Abstract
Antibodies against heat shock or stress proteins (Hsps) have been reported in a number of diseases in which they may be involved in the pathogenesis of the disease or may be of use for prognosis. Heat-induced diseases, such as heat cramps, heat exhaustion, or heat stroke, are frequent in hot working or living environments. There are still few investigations on the presence and possible significance of autoantibodies against Hsps in heat-induced illnesses. Using an immunoblotting technique with recombinant human Hsps, we analyzed the presence and titers of antibodies against Hsp60, Hsp71, and Hsp90α, and Hsp90β in a group of 42 young male patients who presented with acute heat-induced illness during training. We also examined the presence of antibody against Hsp71 in a second group of 57 patients with acute heat-induced illness and measured the changes in titers of anti-Hsp71 antibodies in 9 patients hospitalized by emergency physicians. In the first group of young persons exercising in a hot environment, the occurrence of antibodies against Hsp71 and Hsp90α was significantly higher among individuals with symptoms of heat-induced illness (P < 0.05) than in the matched group of nonaffected exercising individuals. Moreover titers of antibody against Hsp71 were higher in individuals of the severe and mild heat-induced illness groups, the highest titer being found in the most severe cases. The results from the second group of 57 heat-affected patients exposed to extreme heat were similar. Again, patients with the more severe heat-induced symptoms showed a significantly higher incidence of antibodies to Hsp71 than controls and the titer of anti-Hsp71 was higher in the severely affected group. Finally, in a study of 9 patients, it was observed that the titer of anti-Hsp71 decreased during recovery from severe heat symptoms. These results suggest that measurement of antibodies to Hsps may be useful in assessing how individuals are responding to abnormal stress within their living and working environment and may be used as one biomarker to evaluate their susceptibility to heat-induced diseases.
INTRODUCTION
All organisms react to exposure to supraoptimal temperatures by inducing the synthesis of heat shock or stress proteins (Hsps). Synthesis of Hsps is induced not only by heat but also by a variety of noxious stimuli, including physiological stresses such as ischemia, fever, viral infection, and environmental xenobiotics or chemical stressors such as heavy metals, free radicals, and carbon monoxide (Craig 1985; Lindquist 1986; Lindquist and Craig 1988; Morimoto et al 1994; Wu et al 1996). Many of these stimuli are common in the working or living environment. The ubiquitous nature of this response and its phylogenetic conservation suggest that Hsps are essential for cell survival. Hsps function as molecular chaperones, facilitating the synthesis, folding, assembly, and intracellular transport of many proteins (Hightower 1991; reviewed in Morimoto et al 1994; Bohen et al 1995; Hartl 1996). Another important function of Hsps is protection against cell and organ damage. This has been documented for the acquisition of thermotolerance in cultured cells (Landry et al 1982, 1989; Li and Werb 1982; Laszlo 1988; Angelidis et al 1991; Li et al 1991; Rollet et al 1992; Mehlen et al 1995; Parsell and Lindquist 1994) and in transient protection from ischemic injury in whole organs such as the heart, brain, and kidney (Currie et al 1993; Marber et al 1995; Plumier et al 1995; Krueger et al 1999; Beck et al 2000; Morrison et al 2000). In addition, Hsps also seem to play roles in the processes of growth, differentiation, and development (Arrigo and Tanguay 1991; Tanguay et al 1993; Loones et al 1997; Michaud et al 1997).
Many observations have shown links between the aberrant expression of stress proteins and disease states (Welch 1992; Minowada and Welch 1995). Some of the Hsps can also present as self-antigens to the immune system, resulting in the production of autoantibodies to Hsps in patients with inflammatory diseases, autoimmune disorders, hypertension, or atherosclerosis or after various infections caused by viruses, bacteria, mycobacteria, and parasites (reviewed in Burdon 1993; Kaufmann and Schoel 1994; Schett et al 1995; Frosttegard et al 1997; Xu et al 1993, 1999). It has been suggested that antibodies against Hsps might be of significance in the pathogenesis and/or prognosis of some diseases (Jarjour et al 1991; Schett et al 1995; Shingai et al 1995; Wu et al 1998; Xu et al 1993, 1999). However, it has been reported that Hsps and antibodies to Hsps are found in the serum of normal individuals (Pockley et al 1999).
