Abstract
Encysted embryos of the primitive crustacean Artemia franciscana are among the most resistant of all multicellular eukaryotes to environmental stress, in part due to massive amounts of a small heat shock/α-crystallin protein (p26) that acts as a molecular chaperone. These embryos also contain very large amounts of the disaccharide trehalose, well known for its ability to protect macromolecules and membranes against damage due to water removal and temperature extremes. Therefore, we looked for potential interactions between trehalose and p26 in the protection of a model substrate, citrate synthase (CS), against heat denaturation and aggregation and in the restoration of activity after heating in vitro. Both trehalose and p26 decreased the aggregation and irreversible inactivation of CS at 43°C. At approximate physiological concentrations (0.4 M), trehalose did not interfere with the ability of p26 to assist in the reactivation of CS after heating, but higher concentrations (0.8 M) were inhibitory. We also showed that CS and p26 interact physically during heating and that trehalose interferes with complex formation and disrupts CS-p26 complexes that form at high temperatures. We suggest from these results that trehalose may act as a “release factor,” freeing folding intermediates of CS that p26 can chaperone to the native state. Trehalose and p26 can act synergistically in vitro, during and after thermal stress, suggesting that these interactions also occur in vivo.
INTRODUCTION
Molecular chaperones assist the folding of proteins, reduce stress-associated protein denaturation and aggregation, aid in renaturation, and influence the final intracellular location of mature proteins. The literature is massive (Morimoto et al 1994; Fiege et al 1996; Beissinger and Buchner 1998; Ellis and Hartl 1999; Feder and Hofmann 1999; Feldman and Frydman 2000). Chaperones are divided into families, including Hsp100, Hsp90, Hsp70, Hsp60 (the chaperonins), and the small heat shock/α-crystallin proteins (Arrigo and Landry 1994; Heikkila et al 1997; Clark and Muchowski 2000; van den IJssel et al 2000), sometimes referred to as α-Hsps (de Jong et al 1998; MacRae 2000). These proteins range in size from about 10- to 40-kDa monomer molecular mass, but oligomerize into particles of varying monomer numbers (Vanhoudt et al 1998; Ehrnsperger et al 1999). As with many other Hsps, α-Hsps protect cells during stress by preventing aggregation of unfolded proteins (Horwitz 1992; Horwitz et al 1998; Lindner et al 1998) and in some cases assisting in their renaturation (Jakob et al 1993; Liang et al 1997a; Ganea and Harding 2000).
Cells of organisms naturally adapted to survive severe stress commonly contain large amounts of low-molecular-mass “compatible solutes” (Yancey et al 1982; Somero and Yancey 1997) that also play key protective roles. Particularly important among these is trehalose, the nonreducing disaccharide of glucose, which is of widespread occurrence and importance in bacteria, fungi, and a number of multicellular eukaryotes (Clegg 1974; Eleutherio et al 1993; Crowe et al 1997). The protective effects of this sugar are well established (Crowe et al 1992, 1997, 1998; Colaço et al 1994; Welch and Brown 1996; Allison et al 1999). Dramatic results have recently been obtained on the ability of trehalose to greatly improve the survival of cryopreserved cells (Eroglu et al 2000) and even to allow mammalian cells to survive desiccation at room temperature (Guo et al 2000).
We have been studying encysted embryos of the primitive crustacean Artemia franciscana, which contains very large amounts of the α-Hsp known as p26 (making up 12–15% of the total nonyolk protein) and also trehalose (about 15% of the embryo's total dry mass). Previous work on these embryos reveals the individual importance of trehalose (Clegg 1974; Clegg and Conte 1980; Drinkwater and Clegg 1991; Crowe et al 1992, 1997, 1998) and p26 (Clegg et al 1994, 1995, 1999, 2000; Jackson and Clegg 1996; Liang et al 1997a, 1997b; Liang and MacRae 1999), but their potential interaction has not been studied. The latter seemed likely in view of the critical interplay between trehalose and Hsp104 in the heat shock response of the yeast Saccharomyces cerevisiae (Singer and Lindquist 1998a, 1998b) that we will consider in the Discussion section. Appreciation of the results to be presented requires knowledge of Artemia embryos so a brief summary follows.
The genus Artemia is found in harsh, hypersaline habitats worldwide, and its biological structure is well known (Persoone et al 1980; Decleir et al 1987; MacRae et al 1989; Warner et al 1989; Browne et al 1991). Fertilized eggs develop directly into free-swimming larvae that are released from females or development stops at the gastrula stage, with the embryo now being surrounded by a complex shell. These “encysted embryos,” which are composed of about 4000 cells (nuclei), are in diapause (Drinkwater and Clegg 1991), a condition of obligate developmental arrest characterized by a level of metabolic activity so low that its detection becomes a major problem (Clegg et al 1996). Desiccation is often a normal part of this developmental pathway and is one of the conditions that terminates diapause, restoring metabolism and development under permissive conditions of water content, molecular oxygen, and temperature (Slegers 1991; Hand and Hardewig 1996). These embryos are among the most resistant of all multicellular eukaryotes to environmental insults, surviving continuous anoxia for years (Clegg 1997; Clegg et al 1999, 2000), temperature extremes (Clegg and Conte 1980; Clegg and Jackson 1992), dehydration/rehydration cycles (Morris 1971), and exposure to various forms of radiation (Persoone et al 1980; Decleir et al 1987). They endure virtually complete desiccation (Clegg and Drost-Hansen 1990; Crowe et al 1992, 1997) and, when dry, survive the conditions of outer space (Gaubin et al 1983). Trehalose and p26 are both essential components of the adaptive mechanisms underlying these impressive abilities.
To explore further the roles of these 2 critical molecules, we studied their potential interaction in the protection of heat-treated citrate synthase (CS) against aggregation and in its reactivation (renaturation) in vitro. We found that important interactions between CS, p26, and trehalose do indeed take place and that p26 and trehalose have different but overlapping roles in the thermal protection of this enzyme in vitro and, we suppose, of embryonic proteins in vivo.
