Abstract
Induction of heat shock proteins (Hsps) is often associated with a cellular response to a harmful stress or to adverse life conditions. The main aims of the present study were (1) to assess if stress-induced Hsp70 could be used to monitor exposure of the earthworm species Lumbricus terrestris to various soil pollutants, (2) to assess the specificity of pollutants in their tissue targeting and in Hsp70 induction, and (3) to evaluate if dose-response relationships could be established and if the stress-response observed was specific. The midgut/intestinal tissues of L. terrestris are shown to express an inducible member of the Hsp70 family after heat shock treatment in vitro and exposures to different soil toxicants in vivo (re: artificial soil). Short-term (24–72 hours) and long-term (14–16 days) exposures to the chemical standards chloroacetamide and pentachlorophenol and to heavy metals (Pb++, Cd++, Cu++, and Hg++) also affected the earthworms, and Hsp70 was induced in their midgut/intestinal tissues. After a 3-day exposure to heavy metals, the level of Hsp70 induction in the midgut/intestinal tissues appears to correlate well with the reported in vivo and in vitro toxicity data. Comparatively, in proximal and midbody wall muscle tissues of animals exposed to the heavy metals, a decrease in expression of Hsp70 was sometimes detected. Thus Hsp analysis by Western blot in L. terrestris tissues and particularly in the midgut/intestine proved to be a suitable and sensitive assay for adverse effects in earthworms and showed a good level of reproducibility despite some individual variations. The use of pristine/nonexposed animals transposed into contaminated environments as in the present study should therefore be of high ecological relevance. Induction of Hsp70 in earthworms should represent not only a good wide-spectrum biomarker of exposure but also a biomarker of effect since known toxicants altered gene expression in tissues of these animals, as contrasted with a simple accumulation of Hsp. Hence, the detection of Hsp70 in earthworms can constitute an early-warning marker for the presence of potentially deleterious agents in soils, with L. terrestris in particular and earthworms in general acting as potential sentinel animal species.
INTRODUCTION
Heat shock proteins (Hsps) are families of proteins that, when expressed, play an important role of protection and maintenance of many vital cellular functions. Many Hsps play a chaperone role by folding newly synthesized proteins to acquire their proper 3-dimensional conformation and have also been implicated in protein translocation or in protein repair (Lindquist and Craig 1988; Schlesinger 1990; Becker and Craig 1994; Morimoto et al 1994; Hartl 1996; Fink 1999). First described in experiments in which sudden increases in temperature were used, hence their name, it is now well acknowledged that Hsp synthesis can be induced by a wide variety of stress factors (Schlesinger 1990; Nover 1991; Sanders 1993). The main Hsp, Hsp70, is highly conserved among eukaryotes and prokaryotes (Kelley and Schlesinger 1982; Lindquist 1986; Sanders 1990; Ang et al 1991). As members of the Hsp70 are often the prominent proteins to be expressed following environmental insults, these stress proteins are therefore very useful biomarkers that have been used to monitor the impact of environmental factors on various animal species, including many invertebrates (Köhler et al 1992; Sharp et al 1994; De Pomerai 1996; Eckwert and Köhler 1997; Lewis et al 1999).
Earthworms, the major invertebrate biomass of the soil, are one of the first animal species to be affected by soil contaminants, their feeding mode and their elevated surface/volume ratio facilitating the assimilation and the fast distribution of toxicants to target tissues. In addition, these invertebrates present a large diversity and are thus of great ecological interest (Bouché 1972; Abdul-Rida 1994; Edwards 1998). Hence, they constitute an attractive tool and a less controversial surrogate animal species to vertebrates for the biomonitoring of polluted soils (Venables et al 1992; Li et al 1994). Despite recognition of its potential for the evaluation of sublethal effects of soil pollutants (Venables et al 1992), Hsp induction has been studied only by immunochemistry and in the earthworm species Lumbricus rubellus, either after caudal amputation and parasitic infection (Mariño and Morgan 1998) or after exposure to metalliferous soils (Mariño et al 1999).
In the present study, the species Lumbricus terrestris was chosen because its widespread distribution allows in-the-field assessments and its larger size permits given target tissues or cells to be isolated easily (eg, Ireland 1978; Morgan 1981; Prentø 1987; Diogène et al 1997; Affar et al 1998). Secondly, this particular earthworm species can be readily maintained under laboratory conditions for in vitro and in vivo experiments (eg Morgan 1981; Edwards and Coulson 1992; Li et al 1994; Walsh et al 1995; Diogène et al 1997). The midgut/intestinal tissues were the main targets selected in the present study since they are key tissues for the assimilation of soil elements (Prentø 1987). Earthworms are also known to be selective in their ingestion of materials (eg, Morgan and Morgan 1992). Moreover, soil pollutants may cause pathophysiological effects on the midgut (eg, Yongcan et al 1998), and many detoxification mechanisms have been associated with midgut tissues (Ireland 1978; Stenersen 1984; Prentø 1987; Morgan et al 1993; Prentø 1994).
Therefore, using the Western blot technique, the aims of the present study were (1) to assess if stress-induced Hsp70 could be used to monitor exposure of L. terrestris to various soil pollutants, (2) to assess specificity of pollutants in their tissue targeting and in Hsp70 induction, and (3) to evaluate if dose-response relationships could be established and if the stress-response observed was specific to a given pollutant or type of pollutant. The chemical toxicants pentachlorophenol (PCP) and chloroacetamide (CLA) were selected because they are widely used in lethality tests (LC50) and their effects on earthworms are well documented (eg, Edwards and Coulson 1992; van Gestel 1992). Heavy metal toxicity has also been extensively studied in earthworms (eg, Ma 1982; Fugère et al 1996). As these metals can be found in many contaminated industrial sites (eg, Morgan and Morgan 1992; Yongcan et al 1998; Mariño et al 1999), the effects of lead (Pb++), copper (Cu++), mercury (Hg++), and cadmium (Cd++) were also evaluated.
MATERIAL AND METHODS
Products
Basal Eagle culture medium/BME, antibiotic-antimycotic mixture/PSF and Fungizone (Gibco BRL, Burlington, Ontario, Canada); toxic reference chemicals chloroacetamide/CLA and pentachlorophenol/PCP, soybean trypsine inhibitor and phenylmethylsulfonyl fluoride/PMSF, β-mercaptoethanol/β-ME and sodiumdodecyl sulfate/SDS (Sigma-Aldrich Canada, Oakville, Ontario, Canada); and heavy metals as chloride salts (Fisher Scientific, Fair Lawn, NJ, USA, or J.T. Baker Chemical Co, Phillipsburg, NJ, USA); acrylamide, bis-acrylamide, Trans-Blot nitrocellulose or Immun-Blot PVDF 0.2 μm and Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories [Canada] Ltd, Mississauga, Ontario, Canada); Hybond-C nitrocellulose/0.45 μm (Amersham Pharmacia Biotech Inc, Baie d'Ùrfé, Québec, Canada); and bovine serum albumin/BSA and BM Chemiluminescence Western Blotting Kit (Boehringer Mannheim Gmbh, Mannheim, Germany) were obtained as indicated. Other products were reagent grade, and all water-based solutions were prepared with high-quality (≥18 Mohm-cm) UV-treated, demineralized, and ultrafiltered water (Elgastat Maxima Water Purification System, Life Sciences Model, Elga Ltd, Bucks, England).