The temperatures of the living and/or working environments are particularly high in some instances, leading to heat-induced illnesses and heat-related diseases. The former includes heat syncope, heat rash, heat cramps, heat exhaustion, and heat stroke in order of increasing severity. Heat stroke is a medical emergency characterized by hyperpyrexia, impairment of the level of consciousness, and occasionally multiorgan damage and dysfunctions (Clowes et al 1974; Alzeer et al 1997). These conditions may be related to the functions of Hsps.
We have previously reported that workers who experience abnormal chemical stress within their working environment showed a high incidence of antibodies against Hsp71 and suggested that antibodies against Hsps could potentially be useful biomarkers for environmental stress (Wu et al 1995, 1996, 1998). Although Hsps were first discovered in heat-shocked organisms and one of their important functions shown to protect against thermal stress, the possible significance and presence of Hsps and/or antibodies against Hsps in heat-induced diseases have not been documented previously. In the present study, we investigated the presence of antibody against Hsps in plasma from 2 groups of patients with acute heat-induced illness and measured changes in the titer of antibodies to Hsp71 before and after treatment of hospitalized patients.
MATERIALS AND METHODS
Groups
The present study was conducted on 2 types of patients differing in age and in activities (exercise). The first group (group A) consisted of young (18- to 24-year-old) male patients (42 patients) with heat-induced illnesses incurred during exercise in a hot environment (Nanjing) and matched controls (30 persons who exercised in the same hot environment without any illness). The second group (group B) was more heterogeneous, with ages ranging from 25 to 81 years. Group B consisted of 57 patients (33 men and 24 women) with acute heat-induced diseases who were seen and diagnosed by emergency physicians from the 4 central hospitals in Nanjing. Controls consisted of 30 individuals matched for sex (17 men, 13 women) who voluntarily participated in the study and had no detectable diseases after medical examination. All patients were diagnosed according to diagnostic criteria and principles of management of occupational heat illness (see below). These patients were further divided under mild heat-induced and severe heat-induced illness, including heat cramps, heat exhaustion, and heat stroke, according to their heat exposure, oral temperature, and clinical characteristics. These characteristics included painful spasms of the voluntary muscle of the abdomen and extremities, nausea and vomiting, diarrhea, weakness, pale skin, tachycardia, hypotension, headache, dizziness, visual disturbances, confusion, hot and dry skin, and laboratory examination.
Evaluation of health status
Health status was evaluated in all patients and matched controls using a questionnaire and clinical and laboratory examinations. The questionnaire designed according to diagnostic criteria and principles of management of occupational heat illness (GB11508–89) and (Petersdorf and Root 1987) also focused on disease history, lifestyle, and the presence of possible inducing factors of acute heat-induced illness. The questions were directly asked by the emergency physicians to the patient when he or she was conscious or to his or her relatives when the patient was unconscious. The clinical examination included a general examination (signs), determination of oral and rectal temperature, pulse, respiratory rate, and blood pressure. The physical examination was complemented by electrocardiography and chest x-ray examination. Hemoglobin, blood typing, and white cell counts were determined in blood from a finger puncture. Venous blood was also collected and divided into 2 parts, one of which was mixed with heparin to separate plasma. The second part was used for the measurement of electrolytes (Na+, K+, Cl−, Ca++, CO2), urea, glucose, hormones (growth hormone, insulin, cortisol, aldosterone), and activities of alkaline phosphatase (ALP), lactic dehydrogenase (LDH), and creatine kinase (CK), using the corresponding kits from Biochemical Reagents Company (Beijing).
