellonian University, Krakow, Poland). The phenylthiohydantoin derivatives were analyzed by on-line gradient high-performance liquid chromatography (Perkin Elmer, Applied Biosystems).
Western blotting analysis and scanning densitometry
Western blotting was performed as described by Harlow and Lane (1988). Addition of anti-Fda antibody (rabbit) was followed by a peroxidase-coupled goat anti-rabbit second antibody, 4-chloro-1-naphtol, and H2O2 served as substrates. Scanning densitometry of the Fda was carried out using UVP E.A.S.Y. densitometry system (Ultra-Violet Products, Cambridge, UK). In this system, 100% of spot density corresponds to that of the spot of the highest density on the developed immunoblots.
RESULTS
Identification of several protein components of the S fraction
Numerous protein spots of different intensity were discernible on 2D electrophoregram of the S fraction from E coli W3350. With the purpose of selection of native substrates for the heat shock chaperones and/or proteases participating in reactivation or removal of the S fraction, we identified some of its protein components. Four protein spots were arbitrarily chosen, and N-terminal, 10-aa sequences of the proteins were determined: (1) MYVVSTKQMLN, (2) AVTKLVLVRH, (3) SKIFDFVKPG, and (4) MDYTLTRID. The computer search of the Swiss Prot database by FASTA program revealed that the N- terminal sequences were identical with those of (1) tagatose-1,6-bisphosphate aldolase, (2) phosphoglycerate mutase I, (3) fructose-1,6-bisphosphate aldolase, and (4) NADH dehydrogenase I chain B. Fda was chosen as a test substrate for further studies. It is the fda gene product, Fda protein of Mr 39016, active as a homodimeric enzyme (EC 4.1.2.13) with the absolute requirement of the divalent metal ion (Zn2+) for its catalytic activity, thus belonging to the class II aldolases (Alefounder et al 1989; Packman and Berry 1995). It is quite thermoresistant (half-life of the purified Fda: 60 days at 25°C; 6 days at 37°C; 10 minutes at 50°C; Henderson et al 1994). Its crystal structure has been determined recently (Hall et al 1999) by X-ray diffraction studies to a resolution of 2.0 Å.
Role of Hsps in reactivation of thermally inactivated Fda in vivo
E coli strains bearing mutations in dnaK, dnaJ, groES, groEL, and ibpA/B were transformed with the pKEN8- overproducing Fda because the level of activity of Fda in nontransformant cells was too low for reliable measurements.
Since we found Fda in the S fraction, we expected that it would be inactivated in the heat shock conditions (ie, 45°C, 15 minutes) used in previous experiments (Kucharczyk et al 1991; Laskowska et al 1996a; Kędzierska et al 1999). In preliminary experiments, the level of the native Fda activity was determined in all the strains used (transformed with pKEN8-overproducing Fda) (Table 1). The activity was from 1.02 to 1.35 U/mg of total protein with the exception of that in dnaK (0.89 U/mg) and dnaJ (0.65 U/mg) mutant cells. Since these strains have the same genetic background as their parent B178, the low level of Fda activity may be ascribed to the lack of the completely active DnaK and DnaJ chaperone proteins, necessary for the protein maturation.
Table 1.
The Fda activity levels in Escherichia coli strains overproducing Fda from pKEN8 plasmid
Figure 1 presents the levels of the Fda activity in cells before and after heat shock and during reactivation period. In wt cells, the level of Fda activity fell to 80% of its native value after heat shock and was recovering stepwise so that it reached 94% in the 45th minute of the experiment. In contrast, a significant decrease of the Fda activity was observed in the case of mutants dnaK756 (to 54%) and dnaJ259 (to 46%) in the 15th minute after heat shock; moreover, during incubation at 37°C, not only did the reactivation not take place but the activity continued dropping and achieved 39% and 31% of the starting level, respectively, in the 45th minute.