Earthworm source and maintenance
Adult L. terrestris (clitellate, 3–7 g wet body mass) were purchased from a local supplier (Anilab Enr., Ste-Foy, Québec, Canada). The earthworms were housed, fed, and maintained as previously described (Diogène et al 1997; Affar et al 1998). The protocols for the exposures and maintenance were approved by the institutional animal care committee and were conducted according to Canadian guidelines.
In vitro exposure
For the heat shock treatment, the midgut tissue (eg, Prentø 1987)—that is, the total intestine, including the surrounding chloragogenous tissue—was obtained as follows. The earthworms were gently washed under tap water and then anesthetized (≈10 minutes) in an ice-cold 10% ethanol solution as described in Prentø (1987, 1994) but made instead with a modified Hanks' balanced salt solution/MHBSS, pH 7.4, 210 mOsm (Diogène et al 1997; Affar et al 1998). Afterward, the postclitellar region of each earthworm was longitudinally dissected ventral side up, and the midgut tissue below the gizzard was exposed. The intestinal tract was cut open (opposite side of the typhlosole) and as close as possible to the anus. The luminal surface of the intestine was rinsed several times with MHBSS heavily fortified with PSF and Fungizone (2×) to wash away the contents. Then the midgut/intestinal tissue was removed from the body cavity and kept at 15°C (±0.5°C) in fortified MHBSS. During the whole procedure, care was taken to avoid as much as possible damage to the peripheral and typhlosole-associated chloragogenous tissue.
All the subsequent steps were done in a BME culture medium supplemented with 4 mM sodium bicarbonate, PSF (1×), and Fungizone (1×). The midgut/intestinal tissues were first pre-equilibrated for 30 minutes at 15°C. The tissues were then incubated for 1 hour at the selected temperatures of 15°C/control, 27°C, 30°C, 32°C, or 37°C (±0.5°C). Following the heat shock treatment, each tissue was allowed a 1-hour recovery period at 15°C before being processed for Hsp analysis as described in the following.
In vivo exposures
Prior to the exposure, animals were kept for at least 1 week in the artificial soil used for the toxicity tests. The artificial soil composition was 29% peat moss, 60% sand, 10% clay, and a 1% CaCO3 supplement for buffering (eg, Schrader 1992); the soil was humidified (65%–80%) with preboiled tap water to lower the chlorine content. Solutions of pollutants were prepared either in water (CLA and heavy metals) or in organic solvents (ethanol or acetone for PCP). Heavy metal solutions were acidified to ≈pH 4 with a few drops of HCl to facilitate dissolution, stability, and preservation. All solutions were mixed with 250 g of soil (dry weight), and, in order to reduce toxicity, the organic solvents were allowed to evaporate for at least 24 hours prior to the expositions. The soils were then placed into Mason-type glass jars covered with a geotextile cloth perforated with numerous pin-size holes to allow respiration.
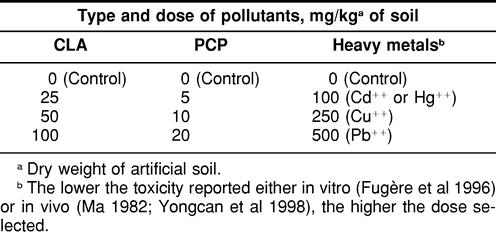
Control soils were prepared accordingly using only the corresponding solvent solution and were used for both unexposed animals as well as for assessing water evaporation. The percentage of humidity of the soil mixture had to be corrected only for the long exposures (>3 days). One or 2 worms/jar were exposed for various time periods (24 hours to 16 days) in a custom-built environmental chamber (Model GH-20-GTL, Foster Refrigerator of Canada, St-Hyacinthe, Québec, Canada), and the exposure parameters were set at 15°C (±2°C) and 95% humidity, with continuous lighting and air circulation with exhaust air evacuation (2 minutes) every hour. No animals died during the in vivo experiments whether exposed for 24 hours (minimum period) or 16 days (maximum period), indicating that the concentrations of the tested chemicals and metals were in sublethal ranges (Table 1).
Table 1.
Concentrations of chloroacetamide (CLA), pentachlorophe~nol (PCP) and heavy metals used for in vivo exposure of Lumbricus terrestris earthworms
After the exposures, the midgut/intestinal tissues of individual earthworm were removed and processed as described previously. However, for the heavy metal exposures, proximal tissues (whole animal from the mouth to the gizzard, ie, including the body wall and internal organs) and a sample of the midbody wall muscle tissues (including the cuticle, some septa, and small blood vessels left in the body cavity) where the midgut/intestine was removed were also retained for Hsp analysis. These latter tissues were selected mainly because some organs, like the calciferous glands in the proximal section of this earthworm species, can concentrate heavy metals (Schrader 1992). The body wall muscles also constitute the first tissues to be exposed to soil pollutants and make up the bulk of the weight of these animals.
Tissue preparation for protein and Hsp analysis
At the end of each in vivo or in vitro exposure, the tissue samples were handled the same way. Freshly dissected tissues were used with a few exceptions where the tissues were quickly frozen in liquid nitrogen before being stored (−30°C). First, the tissue samples were processed with handheld microhomogenizers (Micropestle, Brinkmann Instruments [Canada] Ltd, Mississauga, Ontario, Canada, or Kontes Pellet Pestle, Fisher Scientific) in MHBSS supplemented with 10 μg/mL antitrypsin and 2.97 mM PMSF as protease inhibitors, and 0.12 mM β-ME. The homogenates were then sonicated for 15–30 seconds (W-10 Sonicator, Heat Systems Ultrasonics, Plainview, NY, USA) and centrifuged at 12 000 × g for 3 minutes (Microfuge 12, Beckman Instruments [Canada] Inc, Montréal, Québec, Canada). The tissue supernatants were used for polyacrylamide gel electrophoresis (PAGE) and for protein determination. Protein assays were performed in triplicate using a modified Bradford procedure adapted for measurements in 96 wells plates and BSA as a standard (Vincent and Nadeau 1983; Affar et al 1998).