Determination of antibodies to Hsps in plasma of patients
Recombinant human Hsp60, Hsp71, Hsp90α, and Hsp90β were obtained through the expression of the corresponding complementary DNAs in Escherichia coli BL2 (DE3) cells using a pET vector (Novagen) as described earlier (Tanguay et al 1993). Approximately 10–15 μg of each recombinant human Hsp was loaded on each sodium dodecyl sulfate–polyacrylamide gel electrophoresis without comb and transferred electrophoretically to nitrocellulose membranes (NCs). The transfer was monitored by staining with rouge ponceau S (Carbajal et al 1986). The NC containing a specific Hsp was then cut into 2 by 2-mm pieces and marked with a small red dot on the protein side of the NC. Membrane pieces were placed in individual wells of enzyme-linked immunosorbent assay (ELISA) plates, rinsed with phosphate-buffered saline (PBS), and saturated with 100 μL of blocking buffer (5% skim milk in 0.05% PBS–Tween 80) for 1 hour at 37°C with gentle agitation. After washing with 0.05% PBS–Tween 80 for 5 minutes, the plasma was diluted 1:10, 1:20, 1:40, and 1:80 in 100 μL of blocking buffer, added to the NC pieces containing the Hsps, and incubated at 37°C for 2 hours with gentle agitation. The membranes were washed 6 times (10 minutes each) with 200 μL of 0.05% PBS–Tween 80 before adding 100 μL of horseradish peroxidase–labeled goat anti-human immunoglobulin G (Sigma, dilution 1:2500) in blocking buffer. After incubation at 37°C for 1 hour with gentle agitation, membranes were washed 6 times (10 minutes) with 200 μL of 0.05% PBS–Tween 80. The presence of antibodies to Hsps was revealed with 3,3-diaminobenzidine tetrahydrochloride buffer for 3–5 minutes. A brown band on NC is regarded as positive and no color on band as negative. The method, which is basically a Western blot assay in an ELISA plate to reduce volumes, is cost-effective and, although not the most sensitive technique, gives reproducible and reliable results under the laboratory conditions available.
Statistical analyses
Analysis was carried using a statistical analysis software (SAS/STAT release 6.03) package (SAS Institute Inc, Cary, NC). Other analyses were carried out based on the χ 2 test. Results are given as mean and SD. Statistical inferences are based on the different levels of significance (P < 0.05 or P < 0.01).
RESULTS
Characteristics and symptoms of patients
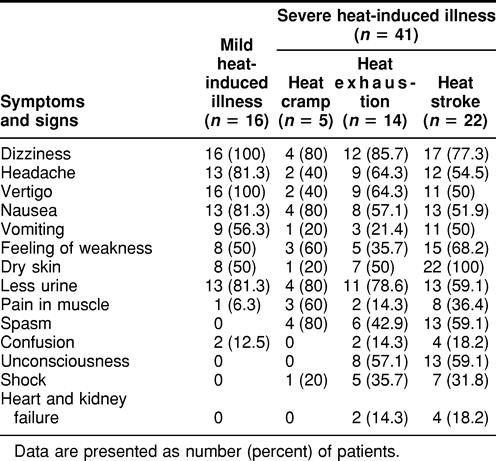
Tables 1 and 2 list some of the symptoms and signs in the 2 groups of patients (group A, young males trainees aged 18–24 years, and group B, mixed sex, aged 25–81 years) with acute heat-induced illness. The patients from group B who were severely affected were further subdivided into 3 groups, heat cramp, heat exhaustion, and heat stroke. As shown in these tables, the main clinical characteristics of these patients were painful spasms of the voluntary muscle of the abdomen and extremities, nausea and vomiting, diarrhea, weakness, pale skin, tachycardia, hypotension, headache, dizziness, visual disturbances, confusion, and hot and dry skin. However, spasm, confusion, unconsciousness, shock, and failure of heart and kidney were more frequently encountered in patients with severe heat-induced illness than in patients with mild heat-induced illness. This trend was seen in both groups, the young group A patients training in a hot environment (Table 1) and the group B patients (Table 2).
Table 1.
Symptoms and characteristics of mild and severe heat-induced illness in young male patients (group A, 18–24 years old) exercising
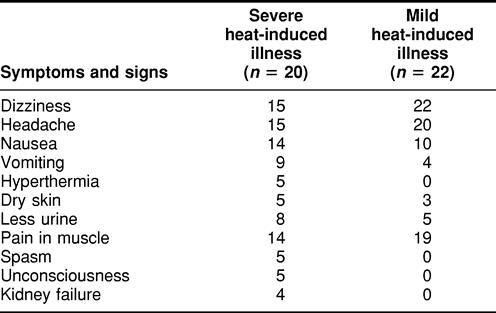
Table 2.