Fig 1. Effect of mutations in Hsp genes on the inactivation and recovery of active Fda in E coli cells submitted to heat shock. E. coli B178 (wt); E coli B178 bearing mutations dnaK756, dnaJ259, groES619, and groEL44; and E coli AJW101ibpA/B, transformed with pKEN8-overproducing Fda, were grown at 30°C. At time 0, the bacteria were submitted to heat shock (45°C, 15 minutes) in the presence of kanamycin or tetracycline and then transferred to 37°C. Samples were collected immediately after the heat shock (15 minutes) or after 10 minutes (25th minute of experiment), 20 minutes (35th minute of experiment), and 30 minutes (45th minute of experiment) further incubation at 37°C. Lightly shadowed bars, native Fda activity just before heat shock (30°C), are taken as 100%, black bars as % of the Fda activity after 15 minutes at 45°C, and shadowed bars as % of the Fda activity during the reactivation by incubation at 37°C. The experiment was done 5 times with each strain, and the deviation in the values was ±5%
The groES619 and groEL44 mutations exerted less pronounced effects. The Fda activity was lowered to 78% and 71%, respectively, but it started to recover in the 45th minute, reaching 85% and 78%. The deletion of the ibpA/ B operon had slight influence, and the Fda activity levels were close to those in the wt strain. These experiments pointed to the particular importance of DnaK and DnaJ proteins for the Fda activity level in cells.
Reactivation in vitro of the Fda present in the S fraction from E coli W3350[pKEN8]
Results of the experiment on reactivation of Fda present in the S fraction (Fig 2) demonstrated that if the aggregated proteins were suspended in Tris buffer without ATP (2 mM), practically no enzyme reactivation was observed after 90 minutes at room temperature (column 4). However, addition of solely ATP brought about reactivation of 0.177 U/mg of the S fraction (column 5). It was interesting that addition of GroEL/GroES or DnaK/DnaJ in the presence of ATP increased the reactivation level only to 0.20 U/mg (columns 2 and 3), while both these Hsps systems together enhanced the level to 0.24 U/mg (column 1). This observation was in accord with the earlier demonstration of the presence of Hsps in the S fraction (Kucharczyk et al 1991) and turned our attention to the fact that the Hsps must be associated so strongly with the S fraction that despite the long procedure of the S fraction isolation, apparently they were still associated and active when only ATP was provided. Their concentration seemed to be so close to the optimal one that the Hsps additions had only a moderate effect.
Fig 2. Reactivation in vitro of the Fda present in the S fraction from E coli W3350 [pKEN8]. The bacteria were grown and submitted to heat shock, and the S fraction was isolated as described in “Cell Fractionation and Isolation of the S Fraction.” For each reaction, 50 μg of the S fraction proteins were used. The reactions were performed in Tris buffer supplemented with MgCl2 and ZnCl2. The control did not contain any additions (column 4); to the other samples, ATP (2 mM) or ATP and Hsps (5 μg of each, except of DnaJ-2.5 μg) were added as indicated. The Fda activity was measured after 90 minutes of incubation at room temperature; no Fda activity was detected in the S fraction before the reactivation. The reactions were carried out 3 times, and the deviation in the values was ±5%
The effect of Hsps on protection of the Fda from aggregation in vitro
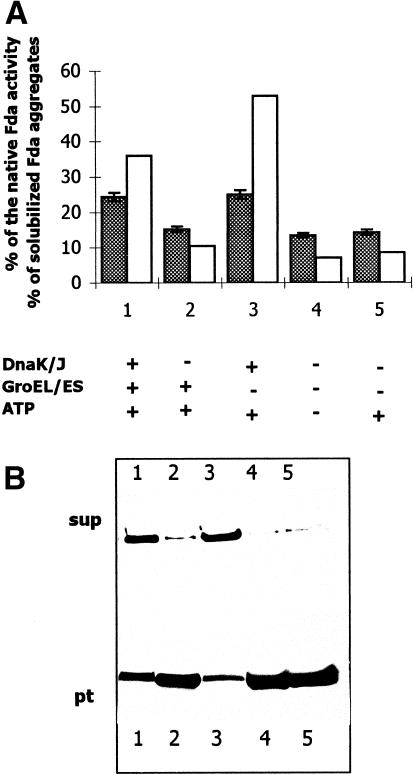