MATERIALS AND METHODS
Materials
Pig heart CS, bovine serum albumin (BSA), acetyl-coenzyme A, adenosine triphosphate (ATP), and guanosine 5′-triphosphate (GTP) were from Sigma (St Louis, MO, USA), (NH4)2SO4 from ICN (Costa Mesa, CA, USA), and trehalose from Pfanstiehl Laboratories (Waukegan, IL, USA). Polyclonal antibodies to CS were from Biogenesis (Kingston, NH, USA) and against p26 as described previously (Clegg et al 1994). All other chemicals were analytical grade.
p26 purification
p26 was purified from encysted embryos of A franciscana (Great Salt Lake) purchased from San Francisco Bay Brand, Newark, CA, USA. Purification steps were performed at 4°C or on ice. Dried embryos (50 g) were hydrated at 4°C for 16 hours in sea water; filtered; washed with cold 40 mM HEPES-KOH, pH 7.5, at 4°C, 70 mM NaCl, and 1 mM EDTA (buffer A); and homogenized in the same buffer with a Retsch motorized mortar and pestle (Brinkman Instruments, Canada). The homogenate was centrifuged (4000 × g, 20 minutes) and the supernatant filtered through 6 layers of cheesecloth, centrifuged again at 16 000 × g for 40 minutes, and then at 23 500 × g for 30 minutes. Solid (NH4)2SO4 was added to 40% saturation in the final supernatant. Precipitated proteins were collected at 10 000 × g for 30 minutes; dissolved in 20 mM Tris-HCl, pH 8.15, 150 mM NaCl, 1 mM MgCl2, and 0.1 mM EDTA (buffer B); and dialyzed overnight against this buffer. After dialysis, the solution was passed through a 0.45-μm filter, applied to a Source 15 Q ion-exchange column (Amersham Pharmacia Biotech), equilibrated, and developed at 2 mL/min in buffer B. The column was washed with buffer B for 30 minutes, and a linear NaCl gradient (150–500 mM) was used for elution of p26 between 235–270 mM NaCl. Fractions containing p26 were pooled; concentrated using Centriprep-30 (Amicon); dialyzed against 40 mM HEPES-KOH, pH 7.5 (buffer C), and 300 mM NaCl; further purified by gel filtration using a TSK-Gel G4000SWXL column (0.78 × 30 cm, Toso Haas, Japan); equilibrated; and developed at 0.5 mL/min in buffer C and 300 mM NaCl. p26 was eluted between 9.5–10.5 mL of the buffer volume, and the resulting protein was more than 95% pure. The protein was concentrated to approximately 1 mg/mL with Centriprep-30; dialyzed against 50 mM Tris-HCl, pH 8, and 2 mM EDTA (TE buffer); and centrifuged at 10 000 × g for 15 minutes. Further concentration and storage in buffer C led to unwanted insoluble aggregates. Aliquots were quick frozen in liquid nitrogen and stored at −70°C. Fractions from each step of purification were checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and/or Western immunoblotting with polyclonal antibody to p26 and then pooled according to purity.
Protein determinations
The concentration of p26 was determined using the Pierce BCA™ protein assay kit with BSA as standard. The concentration of CS was determined at 280 nm (Ehrnsperger et al 1997). Unless stated otherwise, concentrations of CS and p26 given in the text refer to the dimer and 28-mer, respectively.
Electrophoresis and Western blot analysis
Nondenaturing pore-exclusion polyacrylamide gel electrophoresis was performed on 4–10% acrylamide gels (Lee and Vierling 1998), and Western blotting (Towbin et al 1979) used polyclonal antibodies to CS (1:1000) or p26 (1:10 000) and alkaline phosphatase–conjugated secondary antibody from Pierce (Rockford, IL, USA) (1:5000). These antibodies to CS and p26 detect both native and denatured forms (unpublished Western blotting results).
Aggregation assay
CS (7.5 μM) was diluted 1:100 in 40 mM HEPES-KOH (pH 7.5) and equilibrated at 43°C, in the presence and absence of p26, BSA, or trehalose. Light scattering was used to monitor the kinetics of thermal aggregation in a PTI spectrofluorometer (South Brunswick, NJ, USA) in stirred and temperature-controlled quartz cells with excitation and emission wavelengths set to 500 nm and a band pass of 2 nm (Buchner et al 1998).
Inactivation and reactivation
CS (7.5 μM) was diluted 1:100 in 40 mM HEPES-KOH (pH 7.5) in the presence or absence of additional compounds at 25°C. Heat inactivation was started by shifting the sample to 43°C. Reactivation of CS was initiated by lowering the temperature to 25°C, and incubation continued for up to 60 minutes in the absence or presence of trehalose (0.4 or 0.8 M). To determine inactivation kinetics, aliquots were taken at the times indicated and CS activity measured at 25°C as described previously for CS (Robinson et al 1987), whose thermal inactivation kinetics are first order (Buchner et al 1998).
Previous work (Liang et al 1997a) indicated that neither ATP nor GTP was required for chaperone activity of p26 in vitro, and we confirmed that result here. CS was incubated with p26 for 15 minutes at 43°C, then shifted to 25°C for another 30 minutes to enable recovery of activity, and finally incubated at this temperature for another 60 minutes after addition of 3.5 mM MgCl2, 10 mM KCl, and 3.5 mM ATP or GTP.
Size exclusion chromatography
CS (75 nM) was incubated with or without p26 (150 nM) at 43°C for up to 60 minutes. To analyze complex formation between p26 and CS, size exclusion chromatography (SEC) was performed using a TSK GEL G4000SWXL column (Toso Haas, Japan). Chromatography used buffer C and 300 mM NaCl at a flow rate of 1 mL/min and a sample size of 150 μL. Samples were prepared in buffer C supplemented with 300 mM NaCl and injected immediately onto the column without prior centrifugation. Proteins were detected by absorbance at 220 nm. The column was calibrated with molecular mass standards (kDa): blue dextran (2000), thyroglobulin (669), ferritin (443), and alcohol dehydrogenase (150).