Symptoms and characteristics of heat-induced illness in mixed-sex patients (group B, 25–81 years old)
Physical and biochemical characteristics of group B patients with heat-induced illness at admission
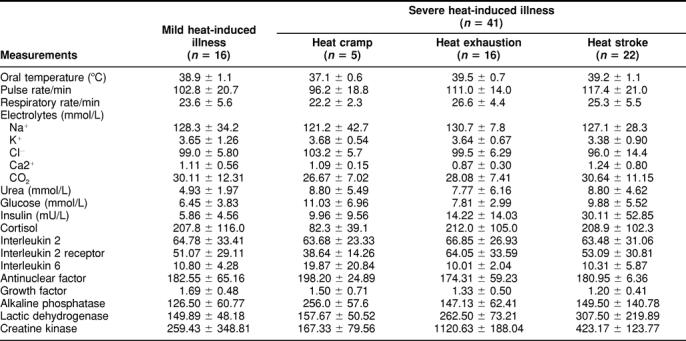
Table 3 lists the physical and biochemical characteristics of patients from group B (aged 25–81 years) with heat-induced diseases at admission. The physical analyses included oral temperature, pulse, and respiratory rate. Statistical analysis shows that there are no significant differences in oral temperature, pulse, and respiratory rate among these subgroups, although these indexes are higher than the normal level. Biochemical analyses included the concentration of electrolytes such as Na+, K+, Cl-, Ca++, and CO2; urea; glucose; hormones such as growth hormone, insulin, cortisol, and interleukin 2; and activities of ALP, LDH, and CK. Again, there were no significant differences in all these biochemical indexes among these subgroups, although there were large differences among individuals mainly in the levels of enzymes (ALP, LDH, CK).
Table 3.
Biochemical characteristics of group B patients with acute heat-induced illness at hospital admission
Presence of antibodies against Hsps in patients from the 2 groups
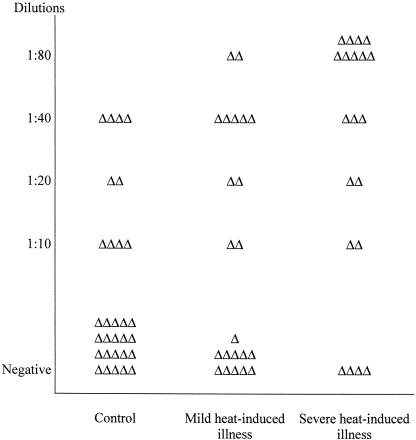
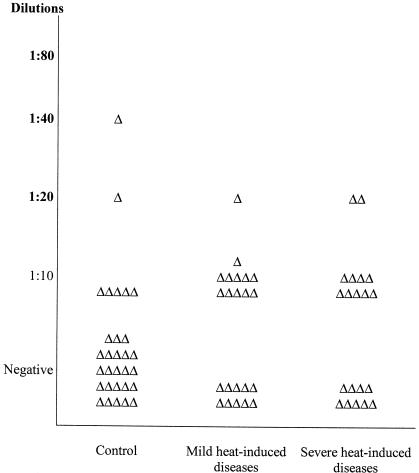
To know whether antibodies against Hsps might have a possible medical significance in patients with acute heat-induced diseases, we investigated the presence and titers of antibodies against Hsp60, the inducible member of the Hsp70 family, Hsp71, and Hsp90α and Hsp90β in the plasma of the 42 male patients in whom heat-induced illness resulted from exercise in a hot environment and in matched controls (group A). Table 4 shows that the occurrence of antibodies against Hsp71 and Hsp90α was significantly higher in the heat-induced illness group (P < 0.05) than in the matched control group. There was a similar trend but no significance difference for antibodies to Hsp60 and Hsp90β. The individual values for the titer of antibodies against Hsp71 and Hsp90α are plotted in Figures 1 and 2, respectively. The titer of antibody against Hsp71 is higher in the plasma of individuals from both the severe and mild heat-induced groups than in controls. Interestingly, the highest titer of antibody to Hsp71 measured (1:80) was only found in patients from the disease groups. Figure 2 shows that there is no significant difference in the distribution of antibody titers of individuals between the control and mild or severe groups, although the number of patients with antibodies against Hsp90α is significantly higher in heat-induced illness groups than in the control one (P < 0.05).
Table 4.