SDS-PAGE was conducted as follows. Tissue supernatants were mixed 2:1 (v/v) with Laemmli sample buffer (Bio-Rad) containing 5% (v/v) β-ME and heated in boiling water for 12 to 15 minutes. Generally, for a given set of experiment, equal amounts of proteins (in 35–40-μL volumes for a 0.75–1.0-mm gel thickness) were separated using a 3% acrylamide stacking gel and a 12% acrylamide resolving gel (Thomas and Kornberg 1975). The electrophoresis was run in a mini-Protean II gel apparatus (Bio-Rad) at constant voltage for about 1 hour (150 V/first 15 minutes, 200 V/∼45 minutes). Molecular weight standards ranging from 14.4 to 94 kDa (Low Molecular Weight Electrophoresis Calibration Kit, Pharmacia Fine Chemicals, Piscataway, NJ, USA) or from 7.6 to 216 kDa (Bio-Rad Kaleidoscope Polypeptide Molecular Weight Protein Standards) were run at the same time on each gel as well as at least 1 Hsp70-positive sample (human MCF7 cells, Drosophila heat-shocked S2 cells, or L. terrestris midgut/intestinal tissue heat-shocked at 30°C). However, the Bio-Rad Kaleidoscope standards should be avoided in this system because its presence caused a very strong false-positive chemiluminescence reaction.
Western blots
The SDS-PAGE resolved proteins were electrotransferred onto nitrocellulose (PCP and CLA) or PVDF membranes (heavy metals) at 100 V for 1 hour, then stored at 4°C in a TRIS-buffered saline (TBS, pH 7.4, 50 mM TRIS, 150 mM NaCl) until immunoblotting. The membranes were thereafter treated essentially according to the manufacturer's instructions (BM kit #1520709). The primary antibody, a rabbit antibody against human Hsp71 (#799) specific for the inducible member of the Hsp70 (Tanguay et al 1993), was diluted 1/5000, and the secondary antibody (BM anti-rabbit/anti-mouse horseradish peroxidase–coupled IgGs) was diluted 1/12 500. The chemiluminescence reaction was revealed by exposing an X-ray film (Fuji Medical Super RX).
RESULTS
Heat shock treatment induces Hsp70
Midgut/intestinal tracts of L. terrestris were shown to respond in vitro to a heat shock treatment (Fig 1). The migration behavior of the L. terrestris Hsp70 more closely resembled that of the Drosophila protein (heat-shocked S2 cells) than that of the human homologue (MCF7 cells). This experiment clearly shows a temperature-dependent induction of Hsp70 in the midgut/intestinal tissues of this invertebrate species, with a probable critical exposure temperature response around 30°C. Above that temperature range, the induction of Hsp70 decreased and almost completely disappeared when the tissue was incubated at 37°C. It is interesting to point out that a heat stress response was not observed at 15°C, a temperature reported to be in the “optimal activity” range for this earthworm species (Fitzpatrick et al 1987).
Fig. 1.
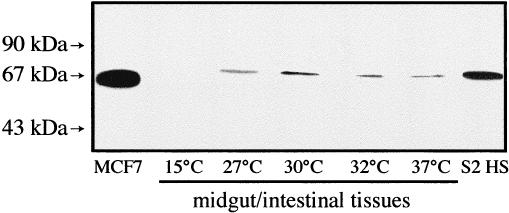
Western blot analysis of Hsp70 expression in the midgut/intestinal tissues of Lumbricus terrestris earthworms after heat shock treatments in vitro. The dissected tissues were heat-shocked for 60 minutes at the indicated temperatures followed by a 60-minute recovery at 15°C. Molecular weight standards are indicated by arrows. S2 HS is Drosophila heat-shocked S2 cells. Human MCF7 cells have also been used as a positive Hsp70 control; 40 μg of midgut/intestinal proteins were loaded on the gel
Exposure to PCP and CLA induces Hsp70
In vivo exposures to the toxicity reference chemical PCP are illustrated in Figure 2. After 24 hours of exposure, maximal induction in the midgut/intestinal tissues was observed at a dose of 10 mg/kg soil (Fig 2A). After a 48-hour exposure period, the tissue of the animal exposed to 20 mg of PCP showed no Hsp70 (Fig 2B). A light induction was also observed in the control animals in these experiments (0 mg PCP, Fig 2A,B). The latter response may be due to the solvent ethanol. Thus, despite 24 hours of evaporation of the ethanol used to prepare the PCP solution, control animals appeared somewhat “sensitized,” as they were found laying at the surface of the artificial soil instead of normally staying buried in the soil. When the solvent of the PCP solution was changed for acetone, the worms showed normal behavior, and expression of Hsp70 in the midgut tissue of control animal was only rarely observed (data not shown). In addition, we noted that an Hsp response in the midgut/intestinal tissues of control earthworms may be linked to the health status of the animal used. Animals that would be in poorer condition and/or possibly wounded or infested (Mariño and Morgan 1998; Mariño et al 1999), or possibly overreacting to handling (Sharp et al 1994) may be more prone to present a stress response apparently not related to the presence of a given toxicant in the soil.
Fig. 2.
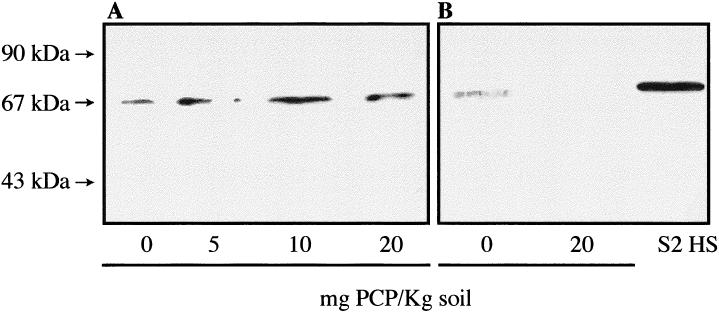
Hsp70 expression in the midgut/intestinal tissues of Lumbricus terrestris earthworms exposed in vivo to the chemical pentachlorophenol (PCP). (A) 24-hour exposure; (B) 48-hour exposure. Molecular weight standards are indicated by arrows. S2 HS is Drosophila heat-shocked S2 cells. The doses of PCP/kg of soil are indicated, and 0 mg represents control animals; 40 μg of proteins were loaded on the gel
After exposure to CLA, maximum expression in the midgut/intestinal tissues was observed at a dose of 25 mg/kg soil after a 24-hour exposure period (Fig 3A). However, after 48 hours of exposure, an earthworm exposed to a 100-mg/kg soil of CLA apparently expressed more Hsp70 than another animal exposed to a dose of 50 mg/kg soil (Fig 3B). Even after 14 days of exposure to CLA, a standardized time period used in earthworm LC50 tests (eg, Edwards and Coulson 1992), expression of Hsp70 in the midgut/intestinal tissues of L. terrestris was still detectable (Fig 3C). After such a long exposure period, the animal exposed to 50 mg/kg soil presented the strongest stress response.
Fig. 3.