RESULTS
p26 protects CS from thermal inactivation and aggregation
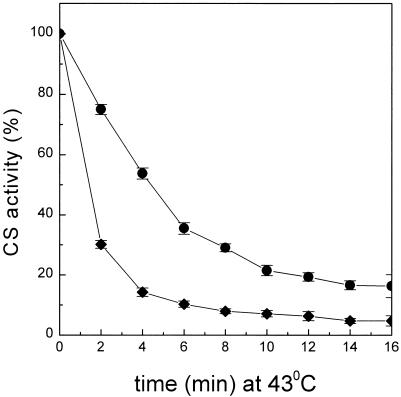
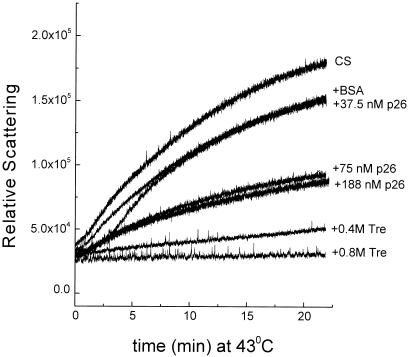
A convenient and widely used assay for chaperone activity involves the thermal inactivation and aggregation of CS (Buchner et al 1998). We know that native p26 can reactivate thermally inactivated CS in vitro (Liang et al 1997a), so the ability of p26 to recognize and bind unfolding intermediates of CS during heating was examined. On incubation at 43°C, CS alone or in the presence of the control protein BSA was inactivated rapidly, and less than 5% of the original CS activity remained after 15 minutes (Fig 1). Inactivation was accompanied by protein aggregation, which approached completion after 20 minutes (Fig 2). BSA alone had only a modest effect on the aggregation kinetics of heat-treated CS. In contrast, p26 decreased the rate of aggregation of CS, providing maximal protection at a concentration of 75 nM and a ratio of 1 CS to 1 p26 (native molecular masses). Lower p26 concentrations were not effective, whereas those higher than a 2-fold molar excess of p26 did not provide greater protection. Under the same heating conditions, p26 is stable since no light scattering was observed (not shown). Results on trehalose shown in Figure 2 will be considered later.
Fig. 1.
Inactivation kinetics of CS in the presence and absence of p26. Inactivation of CS (75 nM) at 43°C in the presence of 188 nM p26 (0.109 mg/mL, •) or 0.1 mg/mL of BSA (♦) was monitored by measuring CS activity at the times indicated. CS activity is shown as a percentage of enzyme activity before heating, which was set to 100%. Data points are means ± standard errors for n = 5
Fig. 2.
Influence of p26 on the thermal aggregation of CS. CS (75 nM) was incubated at 43°C alone or in the presence of p26 (37.5–188 nM) with BSA (0.1 mg/mL) or with trehalose (Tre). Aggregation was measured by light scattering at 500 nm. This experiment is 1 of 4 that produced comparable results
Although p26 suppressed aggregation of CS effectively in stoichiometric concentrations, as shown in Figure 2, the kinetics of the inactivation reaction was not significantly different until a native p26 to CS ratio of at least of 2.5 was reached (Fig 1). The thermal inactivation of CS follows first-order reaction kinetics (Buchner et al 1998), and we obtained apparent rate constants of 8.5 × 10−3/s and 3.9 × 10−3/s for the inactivation of CS in the presence of BSA (0.1 mg/mL) and p26 (188 nM), respectively. It appears that p26 “stabilized” CS by reducing the rate constant of thermal inactivation by a factor of about 2.
Analysis of physical interactions between p26 and CS
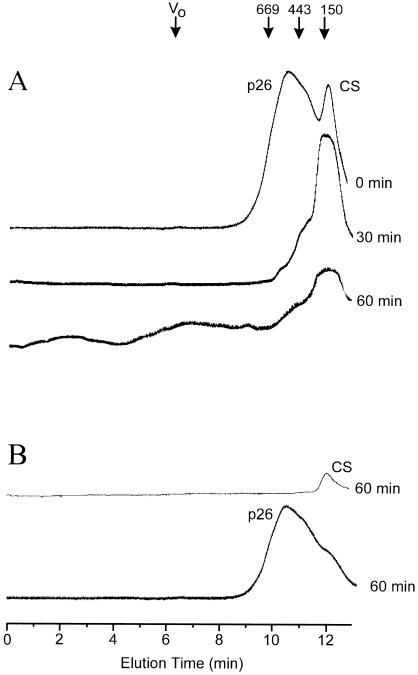
Next we used size-exclusion high-performance liquid chromatography (SEC) (Fig 3) and Western blot analysis of CS and p26 (Fig 4) to show that p26 does indeed form complexes with heated but not unheated CS. When p26 was mixed with CS at 25°C, incubated for 1 hour, and then analyzed by SEC, both proteins eluted with retention times consistent with their native molecular masses (Fig 3A). However, when a mixture of p26 and CS (2:1) was heated up to 60 minutes at 43°C, progressively higher-molecular-weight species appeared. After the first 30 minutes of incubation, complexes of high-molecular mass appeared as a shoulder off the p26 peak. After 60 minutes at this elevated temperature, 2 new broad peaks appeared close to the void volume of the column (Fig 3A). When p26 was heated alone under these same conditions and then analyzed by SEC, no change in retention time or peak area was observed (Fig 3B), indicating that p26 does not change size or become insoluble as a result of this heat treatment. In contrast, CS heated alone yielded insoluble aggregates after centrifugation, and SEC revealed substantial loss of soluble protein (Fig 3B).
Fig. 3.
Detection of p26-CS complexes by SEC. (A) p26 (150 nM) was incubated with 75 nM CS at 43°C for the indicated times, then analyzed on a Toso Haas G 4000 SWXL column as described in the Materials and Methods section. (B) A total of 150 nM p26 and 75 nM CS were incubated alone for 60 minutes at 43°C then analyzed similarly. Proteins were monitored by absorbance at 220 nm. The retention times of protein molecular mass standards (×10−3) are shown above part A. Blue dextran (2000 kDa) indicates the void volume (Vo)
Fig. 4.
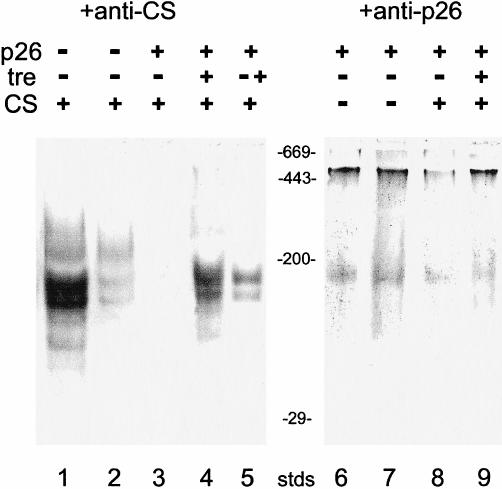
Effects of p26 and trehalose on the thermal unfolding of CS. CS (75 nM) was incubated at 43°C for 60 minutes alone (lane 2) or in the presence of 188 nM p26 (lanes 3 and 7) or with 188 nM p26 and 0.8 M trehalose (lanes 4, 5, and 8). After heating, samples were immediately separated on 4–10% native gels, blotted to nitrocellulose, and stained with antibody to CS (lanes 1–5) or p26 (lanes 6–9). Lane 1: CS was incubated for 60 minutes at 25°C. Lane 5: CS was incubated at 43°C for 30 minutes in the presence of p26, then 0.8 M trehalose was added and incubation continued another 30 minutes. Stds are molecular mass markers (×10−3 kDa). Each lane for native gels received 3.8 μg of CS and 13.7 μg of p26
Complex formation between p26 and CS at elevated temperatures was also investigated by Western blot analysis, using antibodies to CS and p26 (Fig 4). When CS was incubated alone at 43°C for 1 hour, then immediately applied to native polyacrylamide gels, and analyzed by Western blots using antibody to CS, significant loss of native CS was observed (Fig 4, lane 2 vs 1). Curiously, when CS was heated in the presence of p26 (2.5 p26, 1 CS) and analyzed by Western blots using antibody to CS, no signal was detected on the blots (Fig 4, lane 3). That observation most likely results from epitope masking of CS in complexes with p26, which is also the case for p26, but to a lesser extent (Fig 4, lane 8). When these native gels were stained with Coomassie blue, a very broad protein band appeared with apparent molecular masses higher than 700 kDa, which barely entered the gels (not shown). These results, together with those from the SEC experiments (Fig 3A), provide good evidence for the formation of stable complexes between CS and p26 during prolonged incubation at 43°C.