Presence of antibodies against heat shock proteins in plasma of group A patients (18–24 years old) with heat-induced illness and in a matched control group
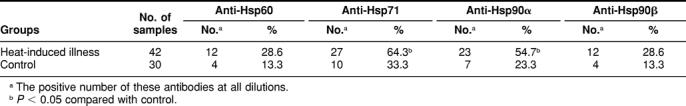
Fig. 1.
Distribution of individual titers of antibodies against Hsp71 in patients of group A (18–24 years old) exercising in a hot environment. Serum samples of individuals were diluted 1:10, 1:20, 1:40, and 1:80 and used in the miniblot assay with recombinant human Hsp71 as the immunogen as described in the Materials and Methods section. Each triangle represents the highest dilution giving a positive reaction in the assay for each individual within the group
Fig. 2.
Distribution of titers of antibodies against Hsp90α in patients of group A (18–24 years old) exercising in a hot environment. Serum samples were diluted as described in the legend of Figure 1 and used in the miniblot assay with Hsp90α as the immunogen. Triangles represents values for individuals as described in the legend of Figure 1.
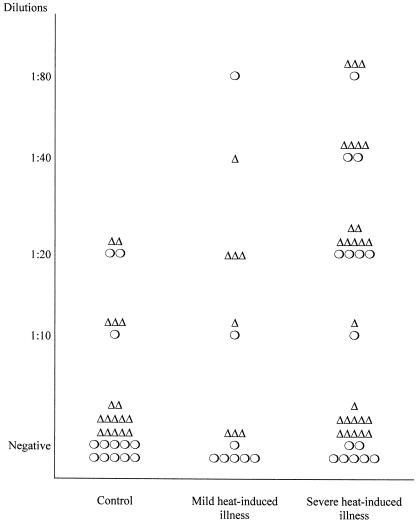
We next investigated the presence and titers of anti-Hsp71 in the plasma of 57 patients from a group of mixed patients with acute heat-induced illness, whose ages ranged from 25–81 years (group B). Illness in these patients occurred in a different hot living or working environment (not exercise). Table 5 summarizes the data on the presence and dilution of antibody against Hsp71 in the plasma of these patients. Antibodies to Hsp71 were found more frequently in the severe (23/41) and mild (7/16) subgroups than in controls (8/30). As shown in Figure 3, the average titer of antibodies to Hsp71 is higher in the severely affected group. However, the distribution of titers in these patients is similar to that found in group A (Fig 1). This figure also shows the distribution of antibody titer according to the sex of the patient. There were more men affected (33) than women (24). There are no clear reasons for this difference. We, therefore, used a matched control group with a similar ratio of men to women. As can be seen in Figure 3, there are no significant differences between sexes with respect to the antibody titers. The number of patients being limited, it was not possible in the present study to divide them into age classes.
Table 5.
Presence and titer of antibody against Hsp71 in plasma of group B patients (25–81 years old) with acute heat-induced illness
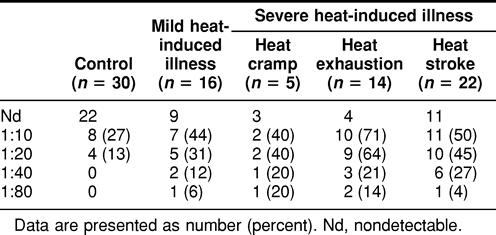
Fig. 3.
Distribution of titers of antibodies against Hsp71 in group B patients (25–81 years old). Serum samples were diluted as described in the legend of Figure 1 and used in the miniblot assay using human Hsp71 as the immunogen. Titers are plotted for individuals. The sex of the patient is indicated ▵, (male); ○, (female)
The titer of antibody against Hsp71 in plasma changes during health recovery
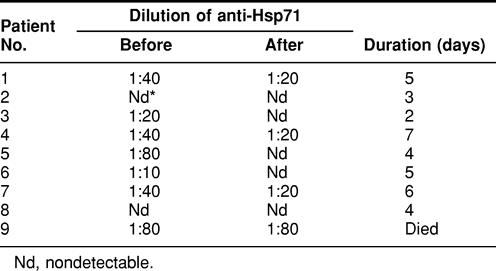
Finally, we investigated changes in the titer of antibody against Hsp71 in the plasma of a limited group of 9 patients during their recovery from heat-induced illness. Table 6 shows the titer of anti-Hsp71 in individuals at admission and release and their recovery time. In most patients, the titer was higher at admission and decreased during recovery. One patient (No. 9) with a high titer (1:80) showed no changes and died after 2 days.
