Abstract
Metallothionein (MT) plays an important role in the detoxification of cadmium.To investigate the usefulness of MT gene expression in peripheral blood lymphocytes (PBLs) as a biomarker of cadmium exposure and susceptibility, reverse transcriptase–polymerase chain reaction was used to measure the MT gene expression in PBLs from cadmium-exposed workers. Both basal and induced MT expressions were found to increase with increased blood cadmium (BCd) and urinary cadmium (UCd) levels. Both basal and induced MT expression levels were significantly correlated with the logarithm of BCd and the logarithm of UCd levels. The dose-response relationship between internal dose of cadmium and MT expression suggested the validity of MT expression in PBLs as a biomarker of cadmium exposure. In vitro induced MT expression level in PBLs was found to be inversely related to the level of renal dysfunction indicator, urinary N-acetyl-β-d-glucosaminidase (UNAG). The latter finding indicates that MT expression in PBLs may be a useful biomarker of susceptibility to renal toxicity of cadmium. (Presented in part at the International Symposium on Metal-Binding Proteins in Biology, Banff, Canada, 1998.)
INTRODUCTION
Metallothioneins (MTs) are a family of stress proteins with a high content of cysteine and divalent metal. MT genes are ubiquitously expressed in many tissues of basal levels, and their expressions are induced readily by many factors, especially by metal ions such as cadmium, zinc, and copper. The physiological functions of MTs are still under study. It has been suggested that MTs are involved in the homeostasis of essential trace elements such as zinc and copper, in the detoxification of certain toxic metals, and as scavengers of free radicals (Aschner et al 1997; Jin et al 1998; Nordberg 1998; Nordberg and Nordberg 2000).
Studies on the metabolism of some heavy metals, especially cadmium, have confirmed the role of MTs in the absorption, transport, and excretion of heavy metals (Nordberg et al 1992; Jin et al 1998; Nordberg and Nordberg 2000). This finding in turn suggested the possibility of using MT expression levels in tissues as a biomarker of metal exposure. Since the detection of MT in urine in an animal model of long-term cadmium exposure (Nordberg and Piscator 1972), interest has increased in the use of MT expression in different tissue as an index of cadmium exposure and toxicity. For example, expression of MT protein in urine was found to increase in cadmium-exposed populations, suggesting MT protein in urine as an indicator of both cadmium exposure and cadmium-caused kidney dysfunction (Nordberg et al 1982; Shaikh and Tohyama 1984; Kawada et al 1990). Peripheral blood lymphocytes (PBLs) provided another candidate cell to analyze MT expression in vivo, since the effects of cadmium on MT expression in blood cells have been studied thoroughly (Hildebrand and Cram 1979; Enger et al 1983; Harley et al 1989). It has been found that MTs are expressed in lymphocytes at a relatively low level (basal expression) but can be induced significantly with cadmium exposure. Since PBLs can easily be collected, it is practically possible to use them to measure MT expression to reflect metal exposure.
To investigate the validity of MT expression in PBLs as a biomarker of cadmium exposure, we measured MT gene expression (messenger RNA [mRNA] level) in PBLs from cadmium-exposed workers using reverse transcriptase–polymerase chain reaction (RT-PCR). In addition, the relationship between MT gene expression and renal dysfunction indicators was examined.
MATERIALS AND METHODS
Studied population
Fifty-nine workers in a cadmium refinery located in Guangxi province, China, were selected as the exposed group. According to cadmium concentration of industrial air in workshops where they worked, they were divided into an intermediately exposed group and a highly exposed group, respectively. Twenty-nine office workers from a company in the same city were selected as the control group. Geographic data suggested no contamination of atmosphere and water from the cadmium refinery; this was evidenced by the low cadmium concentration in rice produced in this region (0.069 mg/kg compared with the limit value, 0.2 mg/kg in Chinese National Environment criteria). All studied subjects were men between the ages of 35 and 45 years. Each subject was questioned according to a questionnaire by a trained questioner. Personal data, such as financial conditions (incomes), lifestyle (including smoking habits, drinking habits), drug-taking history, and heavy metals exposure history, were collected. An experienced physician examined health conditions. Biological samples were collected according to standard methods (for details, see below).