Additional evidence for formation of p26-CS complexes was obtained by heating CS in the presence of p26 and 0.8 M trehalose. Comparison of lanes 3 and 4 in Figure 4 shows that CS neither aggregates nor associates with p26 when trehalose is present in high concentrations. We also found using Western blotting that trehalose added to preformed p26-CS complexes caused the release of CS (Fig 4, lane 5). The foregoing results demonstrate the protection of CS by trehalose but also indicate that trehalose somehow interferes with interactions between CS and p26. Western blot analysis, using antibody to p26, confirmed the SEC results that show that the oligomeric state of p26 did not change on incubation at 43°C. Furthermore, the densities of p26 bands were comparable after incubation at 25°C and at 43°C for up to 60 minutes (Fig 4, lanes 6 and 7).
Collectively, these results indicate that p26 prevents thermal inactivation and aggregation of CS by selectively binding nonnative protein and forming large complexes but that those interactions can be prevented and the complexes disrupted by trehalose when present in high concentrations.
Protection and reactivation of thermally inactivated CS by trehalose
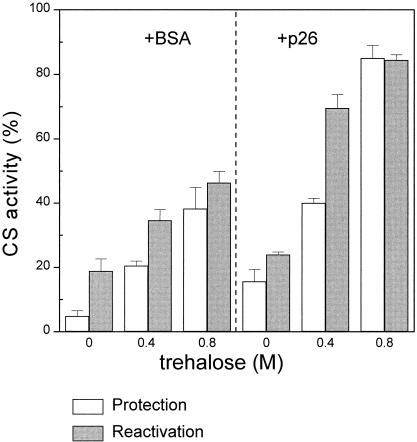
As shown in Figure 2, 0.4 M trehalose suppressed aggregation of CS during heating, and 0.8 M was even more effective. We also know that trehalose hinders the formation of complexes between p26 and CS during heating (Fig 4, lanes 4 and 5 vs 3). Next, we examined the effect of trehalose on CS activity under various heating regimens (Fig 5). We considered the data on CS protection, described by the open bars in Figure 5. As expected, trehalose (0.4 M) protects CS from heat inactivation, which is so whether p26 is present or not. Addition of 0.1 mg/mL of BSA, a mass concentration close to that of p26 (0.109 mg/mL), was used to evaluate nonspecific protein effects (Fig 5). Thus, an increase in protection of about 4-fold was seen for trehalose plus BSA, which was also observed when trehalose was present alone (not shown). In comparison, the corresponding increase in protection by trehalose plus p26 was about 2.5-fold. Increasing the concentration of trehalose to 0.8 M further enhanced protection, with about 40% of CS activity remaining in the presence of BSA (control) and about 85% in the presence of p26 (Fig 5). Thus, under these conditions, it appears that p26 and trehalose might act synergistically to protect CS against thermal denaturation.
Fig. 5.
Effects of p26 and trehalose on the protection and reactivation of thermally inactivated CS. CS (75 nM) was first incubated for 15 minutes at 43°C in the presence of 188 nM p26 or with 0.1 mg/mL of BSA, with or without trehalose as shown (open bars) and CS activity determined immediately after heating. This treatment is referred to as protection. Comparable reaction mixtures were then incubated for an additional 60 minutes at 25°C to enable recovery of enzyme activity (filled bars), a treatment we call reactivation. CS activity before the initial heating was assigned a value of 100%, and other data were normalized to this value (bars are means ± standard errors for n = 4)
In view of these results, we examined the influence of trehalose on the restoration of CS activity following heat treatment (CS reactivation) in the presence and absence of p26. The results of these studies are shown in the solid bars in Figure 5. CS was incubated for 15 minutes at 43°C and reactivation initiated by lowering the temperature to 25°C and incubating for an additional 60 minutes before CS assay. In the presence of BSA, about 14% of the initial CS activity was regained during this time. Although p26 alone provided substantial protection against heating damage, the extent of reactivation was not increased compared with the spontaneous level (Fig 5). When CS was heated at 43°C for 15 minutes in the presence of 0.4 M trehalose plus BSA, then incubated for an additional 60 minutes at 25°C, the levels of CS reactivation were comparable to those obtained without added trehalose (Fig 5). We found (not shown) that the extent of CS reactivation in the presence of 0.4 M trehalose, but without BSA, was essentially the same as the level of CS reactivation in the presence of BSA alone (Fig 5). In other words, trehalose does not seem to be acting as a chemical chaperone (Welch and Brown 1996), at least in terms of the reactivation process. In contrast, when CS was heated with p26 in the presence of 0.4 M trehalose, a 2-fold increase in the extent of reactivation over the spontaneous level was observed, and about 70% of the original activity was recovered after 60 minutes (Fig 5). However, in the presence of 0.8 M trehalose, no activity was regained after heating, with or without BSA and p26, suggesting that high concentrations of trehalose interfered with chaperone-mediated refolding. We note that glycerol, tested in these assays in the same way, had negligible effect on the protection of CS during heating or its restoration after heating (results not shown).