Table 6.
Changes in the titer of anti-Hsp71 in plasma of 9 patients with acute heat-induced illness at admission in hospital and on release
DISCUSSION
On the basis of our previous data that showed the presence of antibodies to Hsps in workers exposed to toxic chemicals (Wu et al 1996, 1997, 1998), we examined whether antibodies would also be found in patients exposed to severe heat stresses. Our results show that in a group of young men exercising in a hot environment (group A), the occurrence of antibodies against Hsp71 and Hsp90α was significantly higher among individuals with symptoms of heat-induced diseases (P < 0.05) than in a matched group of nonaffected individuals exercising in the same environment. Moreover, analysis of results from individuals shows that the titers of antibody against Hsp71 are higher in the plasma of patients from both the severe and mild heat-induced diseases subgroups, the highest titer being found in the most severe cases. A study of a second group of 57 heat-affected patients of mixed sex and age (group B, aged 25–81 years) exposed to an extremely hot living environment and/or having different activity intensity gives highly similar results. Again, patients with the more severe heat-induced symptoms show a significantly higher incidence of antibodies to Hsp71 than matched controls. Moreover, the titer of anti-Hsp71 is higher in the severely affected subgroup. Finally, in a study of 9 hospitalized patients, it was observed that the titer of anti-Hsp71 decreased during recovery from severe heat symptoms.
Many studies have shown that Hsps are involved in cell survival under stressful conditions. Tolerance to stresses such as heat, endotoxin, heavy metals exposure, and oxidants increases as the synthesis of Hsps goes up (Landry et al 1982, 1989; Li and Werb 1982; Laszlo 1988; Angelidis et al 1991; Li et al 1991; Hotchkiss et al 1993). Protection has also been shown to operate in whole organs such as the heart, brain, and kidneys. Currie et al (1993) showed that a hyperthermic pretreatment inducing the expression of Hsp70 in the rabbit myocardium was beneficial in preventing tissue necrosis and improved recovery from ischemia during occlusion/reperfusion injury. Transgenic expression of the human inducible Hsp70 in a mouse model reduced the extent of damage in hippocampal neurons following permanent middle cerebral artery occlusion (Plumier et al 1997). Harmful conditions such as exposure to mercury, CdCl2, heat stress, and osmotic stress also induce Hsps in the kidneys, and some Hsps may have protective functions against these stresses (Gagnon et al 1999; Somji et al 1999; Beck et al 2000; Goering et al 2000).
Hsps are protective in organs such as the brain, heart, kidneys, all of which are involved in the regulation of the human body temperature. As body temperature rises significantly as a result of work, exercise, and/or climatic factors, blood and skin temperature also rise. Skin sensors and the direct effect of warm blood stimulate the thermostatic set point in the hypothalamus, which in turn activates the autonomic nervous system to bring about changes of several systems: nervous, respiratory, circulating, endocrine, and urinary. Whether heat-induced stress in these systems induces Hsps that could then become antigens is unknown. Many investigations have suggested that autoantibodies against Hsps might be of significance in the generation, formation, and prognosis of different diseases. For example, a direct relationship between antibodies against Hsp65 and carotid wall atherosclerosis was documented in a study in which these antibodies were associated with the most severe degree of atherosclerosis and were demonstrated to predict 5-year mortality (Xu et al 1993, 1999). Interestingly, the main systems involved in heat-induced diseases are the nervous, circulating, endocrine, and urinary systems, whose functions and protection are related to Hsps, and the main characteristics and main death causes of heat-induced diseases are the dysfunctions of these systems and organs.