Collection and treatment of biological samples
A total of 5 mL of venous whole blood was collected in an acid-washed, heparin-containing Vacutainer after skin was cleaned with deionized water and followed a protocol for blood sampling (Basun et al 1994). A 1-mL sample was taken for cadmium analyses and stored at −70°C at Shanghai Medical University until analysis. Lymphocytes used for MT mRNA level measurement were isolated from the remaining 4 mL of whole blood by use of Ficoll-Paque according to the method described by Stennard et al (1995).
Spot urine samples were collected in acid-washed containers and stored at −20°C until analysis. Subjects coming directly from the workshop were told to wash their hands carefully before collecting urine sample. Before storage, each sample was divided into 4 parts and taken for measurement of cadmium concentration, β2-microglobulin (B2M), albumin, retinol binding proteins (RBP), and N-acetyl-β-d-glucosaminidase (NAG), respectively. According to the index to be analyzed, the pH value of each sample was adjusted as described (Jin et al 1999).
Exposure estimation
Cadmium exposure consists of occupational and environmental exposure. Because no increase of cadmium concentration in rice and cigarettes was detected, the environmental uptake from food and smoking was found to be negligible compared with occupational exposure. So, we only estimated the occupational exposure in this study.
Occupational uptake was mainly from the cadmium dust in the workplace. Dust samples from 50 L of industrial air in different workshops were collected on membrane filters. Cadmium content of the filters was then measured using atomic absorption spectrometry according to the method described by Adamsson in 1979, which included a quality control program. Cadmium in blood and urine was determined with atomic absorption spectrometry as described previously (Elinder et al 1978; Stoeppler and Brandt 1980).
Effects assessment
Urinary albumin, NAG, B2M, and RBP were used as indices of renal dysfunction. Urinary albumin was measured by enzyme-linked immunosorbent assay (ELISA) and NAG was analyzed as described by Tucker et al (1975). B2M was measured as described by Jin et al (1999). An ELISA-method using RBP kits purchased from Shanghai Second Medical University was used to measure RBP.
Dividing by the amount of creatinine in urine standardized all indices. Creatinine was measured as described by Hare (1950).
Culture and treatment of PBLs
PBLs were isolated using density-gradient centrifugation from 4 mL of freshly collected vein blood. Whole blood was mixed 1:1 with phosphate-buffered saline and laid onto Ficoll-Paque (Amersham Pharmacia Biotech). Lymphocytes were collected at the interface after centrifugation at 1200 rpm for 20 minutes. Lymphocytes were washed twice in phosphate-buffered saline and once in RPMI 1640 (Gibco). Isolated PBLs were then divided into 2 aliquots and incubated in flasks containing RPMI 1640 supplemented with 20% heat-inactivated fetal bovine serum and 2% penicillin-streptomycin. CdCl2 was added to 1 aliquot to the final concentration of 10 μM from a stock solution, and the same volume of saline was added to another aliquot as control. After culture for 6 hours, cells were harvested and total RNA isolated.
RNA isolation and RT-PCR
Total RNA was isolated from harvested cells using TRIzol (Life Technologies, Inc) according to manufacturer's directions. Absorbances at 260 and 280 nm were measured on a Shimadzu UV-16 spectrophotometer to check the concentration of RNA and whether the RNA was degraded.
MT mRNA level was measured by using semiquantitative RT-PCR. One microgram of RNA was reverse transcribed in 20 μL of reaction buffer containing 30 units of avian myeloblastosis virus reverse transcriptase, 40 units of ribonuclease inhibitor (Rnasin), 0.2 mg/mL of oligo (dT)12–18, and 1 mM diethylnitrophenyl thiophosphates (dNTPs) mix (Amersham Pharmacia Biotech). The reaction mixture was incubated for 1 hour at 42°C and then heated to 95°C for 5 minutes to inactivate the reverse transcriptase.