Effects of trehalose on the stability of p26-CS complexes
The stability of p26-CS complexes was studied by Western blot analysis of native gels (Fig 6). CS was incubated for 1 hour at 43°C in the absence or presence of p26 (2.5 p26, 1 CS). Renaturation was initiated by lowering the temperature to 25°C and continuing incubation for 1 hour in the absence or presence of 0.8 M trehalose. Simply lowering the temperature released bound CS from p26, which we suppose also resulted in CS refolding (Fig 6, lane 4). Addition of trehalose after heating did not enhance refolding of heat-denatured CS, either in the absence (Fig 6, lane 3 vs 2) or presence (lane 5 vs 4) of p26. Densitometry of the corresponding protein bands showed the release of at least 15% native CS from p26-CS complexes after 1 hour of incubation at 25°C. Addition of 0.8 M trehalose to these p26-CS complexes did not increase the level of CS refolding (Fig 6). Western blot analysis, using antibody to p26, indicated that trehalose had no effect on the oligomeric state of this protein (Fig 6, lanes 6–10).
Fig. 6.
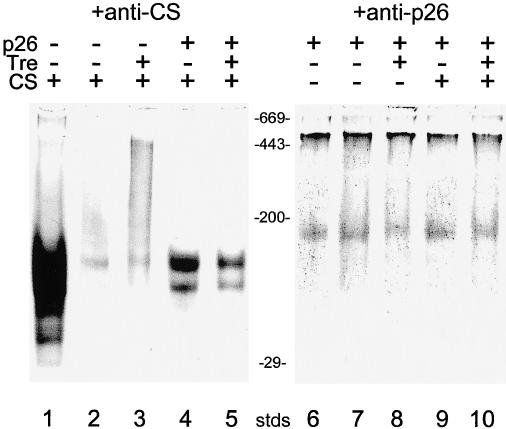
Influence of trehalose on the refolding of heat-denaturated CS. CS was either heated alone (lanes 2 and 3) or with 188 nM p26 (lanes 4, 5, 9, and 10) as described in Figure 4 and then incubated for another 60 minutes at 25°C. Proteins were separated on 4–10% native gels, blotted to nitrocellulose, and stained with antibody to CS (lanes 1–5) or p26 (lanes 6–10). Lane 1: CS incubated for 2 hours at 25°C. Lanes 2 and 3: CS after heating was incubated for 60 minutes at 25°C alone (2) or with 0.8 M trehalose (3). Lanes 4, 5, 9, and 10: CS was heated with 188 nM p26 then incubated for 60 minutes at 25°C in the presence (5 and 10) or absence of 0.8 M trehalose (4 and 9). Lane 6: p26 was incubated for 2 hours at 25°C. Lanes 7 and 8: p26 was heated for 60 minutes at 43°C and then incubated for another 60 minutes at 25°C, either alone (7) or with 0.8 M trehalose (8). Stds are protein standards (×10−3 kDa). Each lane received 3.8 μg of CS and 13.7 μg of p26
DISCUSSION
Since the first demonstrations that small heat shock/α-crystallin proteins act as molecular chaperones (Horwitz 1992; Jakob et al 1993), research in this area has expanded substantially (Heikkila et al 1997; Liang et al 1997a; Horwitz et al 1998; Rao et al 1998; Derham and Harding 1999; Lee and Vierling 2000). In nonlens cells, these proteins are not usually present in appreciable amounts constitutively, but are strongly induced or up-regulated by a variety of stresses (van den IJssel et al 1998; MacRae 2000). Those relationships clearly do not exist in encysted Artemia embryos, which contain massive amounts of p26 that are synthesized as part of their developmental program and not in response to stress (Jackson and Clegg 1996; Clegg et al 1999, 2000; Liang and MacRae 1999). Like other α-Hsps (de Jong et al 1998; MacRae 2000), native p26 is a multimer. Our estimate of 580 kDa for its molecular mass (Figs 3 and 4) corresponds to 28 subunits, each of 20.7 kDa based on amino acid composition (Liang et al 1997b) and in good agreement with previous results (Clegg et al 1994). This protein has been purified previously (Liang et al 1997a), but the procedure developed in the present study is superior in yield and speed, and for those reasons we have described it in some detail in the Materials and Methods section.
To our knowledge, p26 is present in the highest concentration of any stress protein in any nontransfected cell, making up 12% to 15% of the total nonyolk protein (Clegg et al 1994, 1995). That amount can be compared to maximum amounts of α-Hsps up-regulated due to heat shock, reported to be about 0.1% of total cell protein (Arrigo and Landry 1994), and that level was considered to be high. Like many crystallins in other organisms (Heikkila et al 1997; MacRae 2000), p26 is under tight developmental regulation in Artemia, first appearing in the encysted embryo (Jackson and Clegg 1996), then disappearing during the first larval stage (Liang and MacRae 1999). Interestingly, trehalose appears (Clegg et al 1999) and disappears (Slegers 1991) at roughly the same time, supporting the possibility that their functions are related and even synergistic, as some of our results suggest. Thus, unlike the general case for small heat shock/α-crystallins, p26 is present in very large amounts well in advance of environmental stresses that these embryos may encounter soon after release from females or even decades later (Clegg 1974; Clegg and Conte 1980). It appears that the developmental program of Artemia is designed to “premeditate” stress rather than to react to it through the usual stress response, which, by the way, does not appear to be operative in encysted embryos (Clegg et al 1999; Liang and MacRae 1999), unlike larvae (Miller and McLennan 1988) and adults (Frankenberg et al 2000) of this organism.
Previous work (Liang et al 1997a) indicated that p26 had molecular chaperone activity in vitro, and those results have been extended substantially in the present study, even though the assays used to measure chaperone activity differed considerably. One goal of the present study was to explore the mechanism by which p26 protects thermally inactivated CS from aggregation so we could better evaluate the influence of trehalose. Inactivation of CS is slowed appreciably in the presence of p26, indicating that the binding of p26 to folding intermediates of CS is transient and can be reversed partially simply by cooling to 25°C (Fig 5). Stability of p26-CS complexes is significantly increased with increasing time of heat stress (Figs 3 and 4). Those results indicate that p26, in a fashion similar to other small Hsps and α-crystallins (Rao et al 1993; Ehrnsperger et al 1997; Lee et al 1997), traps unfolding proteins (in our case, CS), preventing them from irreversible aggregation and keeping them in a folding-competent state until conditions permissible for refolding occur. Next, we consider the involvement of trehalose and its influence on chaperone activity of p26.