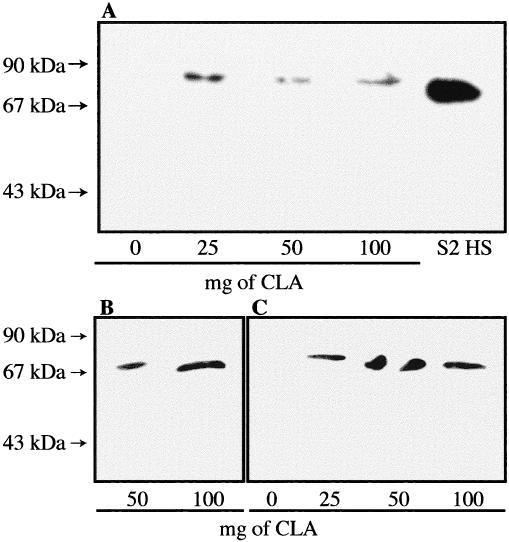
Expression of Hsp70 in the midgut/intestinal tissues of Lumbricus terrestris earthworms exposed in vivo to the chemical chloroacetamide (CLA). The exposure times were (A) 24 hours, (B) 48 hours, and (C) 14 days. Molecular weight standards are indicated by arrows. S2 HS is Drosophila heat-shocked S2 cells. The doses of CLA/kg of soil are indicated, and 0 mg represents control animals; 40 μg of proteins were loaded on the gels
As illustrated in Figure 4, some animal-to-animal variation in stress response is to be expected. A significant difference in Hsp70 induction in the midgut/intestinal tissues can be noted between 2 separate individuals both exposed in the same glass jar to the same dose of 25 mg CLA/kg soil. Therefore, some caution is advisable in the interpretation of the data when only a small number of animals is to be analyzed.
Fig. 4.

Animal variation in midgut/intestinal tissues expression of Hsp70 after in vivo exposure of Lumbricus terrestris earthworms to chloroacetamide (CLA) for 14 days. For each exposure condition, 2 animals were placed in the same glass jar. Molecular weight standards are indicated by arrows. S2 HS is Drosophila heat-shocked S2 cells. The doses of CLA/kg of soil are indicated, and 0 mg represents control animals; 17 μg of proteins from control earthworms were loaded on the gel compared to 35 μg for the CLA-exposed animals
Exposures to heavy metals and induction of Hsp70
Short-term exposure (3 days)
A preliminary test with a dose of 100 mg Pb++/kg soil indicated that the midgut/intestinal tissues of L. terrestris could also present a stress response after a 24-hour exposure to this heavy metal (Fig 5). To assess further the response of various tissues of this earthworm species to different heavy metals, animals were exposed for 3 or 16 days (Figs 6 and 7, respectively) to sublethal concentrations of Pb++, Cd++, Cu++, and Hg++. In the proximal tissues of the control earthworms, whether after 3 or 16 days of confinement in the artificial soil, a strong Hsp70 signal as well as an unexpected reactivity of the anti-human Hsp70 primary antibody with proteins of ≈90 kDa was observed (Figs 6A and 7A, 0 mg). This ≈90-kDa signal may represent a tissue-specific isoform of Hsp70 or an unrelated protein recognized by the rabbit antiserum, its identity remaining undetermined at this time. This Hsp70/≈90-kDa pattern was also observed in the midbody muscle tissues of the control earthworms (Figs 6B and 7B, 0 mg) but not in their corresponding midgut/intestinal tissues (Figs 6C and 7C, 0 mg). In the latter tissues, the control earthworm (Fig 6C, 0 mg) presented a relatively strong Hsp70 band after 3 days. This control animal may have been somewhat more stressed by the experimental conditions and/or was in poorer health at the beginning because a companion earthworm that remained in the same artificial soil and in the same glass jar for 16 days did not present a stress response in its midgut tissue (Fig 7C, 0 mg). This may again represent some individual difference between animals.
Fig. 5.
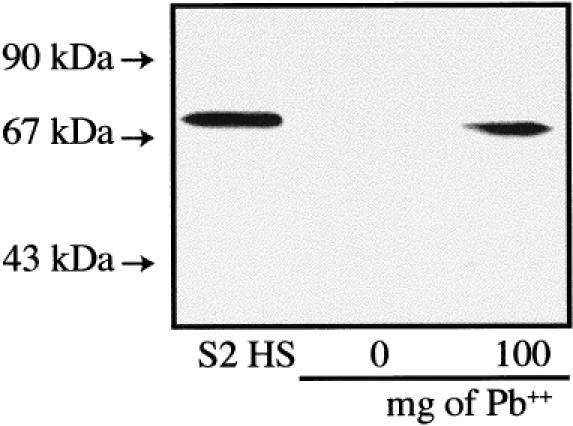
Expression of Hsp70 in the midgut/intestinal tissues of Lumbricus terrestris earthworms exposed in vivo to the heavy metal lead (Pb++). Exposure time was 24 hours, and doses of Pb++/kg of soil are indicated. The control earthworm (0 mg) was not placed in an artificial soil mixture but rather came directly from the animal stock facility. Molecular weight standards are indicated by arrows. S2 HS is Drosophila heat-shocked S2 cells; 40 μg of proteins were loaded on the gel.
Fig. 6.
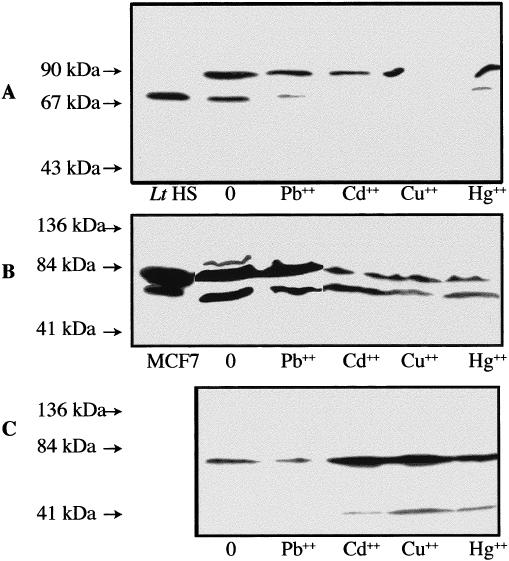
Western blot analysis of Hsp70 and cross-reacting bands in various tissues of Lumbricus terrestris earthworms exposed in vivo to diverse heavy metals. The exposure time to lead (Pb++), cadmium (Cd++), copper (Cu++), and mercury (Hg++) was 3 days, and the proximal (A), midbody wall muscles (B), and midgut/intestinal (C) tissues were analyzed. Molecular weight standards are indicated by arrows. Lt HS is L. terrestris midgut/intestinal tissues heat shocked in vitro at 30°C. Human MCF7 cells have also been used as a positive Hsp70 control. The respective doses of heavy metals in mg/kg of soil are 0 mg (control), 500 mg Pb++, 100 mg Cd++, 250 mg Cu++, and 100 mg Hg++; 60 μg of proteins were loaded on the gels
Fig. 7.