PCR was performed in a 50-μL reaction mixture containing 0.2 mM of dNTPs, 1 pmol of primers both for MT-II and for β-actin, and 5 μL of complementary DNA (cDNA) products. Taq DNA polymerase (Sino-America Biotech) was added after preheating the mixture for 2 minutes at 94°C. The following PCR cycle was used: denaturation at 95°C for 30 seconds, annealing at 60°C for 1 minute, and extension at 72°C for 1 minute. These cycles were repeated 30 times. PCR products (10 μL) were electrophoresed through a nondenaturing 2% agarose gel containing ethidium bromide (1 μg/mL). The result was photographed under UV illumination and subjected to densitometer analysis using a Sixing Image system (Sixing Inc). To normalize the difference in efficiency of reverse transcription and cDNA amplification, MT mRNA levels were calculated as follows: (density of MT-II/198) /(density of β-actin/469). MT mRNA level of PBLs cultured with saline represented the basal expression, whereas MT mRNA level of PBLs treated with CdCl2 represented the (in vitro) induced expression.
PCR primers
Primers specific for MT-II and β-actin were synthesized by the Institute of Biochemistry, Chinese Academy of Science, Shanghai. Primers used to amplify MT-II (Leibbrandt and Koropatnick 1994; Ganguly et al 1996) were 5′-TCTTCAGCACGCCATGGATC-3′ and 5′-CAGGCGCAGCAGCTGCACTT-3′. They yielded a fragment corresponding to 198 bp of coding region of MT-II gene. Primers for β-actin (Liu et al 1997) were 5'-CGGATGTCCACGTCACACTT-3' and 5'-GTTGCTATCCAGGCTGTGCT-3'. They amplified a 469-bp fragment of β-actin gene.
Statistical methods
The database was constructed in a computer using Epi Info (Version 5) (Centers for Disease Control, Atlanta, GA, and World Health Organization, Geneva, Switzerland). χ 2 test was done in the Statcalc program of the Epi-Info package. Regression analyses were done using the SPSS program.
Ethical considerations
The ethics committee of Shanghai Medical University approved this study.
RESULTS
RT-PCR analysis
A pilot study was conducted to test the sensitivity and specificity of the RT-PCR method. MT-II and β-actin were amplified in the same reaction tube, enabling subsequent standardization of MT-II quantification (see also Materials and Methods). This coamplification balances the difference of the amount of total RNA used and the difference of efficiency of transcription between reactions. We found that this method can detect MT expression using as little as 0.1 μg of total RNA (data not shown), and there is no interference between the 2 sets of primers (Fig 1 shows 2 fragments from one reaction of the expected sizes).
Fig. 1.
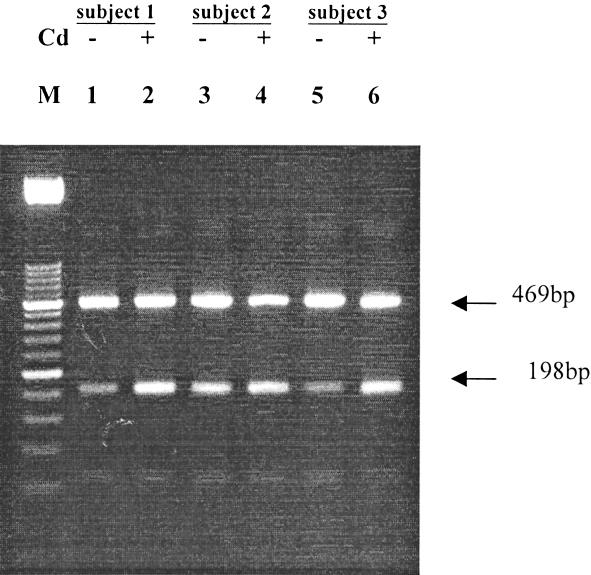
Representative result of a RT-PCR of MT-II and β-actin. PBLs were isolated and divided into 2 aliquots treated for 6 hours either with 10 μM CdCl2 or saline. Cells were harvested and total RNA isolated. One microgram of total RNA was reverse transcribed in 20 μL of reaction buffer using 0.2 mg/mL of oligo (dT)12–18 as primer. A total of 5 μL of cDNA products was amplified in a PCR reaction containing primers both specific for MT-II and for β-actin. PCR products (10 μL) were separated through a nondenaturing 2% agarose gel. This figure shows a representative result for 3 subjects. MT mRNA levels in PBLs cultured with saline represented the basal expression (lanes 1, 3, and 5), whereas MT mRNA level in PBLs treated with CdCl2 represented the induced expression (lanes 2, 4, and 6). Lane M: 50-bp DNA ladder (Amersham Pharmacia Biotech)
Cadmium exposure assessment
Workers from different workshops were divided into intermediate-exposed group (group M) and highly exposed group (group H). Mean value of cadmium concentrations in the industrial air of corresponding workshops showed a significant difference (0.610 vs 3.544 mg/m3). Comparison between the exposed groups and the control group showed no difference in age, income, and smoking habits (data not shown).