To our knowledge, all previous work on interactions between trehalose and any Hsp has used the yeast S cerevisiae. Abundant evidence documents the importance of trehalose for yeast thermotolerance (Eleutherio et al 1993; Hottiger et al 1994; Elliott et al 1996; Iwahashi et al 1998; Singer and Lindquist 1998a, 1998b; Wera et al 1999), although there is not complete agreement about the nature and extent of its participation (Nawaka and Holzer 1997; Alexandre et al 1998; Gross and Watson 1998). The first indication that trehalose and stress proteins somehow complemented each other in dealing with thermal stress came from the study by Elliott et al (1996), who found that mutants with defects in either trehalose metabolism or Hsp104 synthesis showed decreased resistance to heat and the double mutants even more so. These investigations built on previous work that showed that trehalose stabilized proteins and decreased their heat inactivation (Colaço et al 1994; Hottiger et al 1994), whereas Hsp104, which does not protect during heating, played a vital chaperone role afterward, restoring native structure to damaged proteins (Parsell et al 1994). Singer and Lindquist (1998b) then proposed, on the basis of chemical denaturation studies, that trehalose reduced aggregation by maintaining proteins in a nonnative state until they could be refolded by Hsp104, and they also reported that heating produced similar results. High concentrations of trehalose interfered with Hsp104 chaperoning, explaining several puzzling observations in the literature, such as the rapid degradation of trehalose after heat shock. Our results bear both similarities and differences to the situation in yeast. Unlike yeast Hsp104, Artemia p26 protects against aggregation during heating and acts as a chaperone to rescue some of the heat-inactivated CS when returned to recovery conditions (Fig 5). Those data also indicate that trehalose enhances p26 chaperone activity after heating, possibly by increasing the number of CS folding intermediates that p26 can chaperone back to the native state (Figs 4 and 5). Interestingly, trehalose hinders the formation of stable CS-p26 complexes and appears even to disrupt those that have formed (Fig 4, lanes 4 and 5 vs 3). In other words, trehalose might be acting as a “release factor,” much like oxaloacetate does for CS-Hsp25 complexes (Ehrnsperger et al 1997).
The in vitro chaperone activity of p26 does not require addition of ATP or GTP (Liang et al 1997a, 1997b), and we have confirmed that in the present study (Materials and Methods). Other molecular chaperones, such as Hsp70 and 90, require ATP for release of protein substrates. It seems possible that trehalose replaces the need for ATP through its participation as a “releasing factor.” That could be very important in these embryos whose metabolism comes essentially to a reversible standstill under severe stress, resulting in extremely low concentrations of ATP. We suggest that p26 has been adapted, in the evolutionary sense, not only to work in concert with trehalose but even to require it for optimal chaperone function.
It seems certain that trehalose plays a major role during heat stress in vivo, and our in vitro results suggest that p26 contributes to this protection, particularly when the trehalose concentration is not too high. What is the intracellular concentration of trehalose in hydrated encysted embryo cells? The value of 0.4 M used here was based on the total amount of trehalose (about 150 of μg/mg dry weight) and the water content of fully hydrated encysted embryos (about 1.4 g of H2O/g dry weight), which works out to be a bit less than 0.4 M “total concentration.” However, some of the trehalose appears to be sequestered in yolk platelets (Warner et al 1989), and not all intracellular water is available as solvent (Clegg and Drost-Hansen 1990). Thus, a trehalose concentration of 0.4 M is simply our best estimate for physiological conditions.
Trehalose is also an essential substrate for energy metabolism and other metabolic pathways in Artemia embryos, and its concentration decreases as development proceeds (Clegg and Conte 1980; Slegers 1991). Indeed, the application of high temperature to developing embryos that had already used some of their trehalose resulted in a rapid resynthesis of this sugar (Clegg and Jackson 1992). In this respect, the situation in Artemia is much different than in yeast, since trehalose does not interfere with p26 function to the extent that it does with yeast Hsp104.
In summary, the evidence presented here shows that trehalose and p26 interact in a complex way in vitro, suggesting that both are involved in protecting the cells of these embryos from stress-induced damage. Of course, other factors are probably involved in vivo, including the participation of other molecular chaperones (Glover and Lindquist 1998; Ellis 2000; Feldman and Frydman 2000; Lee and Vierling 2000), the specificity of protein substrates for chaperoning (Ellis 2000), and influences that result from the crowded intracellular environment (van den Berg et al 1999; Minton 2000). We are currently exploring these interesting possibilities. However, a principal conclusion from the present study is that p26, trehalose, and their interactions are definitely critical components of the adaptive repertoire of these remarkable embryos.
Acknowledgments
The technical contributions of Susan A. Jackson are appreciated. Diane Cosgrove is thanked for word processing, and we are obliged to Gary Cherr and his laboratory staff for the use of equipment. This study was supported by a grant from the National Science Foundation (MCB-98 07762).