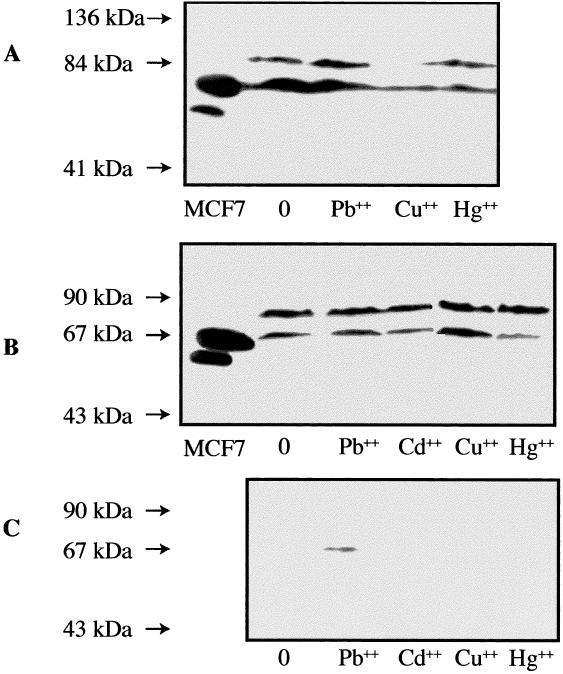
Western blot analysis of Hsp70 and cross-reacting bands in various tissues of Lumbricus terrestris earthworms exposed in vivo to diverse heavy metals. The exposure time to lead (Pb++), cadmium (Cd++), copper (Cu++), and mercury (Hg++) was 16 days, and the proximal (A), midbody wall muscles (B), and midgut/intestinal (C) tissues were analyzed. Molecular weight standards are indicated by arrows. Human MCF7 cells were used as a positive Hsp70 control. The respective doses of heavy metals in mg/kg of soil are 0 mg (control), 500 mg Pb++, 100 mg Cd++, 250 mg Cu++, and 100 mg Hg++; 60 μg of proteins were loaded on the gels
After 3 days of exposure to heavy metals (Fig 6), only the midgut/intestinal tissues of the earthworms presented a clear induction of Hsp70 (Fig 6C) compared to the midbody muscle (Fig 6B) or proximal (Fig 6A) tissues. However, the Hsp70 response in the midgut/intestinal tissues of the earthworm exposed to Pb++ appeared somewhat reduced compared to that of the control animal, which in this experiment presented an unusually strong expression of stress protein as discussed previously (Fig 6C). In the midgut/intestinal tissues of the Cd++-, Hg++-, and Cu++-exposed earthworms (Fig 6C), a reactivity of the anti-human Hsp70 primary antibody with proteins of >40 kDa was observed. The Cu++-exposed animal presented the strongest reactivity. Whether or not the >40-kDa band represents a degradation product of Hsp70 (Becker and Craig 1994), a related protein or a totally unrelated protein is unknown at this time.
In the proximal tissues of the metal-exposed earthworms, a weak expression of Hsp70 was observed after the 3-day exposure to Pb++ and Hg++, but no bands were seen after similar exposures to Cd++ or Cu++ (Fig 6A). One can also note a decreased response of the primary antibody with the ≈90-kDa proteins in the proximal tissues of these metal-treated earthworms. On the other hand, the Hsp70 and ≈90-kDa signals were decreased in the midbody muscle tissues of the Cd++-, Cu++-, and Hg++-exposed earthworms as compared to the control and Pb++-exposed animals (Fig 6B).
Long-term exposure (16 days)
Interestingly, after the 16 days of exposure to the heavy metals (Fig 7), the strong induction of Hsp70 observed in the midgut/intestinal tissues of the treated animals in the short-term exposure was not seen (Fig 7C). One exception was the exposure to Pb++, which still presented a small increase in expression compared to the midgut/intestinal tissues of the control animal (Fig 7C). It is of interest to point out again the absence of the ≈90-kDa signal in the intestinal tissue of all the animals analyzed in the long-term exposure. In the midbody muscle tissues (Fig 7B), only the earthworms treated with Hg++ and to lesser extent Cd++ presented a significant difference in the Hsp70/≈90-kDa pattern with a decrease in expression of Hsp70 compared to the muscle tissues of the control animal.
In long-term–exposed L. terrestris (Fig 7A), the proximal tissues of the animals exposed to Cu++ and Hg++ presented significant decreases in expression of Hsp70, while there was not much difference in expression of Hsp70 in the proximal tissues of Pb++-exposed animal compared to that of the control earthworm. A reduced expression of the ≈90-kDa band was observed in the proximal tissues of the Cu++- and Hg++-treated earthworms. The ≈90-kDa signal in the proximal tissues of the Pb++-treated animal did not differ much from that of the control earthworm (Fig 7A).
DISCUSSION
Hsps are expressed in numerous tissues from several animal species, and their presence is often associated with a response to a harmful stress situation or to adverse life conditions. The highly conserved nature of the Hsp70 was confirmed, as our human anti-Hsp70 primary antibody could detect induced Hsp70 in various tissues of L. terrestris earthworms as well as in a heat-shocked Drosophila fruit fly cell line used as a positive control. In the present study, the midgut/intestinal tissues of L. terrestris earthworms were shown to respond to a heat shock treatment in vitro and to different toxicants in vivo when the latter were added to a laboratory mimic of their natural habitat (re: artificial soil). Unquestionably, the chemicals (CLA and PCP) and the heavy metals (Pb++, Cd++, Cu++, and Hg++) tested also affected the animals, as a stress-induced Hsp70 response was observed. Generally, the stress response observed in L. terrestris earthworms and detected by immunoblot resemble that described in many other animals, including invertebrate species (eg, Köhler et al 1992; Sharp et al 1994; Eckwert and Köhler 1997; De Wachter et al 1998; Lewis et al 1999).
Recently, the induction of the Hsp60, Hsp70, and Hsp90 in several tissues of the earthworm species L. rubellus exposed to heavy metals has been detected by immunohistochemistry (Mariño et al 1999). These authors also reported that Hsc70 levels were generally low in the intestinal tissue of L. rubellus. Since our primary antibody was presumed to be specific for induced Hsp70s (Tanguay et al 1993), we assume that the expression of Hsc70 in the midgut tissue of L. terrestris could not be detected in our blotting assay. On the other hand, notwithstanding possible negative effects due to lower health-related condition and/or handling-related stress induction in some animals, the low levels of Hsp70 that were sometimes observed in the midgut tissue of control earthworms may be indicative of low constitutive levels of Hsp70 in L. terrestris species. Another source of anti-Hsp70 capable of recognizing both the constitutive (Hsc) and the inducible form (Hsp) of Hsp70 (eg, Lewis et al 1999; Mariño et al 1999) should be used to investigate the relative importance of each form in L. terrestris tissues.