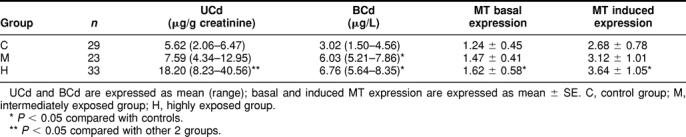
There was a tendency of increase of urinary cadmium (UCd) and blood cadmium (BCd) in differently exposed groups, of which group H has a significantly higher mean value than the controls (Table 1).
Table 1.
Urinary cadmium (UCd), blood cadmium (BCd), and metallothionein (MT) expression in different exposure groups
Effects assessment
The occurrence of complaints about health did not show any significant difference between groups, although there were slightly more complaints of muscle pain in group H. Indicators of renal dysfunction, ie, urinary albumin, NAG, B2M, and RBP showed no significant differences between groups (data not shown).
Relationship between MT gene expression and other indices
To investigate which form of expression, ie, basal expression or induced expression, of MT in PBLs will better reflect exposure (or effect, if it can), we measured both basal and induced MT expression in this study. As shown in Table 1, both basal and induced MT expression increase concurrently with the increase of UCd and BCd in the groups.
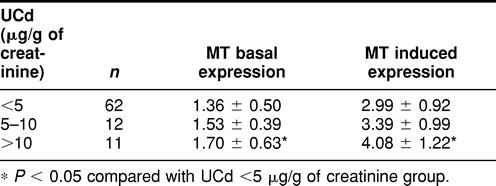
To further elucidate the relationship between MT expression and internal dose index, MT expression was compared between different BCd and UCd groups. As shown in Table 2, there is a statistically significant increase in both basal expression and induced MT expression, in relation to BCd. A similar relationship was observed between basal and induced MT expression and UCd, but the significant increase is only seen in the highest UCd group (Table 3).
Table 2.
Relationship between metallothionein (MT) expression and blood cadmium (BCd)
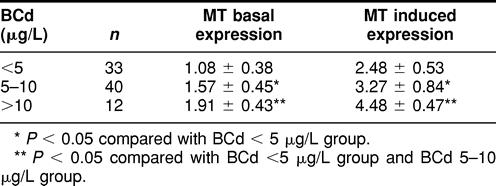
Table 3.
Relationship between metallothionein (MT) expression and urinary cadmium (UCd)
To check whether effect indicators also are closely related to MT expression, partial correlation analysis was also performed between MT expression and internal dose index, effects indicators (urinary albumin, NAG, B2M, and RBP), and other factors that might affect MT expression (age, smoking). It was found that basal MT expression level was significantly correlated with logarithm of BCd (r = 0.465, P < 0.001) and logarithm of UCd (r = 0.314, P = 0.013). Induced MT expression level was also significantly correlated with logarithm of BCd (r = 0.591, P < 0.001) and logarithm of UCd (r = 0.334, P = 0.002). Age and smoking did not correlate with MT expression.