It is reasonable to think that the conditions of extreme heat incurred by the patients under study here are sufficient to induce some Hsps, such as Hsp71 in blood leukocytes and/or in endothelial cells. Heat-induced damage may then trigger an inflammatory response, which may cause necrosis of damaged cells with the release of cellular proteins, including Hsp71. Release of Hsp71 by cell killing and necrosis caused by chemical exposure has been suggested on the basis of high DNA damage and superoxide dismutase levels in workers exposed to benzene (Wu et al 1998). Because Hsp71 somewhat acts as a chemokine when it is outside of the cell, neutralization of this Hsp by antibodies may be a part of a downregulation mechanism of Hsp71 action. Since most persons have had inflammatory responses due to viral, bacterial, or parasitic infection, the immune system is likely primed by Hsps and can rapidly mount an immune response. Although the detailed significance and mechanism of generation of these autoantibodies against Hsps in heat-induced diseases remain to be determined, an interesting alternative model in which Hsps act as danger signals in stressed cells modulating the immune response has recently been proposed to explain the role of exercise in altering immune functions (Moseley 2000). Whether antibodies to Hsps have a physiological role in the response of an individual to stress such as extreme heat remains an intriguing question.
Heat-induced and heat-related illnesses remain high in many working environments. These illnesses include heat syncope, heat rash, heat cramps, heat exhaustion, and heat stroke in order of increasing severity (Alzeer et al 1997). Such diseases can also occur during a sudden increase of ambient temperature or heat wave when acclimatization of the human body to heat is not established. Such adaptation is likely to be related to the roles and functions of Hsps. It will, therefore, be important to understand the mechanisms involved in the generation of antibodies against Hsps and their possible use in diagnosis, prevention, or prognosis of heat-related diseases.
Acknowledgments
We are particularly grateful to all individuals who voluntarily agreed to participate in these studies and to the members of the medical personnel of 4 hospitals in Nanjing for their generous help in the examination and sampling of patients. This work was supported by the National Natural Science Foundation of China (NNSFC to T.W.) and a joint exchange program grant between the NNSFC and the Medical Research Council (MRC) of Canada to T.W. and R.M.T. Work in R.M.T.'s laboratory was supported by the MRC of Canada.
REFERENCES
- Alzeer AH, el-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Biochem. 1997;43:1182–1187. [PubMed] [Google Scholar]
- Angelidis CE, Lazaridis I, Pagoulatos GN. Constitutive expression of heat shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Tanguay RM 1991 Expression of heat shock proteins during development in Drosophila. In: Heat Shock and Development, ed Hightower L, Nover L. Springer-Verlag, Berlin, 106–119. [DOI] [PubMed] [Google Scholar]
- Beck FX, Neuhofer W, Müller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279:F203–F215. doi: 10.1152/ajprenal.2000.279.2.F203. [DOI] [PubMed] [Google Scholar]
- Bohen SP, Kralli A, Yamamoto KR. Hold'em and fold'em: chaperones and signal transduction. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Heat shock proteins in relation to medicine. Mol Aspects Med. 1993;14:83–165. doi: 10.1016/0098-2997(93)90020-e. [DOI] [PubMed] [Google Scholar]
- Carbajal ME, Duband JL, Lettre F, Valet JP, Tanguay RM. Cellular localization of Drosophila 83-kilodalton heat shock protein in normal, heat-shocked, and recovering cultured cells with a specific antibody. Biochem Cell Biol. 1986;64:816–825. doi: 10.1139/o86-110. [DOI] [PubMed] [Google Scholar]
- Clowes GHA Jr,, O'Donnel TF Jr.. Current concepts: heat stroke. N Engl J Med. 1974;291:564–567. doi: 10.1056/NEJM197409122911106. [DOI] [PubMed] [Google Scholar]
- Craig EA. The heat shock response. Crit Rev Biochem. 1985;18:239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG Jr.. Heat-response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Frosttegard J, Lemne C, Andersson B, Zee RVD, Liessling R, Faire U. Association of serum antibodies to heat shock protein 65 with borderline hypertension. Hypertension. 1997;29:40–44. doi: 10.1161/01.hyp.29.1.40. [DOI] [PubMed] [Google Scholar]
- Gagnon F, Orlov SN, Champagne MJ, Tremblay J, Hamet P. Heat stress preconditioning does not protect renal epithelial Na(+), K(+), Cl(−) and Na(+), P(i) cotransporters from their modulation by severe heat stress. Biochim Biophys Acta. 1999;1421:163–174. doi: 10.1016/s0005-2736(99)00127-3. [DOI] [PubMed] [Google Scholar]
- Goering PL, Fisher BR, Noren BT, Papaconstantinou A, Rojko JL, Marler RJ. Mercury induces regional and cell-specific stress protein expression in rat kidney. Toxicol Sci. 2000;53:447–457. doi: 10.1093/toxsci/53.2.447. [DOI] [PubMed] [Google Scholar]