In the intestinal tract of L. rubellus, immunolocalization revealed that the heavy metal–induced Hsp70 response was restricted to the chloragocytes. The alimentary epithelial cells and blood vessels expressed Hsp90 instead of Hsp70 (Mariño et al 1999). Thus, although this will have to be confirmed, it would appear that the Hsp70 response observed in the midgut/intestinal tissues of L. terrestris earthworms exposed to heavy metals, and possibly with the chemicals CLA and PCP, could also have been induced in the chloragogenous tissue. Effectively, this latter tissue accounts for a significant proportion by weight of the midgut/intestinal samples analyzed in this study. Although speculative, this assessment is most likely proper, considering that detoxification by metal sequestration (Ireland 1978; Ireland and Richards 1981; Morgan 1981; Morgan et al 1993) or by uptake and storage of xenobiotics (Prentø 1994) in earthworm chloragosomes is of major importance for these invertebrate animals.
The doses of heavy metals selected in our study were based on the relative in vitro toxicity of these pollutants on L. terrestris coelomocytes (Pb++ < Hg++ ≅ Cd++; Fugère et al 1996), the latter being free-floating cells that can be collected from the coelomic fluid bathing the midgut tissue of earthworms (Diogène et al 1997). In vivo, Cu++ was reported to be less toxic than Cd++ but more toxic than Pb++ (Ma 1982). In the present study, one can note that the expression of Hsp70 in the midgut/intestinal tissues of L. terrestris after 3 days of exposure to these metals correlate fairly well with the aforementioned toxicity studies. Our attempt to equalize the overall toxicity of the metals by using different dosages was somewhat successful at least for Cd++, Cu++, and Hg++, these metals inducing nearly identical strong Hsp70 responses after a 3-day exposure period. Nonetheless, when taking into account the dose of metal used and the level of Hsp70 induction, the stress response ranking obtained for the short-term exposure would be Pb++ < Cu++ ≅ Hg++ < Cd++.
Interestingly, in vivo data in earthworms indicate rates of metal accumulation of the following order: Pb++ ≤ Cu++ < Cd++ (Ma 1982) and concentration coefficients of the order Pb++ < Cu++ < Hg++ < Cd++ (Yongcan et al 1998). The latter authors also reported graded cellular damages to the intestinal mucosa of the earthworms with the level of metal pollution in soils. Soil diplopods exposed to heavy metals also present ultrastructural alterations in their midgut (Köhler et al 1992). In vitro studies in 2 Lumbricus earthworm species have shown that Pb++ was more firmly bound to chloragosomes than Cu++ (Ireland 1978). Moreover, the higher toxicity of Cd++ may be linked to the fact that within the chloragocytes of the chloragonenous tissue, a much higher proportion of this metal might remain soluble in the cell cytoplasm (Ireland and Richards 1981), thus less associated to the calcium-rich chloragosome content.
On exposure to Cu++, Hg++, and Cd++, the net upregulation of Hsp70 observed in the midgut/intestinal tissues of L. terrestris earthworms after 3 days of exposure followed by a downregulation and disappearance after the 16-day exposure period may reflect time-related adaptation/tolerance mechanisms to the stress induced by these pollutants (Mariño et al 1999) and/or some sort of toxic manifestation on protein expression (Bradley et al 1994; Ryan and Hightower 1994). To that effect, high tolerance and bioaccumulation and thus lower toxicity of Pb++ in vivo (Ma 1982) may well account for the more sustained expression of Hsp70 over the 16-day exposure period. It is interesting to point out that this duality of Hsp70 induction was not so evident in the midgut/intestinal tissues of L. terrestris earthworms exposed for 14 days to the chemical CLA, a dose-dependent upregulation being still highly detectable (Fig 3B,C).
Organic compounds can also remain sequestered in the chloragogenous tissue of earthworms for very long periods of time (eg, Prentø 1994). The long-term (14 days) stress response observed with CLA may be indicative of a less deleterious dose effect on the intestine-blood-chloragocytes transport system and/or may be linked to an overall less efficient sequestration capability of the chloragogenous tissue for organic compounds compared to heavy metals. Moreover, with chemical xenobiotics, the balance between chloragocyte sequestration (Prentø 1994) and enzymatic detoxification pathways (Stenersen 1984) is another variable that could modulate Hsp70 expression quite differently than for metal pollutants.
Regarding the other target tissues selected in the exposures of L. terrestris to heavy metals (re: proximal and midbody wall muscle tissues), their cellular heterogeneity renders difficult a fine localization of the stress-response effect by Western blot analysis as compared to an immunocytochemistry approach (eg, Mariño et al 1999). Nonetheless, as our homogenates of the proximal tissues incorporated the calciferous gland-pouch system, this could make the latter tissue one of the potential target organ for an Hsp70 stress response to some metals since this gland can specifically incorporate metal-calcium analogues (Morgan 1981) or heavy metals from contaminated sewage sludge (Schrader 1992). Similarly, the stomach, also being part of proximal tissue homogenates, could be expressing some stress response as well since its mucosa can present pathological changes following heavy metal exposures (Yongcan et al 1998).
The similarities in the pattern of the stress responses observed in the proximal tissue samples and the more refined midbody wall muscle samples may reflect the fact that a very large proportion of the weight of the proximal section of the earthworms that was analyzed can be attributed to the body wall muscles. It is interesting to mention at this time that body wall homogenates can also bind heavy metals, although much less firmly than chloragosomes. Nonetheless, like with chloragosomes, the binding capacity of the body wall tissues for Pb++ was greater than for Cu++ (Ireland 1978). Hence, for L. terrestris, it would appear that the proximal and midbody wall muscle tissues could have much higher levels of Hsp70 and that the main effect of heavy metals would be one of downregulation of protein synthesis by toxicity. With regards to the high levels of Hsp70/≈90-kDa seen in these latter tissues of all the earthworms exposed to heavy metals, including the control animals, this may indicate a stress response of the earthworms to the alcohol used for anesthesia (10%). Effectively, ethanol (6%) can induce the synthesis of 70-, 87-, and 97-kDa proteins in fibroblasts (Li 1983).
As the body wall would be the first protective tissue barrier against any harmful compound present in the soil or ethanol for that matter, the muscle tissues of L. terrestris may already express higher levels of Hsc70 that could provide earthworms with a rapid protection against sudden stresses (Yu 1994). Hence, with the exception of toxic effects causing inhibition of protein synthesis, an induced stress response as indicated by induction of Hsp70 in the body wall muscle tissues of many earthworm species following a toxic exposure could be quite low and be barely detectable (Yu 1994). Methodologies providing quantitative data (eg, Lewis et al 1999) would be more appropriate to demonstrate such low effects. Moreover, regarding the level of stress response induced in the proximal section of L. terrestris, a more specific selection of organs (eg, calciferous glands, gizzard, esophagus, stomach, or reproductive apparatus) appears warranted. Effectively, as observed with the midgut/intestinal tissues, other internal organs should be less prone to effects of an external anesthetic. In order to test these hypotheses, other ethical forms of anesthesia or sacrifice (by decapitation?) and different exposures and detection protocols would have to be investigated.