To test the Fda protection from thermal aggregation and inactivation, the Hsps in different combinations (as indicated in Fig 3) were mixed with Fda and ATP before heat shock (55°C for 10 minutes). The aggregated Fda was sedimented. The supernatant and the sediment were analyzed by 0.1%SDS-12%PAGE followed by immunoblotting (with anti-Fda antibodies) and densitometric estimations (Fig 3B). Concomitantly, Fda activity was determined (Fig 3A). It was found that the pellets did not contain the Fda activity (results not shown). In the control samples, containing only Fda in buffer or Fda supplemented with ATP, 13% to 15% of activity remained after heat shock (Fig 3A, columns 4 and 5, shaded areas). In the presence of DnaK/DnaJ, 25% of the Fda activity remained (column 3, shaded). The presence of GroEL/ GroES (column 2, shaded) did not protect appreciably the Fda activity level, which was only 2% to 3% higher than that of the control. Contrary to expectation, the simultaneous presence of DnaK/DnaJ and GroEL/GroES (column 1) did not increase the protective effect over that provided by DnaK/DnaJ alone (column 3). However, maintenance of Fda in a soluble form appeared as another aspect of the experiment (Fig 3A, open areas, and 3B, the corresponding lanes on immunoblots). The best protection of Fda from aggregation at 55°C was provided by DnaK/DnaJ (Fig 3A, column 3, and 3B, lane 3), resulting in 53% of the protein remaining soluble (though partly inactive). It was evident that GroEL/GroES (Fig 3A, column 2) had little influence on preserving the soluble form of Fda. The addition of GroEL/GroES (Fig 3A, column 1) together with DnaK/DnaJ not only did not increase the amount of the Fda protected by DnaK/DnaJ alone but even decreased it to about 36% of Fda remaining soluble. However, this result could be an artifact since the GroEL/GroES, active in forms of oligomeric ring structure (Horowich et al 1999), might be affected by such an extreme temperature (55°C) and undergo disassembly and denaturation. If so, they might enlarge the fraction of aggregated proteins, thus obscuring the results.
Fig 3. The effect of DnaK/DnaJ and GroEL/GroES chaperone systems on the protection of Fda from thermal aggregation in vitro. Fda in Tris buffer was first preincubated at room temperature for 10 minutes in the presence of the Hsps (as indicated below the figure and in molar ratios as given in “Thermal inactivation and reactivation of Fda in vitro”), then inactivated at 55°C. After the denaturation, the aggregated protein was sedimented. The supernatants and the pellets were used for Fda activity determination (A, shadowed bars). The pellets did not contain the Fda activity. For estimation of the solubilization of Fda aggregates (A, open bars), the supernatants (sup) and the pellets (pt) were also analyzed by Western blotting (B) with the anti-Fda serum followed by scanning densitometry. The reactions were carried out 3 times, and the deviation in the values was ±5%
DnaK/DnaJ system appeared to play the key role in Fda protection from aggregation and to a lesser degree from inactivation of enzymatic activity in the conditions of the experiment. In vivo, the denaturation would occur at the lower temperature (45°C), and the loss of activity of cooperating GroEL/GroES system might not occur, but, even if so, the loss would be compensated by induced synthesis of the Hsps. Therefore, in the next experiment the Hsps were added after heat shock, which should reveal their effect on the reactivation of the previously denatured Fda.