- Hartl F-U. Molecular chaperones in protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hypothermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–57. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Jarjour WN, Jeffries BD, Davis JS, Welch WJ, Mimura T, Winfield JD. Autoantibodies to human stress proteins. Arthritis Rheum. 1991;34:1133–1138. doi: 10.1002/art.1780340909. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, Schoel B 1994 Heat shock proteins as antigens in immunity against infection and self. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, New York, 495–531. [Google Scholar]
- Krueger AMR, Armstrong JN, Plumier JC, Robertson HA, Currie RW. Cell specific expression of Hsp70 in neurons and glia of the rat hippocampus after hyperthermia and kainic acid-induced seizure activity. Brain Res Mol Brain Res. 1999;71:265–278. doi: 10.1016/s0169-328x(99)00198-9. [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Chrétien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42:2457–2461. [PubMed] [Google Scholar]
- Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo A. Evidence for two states of thermotolerance in mammalian cells. Int J Hyperthermia. 1988;4:513–526. doi: 10.3109/02656738809027695. [DOI] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Li LY, Liu K, Mak JK, Chen L, Lee WMF. Thermal response of rat fibroblasts stably transfected with the human 70kDa heat shock protein encoding gene. Proc Natl Acad Sci U S A. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Loones MT, Rallu M, Mezger V, Morange M. HSP gene expression and HSF2 in mouse development. Cell Mol Life Sci. 1997;53:179–190. doi: 10.1007/PL00000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;96:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo A-P. Constitutive expression of human hsp27, Drosophila hsp27 and human α-B crystallin confers resistance to tumor necrosis factor-α and oxidative stress- induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:364–374. [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay RM. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C. ed. . 1994 The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Morrison AJ, Rush SJ, Brown IR. Heat shock transcription factors and the hsp70 induction response in brain and kidney of the hyperthermic rat during postnatal development. J Neurochem. 2000;75:363–372. doi: 10.1046/j.1471-4159.2000.0750363.x. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Exercise, stress, and the immune conversation. Exerc Sport Sci Rev. 2000;28:128–132. [PubMed] [Google Scholar]
- Parsell DA, Lindquist S 1994 Heat shock proteins and stress tolerance. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, New York, 457–494. [DOI] [PubMed] [Google Scholar]
- Petersdorf RG, Root RK 1987 Alterations in body temperature. In:Harrion's Principles of International Medicine. eds Braunwald E, Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, and Fauci AS, McGraw Hill Book Co, 43–49. [Google Scholar]
- Plumier C, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expression of the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier C, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expression the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollet E, Landry J, Lavoie J, Tanguay RM. Expression of Drosophila 27kDa heat shock protein in rodent cells confers thermal resistance. Biochem Biophys Res Commun. 1992;185:116–120. doi: 10.1016/s0006-291x(05)80963-5. [DOI] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van Der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 65 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai R, Maeda T, Onishi S, Yamamoto Y. Autoantibody against 70 kD heat-shock protein in patients with autoimmune liver diseases. J Hepatol. 1995;23:382–390. doi: 10.1016/0168-8278(95)80195-2. [DOI] [PubMed] [Google Scholar]
- Somji S, Todd JH, Sens MA, Garrett SH, Sens DA. Expression of the constitutive and inducible forms of heat shock protein 70 in human proximal tubule cells exposed to heat, sodium arsenite, and CdCl2. Environ Health Perspect. 1999;107:887–893. doi: 10.1289/ehp.99107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wu T, He H, Tanguay RM, Wu Y, Xu D, Currie RW, Qu S, Feng J, Zhang G. The combined effects of high temperature and carbon monoxide on heat stress response. J Tongji Med Univ. 1995;15:178–183. doi: 10.1007/BF02888231. [DOI] [PubMed] [Google Scholar]
- Wu T, Tanguay RM, Wu Y, He H, Xu D, Feng J, Shi W, Zhang G. Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci. 1996;9:370–379. [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:poaths>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169. [DOI] [PubMed] [Google Scholar]
- Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl AS, Stulning T, Luef G, Wick G. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]