As far as metal exposures are concerned, due in part to a possible tolerance phenomenon in soil invertebrates (Eckwert and Köhler 1997; Mariño et al 1999), this study would also raise some caution as to the usefulness of Hsp induction measurements for pollution monitoring since time-dependence regulation may confound exposure-effect relationships. Notwithstanding, early detection (24 to 72 hours) after exposure may prove to be more informative than an assessment after long-term exposures as after 14 days in LC50 toxicity assays. In the soil invertebrate Oniscus asellus (Isopoda) exposed to Pb++, Hsp70 induction could be detected as early as 1 hour after treatment (Köhler et al 1992). Our data also demonstrated that a stress response could be detected very early in the midgut/intestinal tissues of the L. terrestris, as early as 1 hour for a heat shock treatment in vitro (Fig 1) or 24 hours after in vivo exposures to the chemicals CLA and PCP (Figs 2 and 3) and 3 days after in vivo exposure to heavy metals (Fig 7).
In summary, Hsp70 detection by Western blot in the earthworm species L. terrestris not only proved to be suitable and sensitive but also showed a good level of reproducibility despite some individual variations. The use of nonexposed animals transposed into contaminated environments as in this study should therefore be highly ecologically relevant. Moreover, this kind of in vivo toxicity test conducted in the laboratory is also fast, easy to achieve, and relatively inexpensive. Careful control of the health status of the animals and/or proper handling before, during, and after exposure is nonetheless judged imperative for maximum exploitation of the results. The choice of particular target organs would also be highly critical and relevant (this study; Lewis et al 1999; Mariño et al 1999). Hence, it is confirmed that induction of Hsp70 represents a good wide-spectrum biomarker of toxic exposure and that the detection of this Hsp family in earthworms can constitute an early-warning marker for the presence of potentially deleterious elements in soils. Since it was shown that the pollutants selected in this study altered Hsp gene expression in these animals as opposed to a simple accumulation in tissues, the inducible form of Hsp70 can be considered not only a biomarker of exposure but also a biomarker of effect. As biomarkers of effect are more powerful indicators because they imply that a given animal will respond in a measurable way to the action of a pollutant, this could then qualify L. terrestris in particular and earthworms in general as potential sentinel animal species.
Consequently, an Hsp70 biomarker can be used for at least a qualitative assessment of the quality of soils either with pristine earthworms exposed under laboratory conditions (re: to artificial soils spiked with contaminants, to contaminated soils, and/or to mixtures of artificial and contaminated soils), with pristine animals transposed in situ to contaminated soils, or with exposed earthworms collected from the field. Semiquantitative or quantitative assessments by immunoblot could even be attained (Ryan and Hightower 1994; De Wachter et al 1998; Lewis et al 1999), and 1 application of this procedure would be, for example, the evaluation of the environmental impact of a given contaminated soil before, during, and after soil remediation procedures. Effectively, preliminary data seems to indicate that Hsp70 expression studies by immunoblot could be extended to other earthworm species, such as Eisenia fetida (D. Nadeau, data not shown), and hence complement the commonly used LC50 test (eg, Edwards and Coulson 1992) or other toxicity endpoints, such as DNA adducts measurements (Walsh et al 1995, 1997).
Acknowledgments
This work was supported by grants from the NSERC of Canada (D.N.; 0GP0157089) and the MRC of Canada (R.M.T.; MT-14369). These results were presented in part at the 32nd Annual Symposium of the Society of Toxicology of Canada, 2–3 December 1999, Montréal, Québec, Canada.
REFERENCES
- Abdul-Rida AMM. Les vers de terre et l'environnement. La Recherche. 1994;25:260–267. [Google Scholar]
- Affar EB, Dufour M, Poirier GG, Nadeau D. Isolation, purification and partial characterisation of chloragocytes from the earthworm Lumbricus terrestris. Mol Cell Biochem. 1998;185:123–133. doi: 10.1023/a:1006882207581. [DOI] [PubMed] [Google Scholar]
- Ang D, Liberek K, Skowyra D, Zylicz M, Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins (Minireview) J Biol Chem. 1991;266:24233–24236. [PubMed] [Google Scholar]
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones (Review) Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Bouché MB 1972 Lombriciens de France. Écologie et Systématique Vol I.N.R.A. Pub. 72-2. Institut National de la Recherche Agronomique (I.N.R.A.), Paris, France. [Google Scholar]
- Bradley BP, Gonzalez CM, Bond JA, Tepper BE. Complex mixture analysis using protein expression as a qualitative and quantitative tool. Environ Toxicol Chem. 1994;13:1043–1050. [Google Scholar]
- De Pomerai D. Heat-shock proteins as biomarkers of pollution. Hum Exp Toxicol. 1996;15:279–285. doi: 10.1177/096032719601500401. [DOI] [PubMed] [Google Scholar]
- De Wachter B, Scholliers A, Blust R. Semiquantitative immunoblot detection of 70kDa stress proteins in the carp Cyprinus carpio. Bull Environ Contam Toxicol. 1998;60:37–44. doi: 10.1007/s001289900588. [DOI] [PubMed] [Google Scholar]
- Diogène J, Dufour M, Poirier GG, Nadeau D. Extrusion of earthworm coelemocytes: comparison of the cell populations recovered from the species Lumbricus terrestris, Eisenia fetida and Octalasion tyrtaeum. Lab Animals. 1997;31:326–336. doi: 10.1258/002367797780596068. [DOI] [PubMed] [Google Scholar]
- Eckwert H, Köhler HR. The indicative value of the hsp70 stress response as a marker for metal effects in Oniscus asellus(Isopoda) field populations: variability between populations from metal-polluted and uncontaminated sites. Appl Soil Toxicol. 1997;6:275–282. [Google Scholar]
- Edwards CA. ed. 1998 Earthworm Ecology. CRC Press, Boca Raton, FL, USA. [Google Scholar]
- Edwards PJ, Coulson JM 1992 Choice of earthworm species for laboratory tests. In: Ecotoxicology of Earthworms, ed Greig-Smith PW, Becker H, Edwards PJ, Heimbach F. Intercept, Ltd, Andover, Hants, UK, 36–43. [Google Scholar]
- Fink AF. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LC, Goven AJ, Earle B, Rodriguez J, Briceño J, Venables BJ. Thermal acclimation, preference and effects on VO2 in the earthworm. Lumbricus terrestris. Comp Biochem Physiol. 1987;87A:1015–1016. [Google Scholar]