Reactivation of heat-denatured Fda in vitro by Hsps
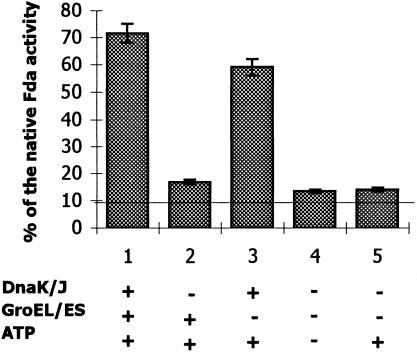
The reactivation of Fda in vivo required mainly the assistance of DnaK/DnaJ, as was inferred from the previous experiments (Figs 1 and 3). To confirm this in vitro, the heat-denatured Fda was incubated with DnaK/DnaJ or GroEL/GroES or with both these systems in the presence of ATP, at room temperature, for 60 minutes (Fig 4). In the absence of the chaperones (control samples), denatured Fda regained 2% to 3% of its activity in the presence or in the absence of ATP (Fig 4, columns 4 and 5). The reactivation occurred and reached 59% in the presence of DnaK, DnaJ, and ATP (column 3). The process was enhanced to 71.5% (column 1) by addition of GroEL/ GroES. However, the denatured Fda practically was not reactivated by GroEL/GroES system alone (column 2). These results demonstrated the dominant role of the DnaK/DnaJ system in the reactivation of heat-denatured Fda in vitro. The addition of high concentrations of the Hsps, up to a 100-fold molar excess, did not affect significantly the reactivation level of the denatured Fda (data not shown).
Fig 4. Reactivation in vitro of thermally inactivated Fda in the presence of ATP and Hsps: DnaK/DnaJ or GroEL/GroES or both systems together. Fda in Tris buffer was denatured at 55°C, then supplemented with MgCl2, ZnCl2, ATP, and Hsps (as indicated at the bottom and in molar ratios as given in “Thermal inactivation and reactivation of Fda in vitro”). The reaction mixtures were left for 60 minutes at room temperature for Fda reactivation, then Fda activity was measured. Other details are given in “Thermal inactivation and reactivation of Fda in vitro.” Native Fda activity just before the denaturation at 55°C is taken as 100%; % of the Fda activity remaining after the denaturation is marked by the broken line; the bars represent the % of the Fda regaining activity. The reactions were carried out 3 times, and the deviation in the values was ±5%
DISCUSSION
According to the in vivo and in vitro experiments reported here, the DnaK and DnaJ chaperones are essential for the resolubilization and reactivation of an endogenous protein, the heat-inactivated and aggregated Fda.
The inability of the dnak756 and dnaJ259 mutants to reactivate Fda (Fig 1) and the low levels of Fda in these strains (Table 1) may also concern other cellular proteins and account for the low viability of the mutants at elevated temperature. Supposedly, the denatured proteins generated in the absence of active DnaK and DnaJ are degraded.
The in vitro experiments establish the dominant role of DnaK/DnaJ in the protection from aggregation and in reactivation of denatured Fda (Figs 3 and 4). These molecular chaperones bind to unfolded proteins, protect them from illegitimate interactions, and promote their refolding into active structures (Langer et al 1992). Our results illustrate the disaggregating capacity of the DnaK/ DnaJ chaperone system. Such activity had been previously demonstrated in vitro only for 2 bacterial proteins: RNA polymerase (Skowyra et al 1990) and glucose-6- phosphate dehydrogenase (Diamant et al 2000). The disaggregating activity of DnaK could rely on its ability to disassemble protein oligomers (Parsell et al 1994). The action of DnaK/DnaJ was slightly enhanced by GroEL/ GroES, while GroEL/GroES alone were inefficient in solubilizing or reactivating thermally aggregated Fda (Figs 3 and 4).