A possible quantitative relationship between MT expression and internal dose indices was examined by multivariate regression analysis using MT expression as the dependent variable and age, cigarette consumption, logarithms of BCd, UCd, urinary B2M, urinary RBP, and urinary NAG as the independent variables. In accordance with results in the partial correlation analysis, logarithms of BCd and UCd showed significant effects on MT expression, whereas for all the other variables this was not found. Stepwise regression analysis (Penter = 0.05, Pexit = 0.10) yielded equations as follow: Basal MT Expression = 0.825 + 0.488 × lgBCd Induced MT Expression = 0.536 + 0.538 × lgBCd + 0.214 × lgUCd.
There was no significant correlation between MT expression and renal effect indicators when all subjects were analyzed. Subjects who have elevated UCd levels (>10 μg/g of creatinine) are prone to renal dysfunction. We further checked the relationship between MT expression and effect indicators in a subpopulation with UCd levels higher than 10 μg/g of creatinine. Interestingly, urinary NAG now showed significant negative correlation with MT-induced expression (r = −0.256, P < 0.05), but not with basal expression. The correlation between MT expression and an indicator of renal function (urinary NAG) seems to be diluted when analyzed in the mixture of subjects who have and do not have renal dysfunction. However, other indicators of renal function, ie, urinary B2M and RBP, were not related to MT expression.
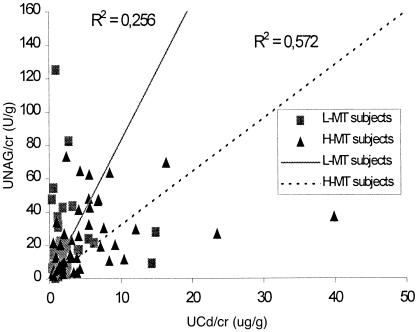
To further investigate the effect of MT-induced expression on the renal dysfunction caused by cadmium exposure, the whole population was divided into 2 groups with different levels of MT-induced expression. Urinary NAG was plotted against UCd for each individual (Fig 2). There was a shift of urinary NAG level between the 2 groups with different MT-induced expression levels, ie, the subjects with low MT-induced expression had higher urinary NAG levels than the subjects with high MT expression when they had the same UCd levels.
Fig. 2.
The effect of MT expression on the renal dysfunction induced by cadmium exposure. The whole studied population was divided into 2 groups with different levels of induced MT expression (▪, low induced MT expression group; ▴, high induced MT expression). Urinary NAG was plotted against UCd for each individual. The trend lines for each group (solid line for L-MT subjects and broken line for H-MT subjects) were predicted according to linear model between urinary NAG and UCd
DISCUSSION
Because of its exceptionally long half-time in humans (10–30 years), cadmium is now considered an important worldwide pollutant. Cadmium exposure gives rise to adverse health effects (World Health Organization 1992). Independent of exposure route, renal dysfunction may develop after cadmium exposure; thus, the kidney is the critical organ (Nordberg 1992). Studies on metabolism and toxicity of cadmium have clearly shown that MT plays an important role in the detoxification of cadmium. MT is synthesized and binds with cadmium in liver on cadmium induction. Cadmium MT can then enter the blood stream and be filtered through the kidney glomerular membrane. In tubular cells, cadmium MT is degraded and free cadmium ion released. Although tubular cells are able to synthesize MT themselves, the free cadmium ion will still exert adverse effects when it exceeds the synthetic capacity of tubular cells (Nordberg et al 1992; Jin et al 1999; Nordberg and Nordberg 2000). Because MT is induced in tissues by cadmium exposure and is involved in the metabolism and detoxification of cadmium, the expression level of MT in tissues and its excretion from the organisms should reflect the exposure and adverse effects of cadmium.