- Fugère N, Brousseau P, Krzystyniak K, Coderre D, Fournier M. Heavy metal-specific inhibition of phagocytosis and different in vitro sensitivity of heterogenous coelomocytes from Lumbricus terrestris (Oligochaeta) Toxicology. 1996;109:157–166. doi: 10.1016/0300-483x(96)03315-x. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Ireland MP. Heavy metal binding properties of earthworm chloragosomes. Acta Biol Acad Sci Hung. 1978;29:385–394. [PubMed] [Google Scholar]
- Ireland MP, Richards KS. Metal content, after exposure to cadmium of two species of earthworms of known differing calcium metabolic activity. Environ Pollut A. 1981;26:69–78. [Google Scholar]
- Kelley PM, Schlesinger MJ. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982;2:267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler HR, Triebskorn R, Stöcker W, Kloetzel PM, Alberti G. The 70 kD heat shock protein (hsp 70) in soil invertebrates: a possible tool for monitoring environmental toxicants. Arch Environ Contam Toxicol. 1992;22:334–338. doi: 10.1007/BF00212095. [DOI] [PubMed] [Google Scholar]
- Lewis S, Handy RD, Cordi B, Billinghurst Z, Depledge MH. Stress proteins (hsp's): methods of detection and their use as an environmental biomarker. Ecotoxicology. 1999;8:351–368. [Google Scholar]
- Li GC. Induction of thermotolerance and enhanced heat shock protein synthesis in chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983;115:116–122. doi: 10.1002/jcp.1041150203. [DOI] [PubMed] [Google Scholar]
- Li W, Chien PK, Furst A. Evaluation of three antidotes on arsenic toxicity in the common earthworm (Lumbricus terrestris) J Appl Toxicol. 1994;14:181–183. doi: 10.1002/jat.2550140306. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Ma WC. The influence of soil properties and worm-related factors on the concentration of heavy metals in earthworms. Pedobiologia. 1982;24:109–119. [Google Scholar]
- Mariño F, Morgan AJ 1998 Immunohistochemical detection of heat shock proteins expression in stressed earthworms. In: Advances in Earthworm Ecotoxicology, ed Sheppard SC, Bembridge ID, Holmstrop M, Posthuma L. Society of Environmental Toxicology and Chemistry (SETAC), Pensacola, FL, USA, 199–214. [Google Scholar]
- Mariño F, Winters C, Morgan AJ. Heat shock protein (hsp60, hsp70, hsp90) expression in earthworms exposed to metal stressors in the field and laboratory. Pedobiologia. 1999;43:615–624. [Google Scholar]
- Morgan AJ. A morphological and electron-microprobe study of the inorganic composition of the mineralized secretory products of the calciferous gland and chloragogenous tissue of the earthworm, Lumbricus terrestris L.: the distribution of injected strontium. Cell Tissue Res. 1981;220:829–844. doi: 10.1007/BF00210465. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Morgan JE, Turner M, Winters C, and Yarwood A 1993 Metal relationship of earthworms. In: Ecotoxicology of Metals in Invertebrates, ed Dallinger R, Rainbow PS. Lewis Publishers, Boca Raton, Fl, USA, 333–358. [Google Scholar]
- Morgan JE, Morgan AJ. Heavy metal concentrations in the tissues, ingesta and faeces of ecophysiologically different earthworm species. Soil Biol Biochem. 1992;24:1691–1697. [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C 1994 The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA. [Google Scholar]
- Nover L. ed. 1991 The Heat Shock Response. CRC Press, Boca Raton, FL, USA. [Google Scholar]
- Prentø P. Distribution of 20 enzymes in the mid-gut region of the earthworm, Lumbricus terrestris L., with particular emphasis on the physiological role of the chloragog tissue. Comp Biochem Physiol. 1987;87A:135–142. [Google Scholar]
- Prentø P. Uptake and long-time storage of natural and synthetic dyes by earthworm chloragocytes: in vivo and in vitro investigations. Comp Biochem Physiol. 1994;109A:805–816. [Google Scholar]
- Ryan JA, Hightower LE. Evaluation of heavy-metal ion toxicity in fish cells using a combined stress protein and cytotoxicity assay. Environ Toxicol Chem. 1994;13:1231–1240. [Google Scholar]
- Sanders BM 1990 Stress proteins: potential as multitiered biomarkers. In: Biomarkers of Environmental Contamination, ed McCarthy J, Shugart LR. Lewis Publishers, Boca Raton, FL, USA, 165–191. [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- Schlesinger MJ. Heat shock proteins (Minireview) J Biol Chem. 1990;265:12111–12114. [PubMed] [Google Scholar]
- Schrader S. Energy-dispersive microanalysis of the calciferous glands of Lumbricus terrestris L. (Oligochaeta) contaminated with heavy metals. Soil Biol Biochem. 1992;24:1755–1759. [Google Scholar]
- Sharp VA, Miller D, Bythell JC, Brown BE. Expression of low molecular weight HSP 70 related polypeptides from the symbiotic sea anemone Anemonia viridis Forskall in response to heat shock. J Exp Mar Biol Ecol. 1994;179:179–193. [Google Scholar]
- Stenersen J. Detoxication of xenobiotics by earthworms (Minireview) Comp Biochem Physiol. 1984;78C:249–252. doi: 10.1016/0742-8413(84)90078-1. [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Kornberg RD. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975;72:2226–2230. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel CAM 1992 The influence of soil characteristics on the toxicity of chemicals for earthworms: a review. In: Ecotoxicology of Earthworms, ed Greig-Smith PW, Becker H, Edwards PJ, Heimbach F. Intercept, Ltd, Andover, Hants, UK, 44–54. [Google Scholar]
- Venables BJ, Fitzpatrick LC, and Goven AJ 1992 Earthworms as indicators of ecotoxicity. In: Ecotoxicology of Earthworms, ed Greig-Smith PW, Becker H, Edwards PJ, Heimbach F. Intercept, Ltd, Andover, Hants, UK, 197–206. [Google Scholar]
- Vincent R, Nadeau D. A micromethod for the quantitation of cellular proteins in Percoll with the Coomasie Brilliant Blue dye-binding assay. Anal Biochem. 1983;135:355–362. doi: 10.1016/0003-2697(83)90696-6. [DOI] [PubMed] [Google Scholar]
- Walsh P, El Adlouni C, Nadeau D, Fournier M, Coderre D, Poirier GG. DNA adducts in earthworms exposed to a contaminated soil. Soil Biol Biochem. 1997;29:721–724. [Google Scholar]
- Walsh P, Lagueux J, Diogène G, Poirier GG, and Nadeau D 1995 DNA adduct measurements in the earthworm Lumbricus terrestris as a sensitive genotoxicity biomarker for contaminated soil assessment. In: Hydrocarbon Contaminated Soils, vol 5, ed Kostecki PT, Calabrese EJ, Bonazountas M. Amherst Scientific Publisher, Amherst, MA, USA, 207–216. [Google Scholar]
- Yongcan G, Zhenzhong W, Youmei Z, Xiaoyang M. Bioconcentration effects of heavy metal pollution in soil on the mucosa epithelia cells ultrastructure injuring of the earthworm's gastrointestinal tract. Bull Environ Contam Toxicol. 1998;60:280–284. doi: 10.1007/s001289900622. [DOI] [PubMed] [Google Scholar]
- Yu ZY. Monoclonal antibody ELISA test indicates that large amounts of constitutive hsp-70 are present in salamanders, turtle and fish. J Therm Biol. 1994;19:41–53. [Google Scholar]