Addition of ATP to the S fraction from wt cells is sufficient to reactivate a considerable part of Fda. The S fraction contains DnaK and DnaJ (Kucharczyk et al 1991), and its chaperone content is adequate for reactivation of the aggregated proteins. This observation accounts for the rapid disappearance of the S fraction when the culture is transferred to 37°C. It also sheds light on the specific properties of the S fraction proteins (Kędzierska et al 1999). The conformational stability of the S fraction proteins might differ from that of the in vitro denatured proteins because of the high in vivo and low in vitro levels of Hsps during the heat treatment. DnaK and DnaJ may preserve proteins in an appropriate structure, competent for refolding. In vivo, these Hsps are rapidly induced along the thermal denaturation of the cellular proteins. In a cell-free system, denaturation proceeds with the constant basal level of Hsps initially present in the extract.
It was shown previously (Kędzierska et al 1999) that the accumulation of the S fraction in E coli wt cells results from a transient insufficient supply of Hsps just after heat shock. The S fraction does not form when a 4-fold excess of DnaK/DnaJ or GroEL/GroES system was artificially expressed in cells without heat shock. Hence, we propose that the denaturation and aggregation of Fda inside cells occurring at the temperature lower than in vitro results from the limited availability of Hsps rather than from a direct temperature effect.
Acknowledgments
We are grateful to B. Lipińska, M. Żylicz, and P. Lund for E coli strains and T. Kaczorowski for M13/T7 phage. We thank K. Liberek and M. Żylicz for generous gifts of purified DnaK, DnaJ, GroES, and GroEL proteins. This work was supported by the Polish State Committee for Scientific Research (grant 386/PO4/97/13 to A.T.) and UG grant BW/1160-5-0125-0 (to S.K.).
REFERENCES
- Alefounder PR, Baldwin SA, Perham RM, Short NJ. Cloning, sequence analysis and overexpression of the gene for the class II fructose 1,6-bisphosphate aldolase of Escherichia coli. Biochem J. 1989;257:529–534. doi: 10.1042/bj2570529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU 1984 Methods of Enzymatic Analysis, vol 4. Verlag Chemie, Weinheim. [Google Scholar]
- Berry A, Marshall KE. Identification of zinc-binding ligands in the class II fructose-1,6-bisphosphate aldolase of Escherichia coli. FEBS Lett. 1993;318:11–16. doi: 10.1016/0014-5793(93)81317-s. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell A. Sensitive mutants of bacteriophage lambda. Virology. 1961;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Diamant S, Peres Ben-Zvi A, Bukau B, Goloubinoff P. Size- dependent disaggregation of stable protein aggregates by the DnaK chaperone machinery. J Biol Chem. 2000;275:21107–21113. doi: 10.1074/jbc.M001293200. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. A new bacterial gene (groPC) which affects DNA replication. Mol Gen Genet. 1977;151:35–39. doi: 10.1007/BF00446910. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Herskovitz I 1971 Escherichia coli mutants blocked in lambda DNA synthesis. In: The Bacteriophage lambda, ed Hershey AD. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 553–564. [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, Mogk A, Peres Ben-Zvi A, Tomoyasu T, Bukau B. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc Natl Acad Sci USA. 1999;96:13732–13737. doi: 10.1073/pnas.96.24.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragerov A, Nudler E, Komissarova N, Gaitanaris GA, Gottesman ME, Nikiforov V. Cooperation of GroEL/GroES and DnaK/DnaJ heat shock proteins in preventing protein misfolding in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:10341–10344. doi: 10.1073/pnas.89.21.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DR, Leonard GH, Reed CD, Watt CI, Berry A, Hunter WN. The crystal structure of Escherichia coli class II fructose- 1,6-bisphosphate aldolase in complex with phosphoglycolohydroxamate reveals details of mechanism and specificity. J Mol Biol. 1999;287:383–394. doi: 10.1006/jmbi.1999.2609. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D 1988 Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Henderson I, Garcia-Junceda E, Liu KK-C, Chen Y-L, Shen G-J, Wong C-H. Cloning, overexpression and isolation of the type II FDP aldolase from E. coli specificity study and synthetic application. Bioorg Med Chem. 1994;2:837–843. doi: 10.1016/s0968-0896(00)82183-5. [DOI] [PubMed] [Google Scholar]