REFERENCES
- Allison SD, Chang B, Randolph TW, Carpenter JF. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch Biochem Biophys. 1999;365:289–298. doi: 10.1006/abbi.1999.1175. [DOI] [PubMed] [Google Scholar]
- Alexandre H, Plourde L, Charpentier C, Francois J. Lack of correlation between trehalose accumulation, cell viability and intracellular acidification as induced by various stresses in Saccharomyces cerevisiae. Microbiology. 1998;144:1103–1111. doi: 10.1099/00221287-144-4-1103. [DOI] [PubMed] [Google Scholar]
- Arrigo A-P, Landry J 1994 Expression and function of the low-molecular-weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, New York, 335–373. [Google Scholar]
- Beissinger M, Buchner J. How chaperones fold proteins. Biol Chem. 1998;379:245–259. [PubMed] [Google Scholar]
- Browne RA, Sorgeloos P, and Trotman CNA 1991 Artemia Biology. CRC Press, Boca Raton, FL. [Google Scholar]
- Buchner J, Grallert M, Jakob U. Analysis of chaperone function using citrate synthase as non-native protein. Methods Enzymol. 1998;290:323–339. doi: 10.1016/s0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- Clark JI, Muchowski PJ. Small heat shock proteins and their potential role in human disease. Curr Opin Struct Biol. 2000;10:52–59. doi: 10.1016/s0959-440x(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Clegg JS. Biochemical adaptations associated with the embryonic dormancy of Artemia salina. Trans Am Microsc Soc. 1974;93:481–490. [Google Scholar]
- Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Conte FP 1980 A review of the cellular and developmental biology of Artemia. In: The Brine Shrimp Artemia, vol 7, ed Persoone G, Sorgeloos P, Roels O, Jaspers E. Universa Press, Wetteren, Belgium, 11–54. [Google Scholar]
- Clegg JS, Drost-Hansen W 1990 On the biochemistry and cell physiology of water. In: Biochemistry and Molecular Biology of Fishes, vol 1, ed Hochachka PW, Mommsen TP. Elsevier Science Publishers, Amsterdam, 1–23. [Google Scholar]
- Clegg JS, Jackson SA. Aerobic heat shock activates trehalose synthesis in embryos of Artemia franciscana. FEBS Lett. 1992;303:45–47. doi: 10.1016/0014-5793(92)80474-u. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Warner AH. Extensive intracellular translocations of a major protein accompany anoxia in embryos of Artemia franciscana. Exp Cell Res. 1994;212:77–83. doi: 10.1006/excr.1994.1120. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Liang P, MacRae TH. Nuclear-cytoplasmic translocations of protein p26 during aerobic-anoxic transitions in Artemia franciscana. Exp Cell Res. 1995;219:1–7. doi: 10.1006/excr.1995.1197. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Drinkwater LE, Sorgeloos P. The metabolic status of diapause embryos of Artemia franciscana. Physiol Zool. 1996;69:49–66. [Google Scholar]
- Clegg JS, Willsie JK, Jackson SA. Adaptive significance of a small heat shock/alpha-crystallin protein in encysted embryos of the brine shrimp, Artemia franciscana. Am Zool. 1999;39:836–847. [Google Scholar]
- Clegg JS, Jackson SA, Popov VI. Long-term anoxia in encysted embryos of Artemia franciscana: viability, ultrastructure, and stress proteins. Cell Tissue Res. 2000;301:433–446. doi: 10.1007/s004410000249. [DOI] [PubMed] [Google Scholar]
- Colaço CALS, Smith CJS, Sen S, Roser DH, Newman Y, Ring S, and Roser BJ 1994 Chemistry of protein stabilization by trehalose. In: Formulation and Delivery of Proteins and Peptides, ed Cleland JL, Langer R. American Chemical Society, Washington, DC, 222–240. [Google Scholar]
- Crowe JH, Hoekstra SA, Crowe LM. Anhydrobiosis. Ann Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Clegg JS, and Crowe LM 1998 Anhydrobiosis: the water replacement hypothesis. In: The Properties of Water in Foods (ISOPOW 6), ed Reid DS. Chapman and Hall, New York, 440–455. [Google Scholar]
- Crowe JH, Crowe LM, Petrelski S, Hoekstra FA, Araujo PD, and Panek AD 1997 Anhydrobiosis: cellular adaptation to extreme dehydration. In: Handbook of Physiology, section 13, vol II, ed Dantzler WH. Oxford University Press, New York, 1445–1477. [Google Scholar]
- Decleir W, Moens L, Slegers H, Sorgeloos P, and Jaspers E 1987 Artemia Research and Its Applications,vol 2. Universa Press, Wetteren, Belgium. [Google Scholar]
- de Jong WW, Caspers G-J, Leunissen JAM. Genealogy of the alpha-crystallin/small heat-shock protein super family. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Derham BK, Harding JJ. Alpha-crystallin as a molecular chaperone. Prog Retin Eye Res. 1999;18:463–509. doi: 10.1016/s1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- Drinkwater L, Clegg JS 1991 Experimental biology of cyst diapause. In: Artemia Biology, ed Browne RAP, Sorgeloos P, Trotman CNA. CRC Press, Boca Raton, FL, 93–118. [Google Scholar]
- Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Lilie H, Gaestel M, Buchner J. The dynamics of Hsp25 quaternary structure: structure and function of different oligomeric species. J Biol Chem. 1999;274:14867–14874. doi: 10.1074/jbc.274.21.14867. [DOI] [PubMed] [Google Scholar]
- Eleutherio ECA, Araujo P, Panek A. Protective role of trehalose during heat stress in Saccharomyces cerevisiae. Cryobiology. 1993;30:591–596. doi: 10.1006/cryo.1993.1061. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Chaperone substrates inside the cell. Trends Biochem Sci. 2000;25:210–212. doi: 10.1016/s0968-0004(00)01576-0. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hartl FU. Principles of protein folding in the cellular environment. Curr Opin Struct Biol. 1999;9:102–110. doi: 10.1016/s0959-440x(99)80013-x. [DOI] [PubMed] [Google Scholar]
- Elliott B, Haltiwanger RS, Futcher B. Synergy between trehalose and Hsp104 for thermotolerance in Saccaromyces cerevisiae. Genetics. 1996;144:923–933. doi: 10.1093/genetics/144.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu A, Russo MJ, Bieganski R, Fowler A, Cheley S, Bayley H, Toner M. Intracellular trehalose improves survival of cryopreserved mammalian cells. Nat Biotech. 2000;18:163–167. doi: 10.1038/72608. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr Opin Struct Biol. 2000;10:26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Fiege U, Morimoto RI, Yahara I, and Polla BS 1996 Stress-Inducible Cellular Responses. Birkhäuser-Verlag, Berlin. [PubMed] [Google Scholar]
- Frankenberg MM, Jackson SJ, Clegg JS. The heat shock response of adult Artemia franciscana. J Therm Biol. 2000;25:481–490. doi: 10.1016/s0306-4565(00)00013-9. [DOI] [PubMed] [Google Scholar]
- Ganea E, Harding JJ. α-Crystallin assists in the renaturation of glyceraldehyde-3-phosphate dehydrogenase. Biochem J. 2000;345:467–472. [PMC free article] [PubMed] [Google Scholar]
- Gaubin Y, Planel H, and Gasset G. et al. 1983 Results on Artemia cysts, lettuce and tobacco seeds in the Biobloc 4 experiment flown aboard Soviet Cosmos 1129. Adv Space Res. 3:135–140. [DOI] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Gross C, Watson K. Transcriptional and translational regulation of major heat shock proteins and patterns of trehalose mobilization during hyperthermic recovery in repressed and derepressed Saccharomyces cerevisiae. Can J Microbiol. 1998;44:341–350. doi: 10.1139/w98-006. [DOI] [PubMed] [Google Scholar]
- Guo N, Puhlev I, Brown DR, Mansbridge J, Levine F. Trehalose expression confers desiccation tolerance on human cells. Nat Biotech. 2000;18:168–171. doi: 10.1038/72616. [DOI] [PubMed] [Google Scholar]
- Hand SC, Hardewig I. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Ann Rev Physiol. 1996;58:539–563. doi: 10.1146/annurev.ph.58.030196.002543. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ, Ohan N, Tam Y, Ali A. Heat shock protein gene expression during Xenopus development. Cell Mol Life Sci. 1997;53:114–121. doi: 10.1007/PL00000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J, Huang Q-L, Ding L, Bova MP. Lens α-crystallin: chaperone-like activities. Methods Enzymol. 1998;290:365–408. doi: 10.1016/s0076-6879(98)90032-5. [DOI] [PubMed] [Google Scholar]
- Hottiger T, De Virgilio C, Hall MN, Boller T, Wiemken A. The role of trehalose for the acquisition of thermotolerance in yeast, II: physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem. 1994;219:187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- Iwahashi H, Nwaka S, Obuchi K, Komatsu Y. Evidence for the interplay between trehalose metabolism and Hsp104 in yeast. Appl Environ Microbiol. 1998;64:4614–4617. doi: 10.1128/aem.64.11.4614-4617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SA, Clegg JS. Ontogeny of low molecular weight stress protein p26 during early development of the brine shrimp, Artemia franciscana. Dev Growth Differ. 1996;38:153–160. doi: 10.1046/j.1440-169X.1996.t01-1-00004.x. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000;122:189–197. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. Expression, purification, and molecular chaperone activity of plant recombinant small heat shock proteins. Methods Enzymol. 1998;290:350–364. doi: 10.1016/s0076-6879(98)90031-3. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Amons R, MacRae TH, Clegg JS. Purification, structure and molecular chaperone activity in vitro of Artemia p26, a small heat shock/α-crystallin protein. Eur J Biochem. 1997a;243:225–232. doi: 10.1111/j.1432-1033.1997.0225a.x. [DOI] [PubMed] [Google Scholar]
- Liang P, Amons R, Clegg JS, MacRae TH. Molecular characterization of a small heat-shock/α-crystallin protein from encysted Artemia embryos. J Biol Chem. 1997b;272:19051–19058. doi: 10.1074/jbc.272.30.19051. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- Lindner RA, Kapur A, Mariani M, Titmuss SJ, Carver JA. Structural alterations of α-crystallin during its chaperone action. Eur J Biochem. 1998;258:170–183. doi: 10.1046/j.1432-1327.1998.2580170.x. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH, Bagshaw JC, and Warner AH 1989 Biochemistry and Cell Biology of Artemia. CRC Press, Boca Raton, FL. [Google Scholar]
- Miller D, McLennan AG. The heat shock response of the cryptobiotic brine shrimp, Artemia salina I: thermotolerance. J Therm Biol. 1988;13:119–123. [Google Scholar]
- Minton AP. Implications of macromolecular crowding for protein assembly. Curr Opin Struct Biol. 2000;10:34–39. doi: 10.1016/s0959-440x(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C 1994 The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Morris JE. Hydration, its reversibility, and the beginning of development in the brine shrimp, Artemia salina. Comp Biochem Physiol. 1971;39A:843–857. [Google Scholar]
- Nawaka S, Holzer H. Molecular biology of trehalose and trehalases in the yeast Saccharomyces cerevisiae. Prog Nucl Acid Res Mol Biol. 1997;58:197–237. doi: 10.1016/s0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S. Protein disaggregation by heat-shock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- Persoone G, Sorgeloos P, Roels O, and Jaspers E 1980 The Brine Shrimp, Artemia,vol 2. Universa Press, Wetteren, Belgium. [Google Scholar]
- Rao V, Horwitz J, Zigler JS. Alpha-crystallin, a molecular chaperone, forms a stable complex with carbonic anhydrase upon heat denaturation. Biochem Biophys Res Commun. 1993;190:786–793. doi: 10.1006/bbrc.1993.1118. [DOI] [PubMed] [Google Scholar]
- Rao CM, Raman B, Ramakrishna T, Rajaraman K, Ghosh D, Datta S, Trivedi VD, Sukhaswami MB. Structural perturbation of α-crystallin and its chaperone-like activity. Int J Biol Macromol. 1998;22:271–281. doi: 10.1016/s0141-8130(98)00025-7. [DOI] [PubMed] [Google Scholar]
- Robinson JB, Brent LG, Sumegi B, and Srere PA 1987 Enzymatic approach to the study of the Krebs tricarboxylic acid cycle. In: Mitochondria—a Practical Approach, ed Darley-Usmar VM, Rickwood D, Wilson MT. Plenum Press, New York, 160–161. [Google Scholar]
- Singer MA, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998a;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- Singer MA, Lindquist S. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotech. 1998b;16:460–468. doi: 10.1016/s0167-7799(98)01251-7. [DOI] [PubMed] [Google Scholar]
- Slegers H 1991 Enzyme activities through development: a synthesis of the activity and control of the various enzymes as the embryo matures. In: Artemia Biology, ed Browne RAP, Sorgeloos P, Trotman CNA. CRC Press, Boca Raton, FL, 37–74. [Google Scholar]
- Somero GN, Yancey PH 1997 Osmolytes and cell volume regulation: physiological and evolutionary principles. In: Handbook of Physiology,section 14, ed Hoffman JF, Jamieson JD. Oxford University Press, New York, 441–484. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B, Ellis RJ, Dobson CM. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 1999;18:6927–6933. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den IJssel P, Norman DG, Quinlan RA. Molecular chaperones: small heat shock proteins in the limelight. Curr Biol. 1999;9:R103–R105. doi: 10.1016/s0960-9822(99)80061-x. [DOI] [PubMed] [Google Scholar]
- Vanhoudt J, Aerts T, Abgar S, Clauwaert J. Quaternary structure of bovine α-crystallin: influence of temperature. Int J Biol Macromol. 1998;22:229–237. doi: 10.1016/s0141-8130(98)00020-8. [DOI] [PubMed] [Google Scholar]
- Warner AH, MacRae TH, and Bagshaw JC 1989 Cell and Molecular Biology of Artemia Development. Plenum Press, New York. [Google Scholar]
- Warner AH, Jackson SA, Clegg JS. Effect of anaerobiosis on cysteine protease regulation during the embryonic-larval transition in Artemia franciscana. J Exp Biol. 1997;200:897–908. doi: 10.1242/jeb.200.5.897. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wera S, De Schrijver E, Geyskens I, Nwaka S, Thevelein JM. Opposite roles of trehalase activity in heat-shock recovery and heat-shock survival in Saccharomyces cerevisiae. Biochem J. 1999;343:621–626. [PMC free article] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero G. Living with water stress: evolution of osmolytes systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]