- Horowich AL, Weber-Ban EU, Finley D. Chaperone rings in protein folding and degradation. Proc Natl Acad Sci U S A. 1999;96:11033–11040. doi: 10.1073/pnas.96.20.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JE, Hartley DL. Formation of recombinant protein inclusion bodies in E. coli. Trends Biotechnol. 1988;6:95–101. [Google Scholar]
- Kędzierska S, Staniszewska M, Wegrzyn A, Taylor A. The role of DnaK/DnaJ and GroEL/GroES systems in the removal of endogenous proteins aggregated by heat-shock from Escherichia coli cells. FEBS Lett. 1999;446:331–337. doi: 10.1016/s0014-5793(99)00154-4. [DOI] [PubMed] [Google Scholar]
- Kucharczyk K, Laskowska E, Taylor A. Response of Escherichia coli cell membranes to induction of lambda cI857 prophage by heat shock. Mol Microbiol. 1991;12:2935–2946. doi: 10.1111/j.1365-2958.1991.tb01853.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer T, Lu C, Echols H, Flanagan J, Hayer MK, Hartl FU. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Laskowska E, Wawrzynów A, Taylor A. IbpA and IbpB, the new heat-shock proteins, bind to endogenous Escherichia coli proteins aggregated intracellularly by heat shock. Biochimie. 1996a;78:117–122. doi: 10.1016/0300-9084(96)82643-5. [DOI] [PubMed] [Google Scholar]
- Laskowska E, Kuczyńska-Wiśnik D, Skórko-Glonek J, Taylor A. Degradation by proteases Lon, Clp and HtrA, of Escherichia coli proteins aggregated in vivo by heat shock; HtrA protease action in vivo and in vitro. Mol Microbiol. 1996b;22:555–571. doi: 10.1046/j.1365-2958.1996.1231493.x. [DOI] [PubMed] [Google Scholar]
- Lipińska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Tomoyasu T, Goloubinoff P, Rüdiger S, Röder D, Langen H, Bukau B. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999;18:6934–6949. doi: 10.1093/emboj/18.24.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Packman LC, Berry A. A reactive, surface cysteine residue of the class-II fructose-1,6-bisphosphate aldolase of Escherichia coli revealed by electrospray ionisation mass spectrometry. Eur J Biochem. 1995;227:510–515. doi: 10.1111/j.1432-1033.1995.tb20417.x. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, and Maniatis T 1989 Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Schröder H, Langer T, Hartl FU, Bukau B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein. EMBO J. 1993;12:4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Georgopoulos C, Żylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990;62:939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Spiess C, Bell A, Ehrmann E. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- Strauch KL, Johnson K, Beckwith J. Characterisation of DegP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KJ, Yuan PM 1989 Protein and peptide purification. In: Protein Sequencing: A Practical Approach, ed Findlay JBC, Geisow MJ. Oxford University Press, New York, NY, 1–41. [Google Scholar]
- Witholt B, Boekhout M, Broek M, Kingma J, van Heerikhuizen H, de Leij L. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976;74:160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Yochem J, Uchida H, Sunshine M, Georgopoulos C. Genetic analysis of two genes, dnaJ and dnaK, necessary for Escherichia coli and bacteriophage lambda DNA replication. Mol Gen Genet. 1978;164:9–14. doi: 10.1007/BF00267593. [DOI] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J, Fayet O, Baird L, Georgopoulos C. Sequence analysis and phenotypic characterization of groEL mutations that block and T4 bacteriophage growth. J Bacteriol. 1993;180:1134– 1143. doi: 10.1128/jb.175.4.1134-1143.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkiewski M. ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation: a novel multi-chaperone system from Escherichia coli. J Biol Chem. 1999;274:28083–28086. doi: 10.1074/jbc.274.40.28083. [DOI] [PubMed] [Google Scholar]