There have been studies on the application of urinary MT excretion as an indicator of cadmium exposure, which suggested the possibility of using urinary MT as a biomarker of cadmium exposure and renal dysfunction (Nordberg et al 1982; Shaikh and Tohyama 1984; Shaikh et al 1987). However, it would be a more straightforward indicator if MT expression could be measured in human tissues. PBLs provide suitable indicator cells to analyze MT expression, because they possess a complete genome that is involved in detoxification of chemicals and are easy to obtain (Lucier and Thompson 1987). Most cadmium in blood is found in blood cells, and PBLs provide a good source for measuring induced MT synthesis. Previous studies (Hilderbrand and Cram 1979; Harley et al 1989) have shown that MT is expressed in a basal level in PBLs and can be induced markedly when cadmium is added to the culture medium. On the contrary, erythrocytes do not exhibit any MT expression, even on cadmium exposure.
Previous studies showed that MT mRNA levels in PBLs are related to cadmium exposure, suggesting this measurement could be an indicator of cadmium exposure. However, there is disagreement on whether basal or induced MT mRNA levels better reflect the cadmium exposure. Ganguly et al (1996) showed that basal MT mRNA levels in PBLs of highly cadmium-exposed workers are significantly higher than of low cadmium-exposed workers. Furthermore, basal MT mRNA levels in PBLs from exposed workers are correlated with airborne cadmium concentrations where they worked. On the contrary, Stennard et al (1995) showed that in vitro induced MT mRNA levels in PBLs correlate positively with cadmium exposure but not basal MT mRNA levels.
In the present study, the status of MT mRNA, ie, both basal and in vitro induced, was measured. Both basal and induced MT mRNA levels in PBLs were found to be significantly elevated in the highly exposed group compared with the control group. This result is consistent with other reports (Stennard et al 1995; Ganguly et al 1996). Further analysis showed significant correlation between MT mRNA levels and internal dose index, ie, BCd and UCd. BCd mainly reflects current and recent exposure, whereas UCd mainly reflects body burden (Nordberg et al 1985; World Health Organization 1992). It is self-evident that cadmium in blood will influence the MT mRNA levels in PBLs, since blood provides the microenvironment in which PBLs exist. Since MT expression levels in PBLs increase before any significant increase in the level of renal effect indicators, this measurement appears to be sensitive enough to detect exposure before any renal effects occur.
Age has been reported to influence the MT level in kidney and liver, because cadmium accumulates with age (Drasch et al 1988; Yoshida et al 1998). Smoking also causes increased cadmium levels in blood, renal cortex, whole body, and urine (Koyama et al 1992; Bem et al 1993) since tobacco contains cadmium. In view of these facts, it may seem peculiar that age and smoking did not have any impact on the expression of MT in this study. One explanation for this is that the age of the subjects in this study is between 35 and 45 years, a range too narrow for the general age effects to be seen. Another reason could be that two-thirds of the subjects were occupationally exposed to cadmium, which provided considerably higher cadmium exposure than that from food and cigarettes. Actually MT expression is higher in smokers than in nonsmokers if the control group is analyzed, although the difference is not statistically significant.
Taken together, our result suggests the validity of MT expression in PBLs as a biomarker of cadmium exposure. A recent study by Yurkow and DeCoste (1999) showed that zinc, another well-known inducer of MT synthesis, does not markedly increase MT in lymphocytes, whereas cadmium causes dramatic induction of MT in lymphocytes. These results suggest that MT expression in lymphocytes may be a specific biomarker of cadmium exposure.
The close relationship between MT mRNA levels in PBLs and UCd is intriguing, since there is no such close physiological relationship between PBLs and kidney. UCd is not only an indicator of cadmium exposure, but also an indicator of renal injury when renal dysfunction occurs (Shaikh and Tohyama 1984; Nordberg et al 1985; Shaikh et al 1987; World Health Organization 1992). One possible explanation is that the ability of PBLs to synthesize MT reflects the ability of the body (including kidney) to synthesize MT on cadmium exposure. Because MT protein in tubular cells plays a pivotal role in detoxifying cadmium (Jin et al 1987a, 1987b), MT mRNA levels in PBLs represent the capability of the kidney to cope with the cadmium accumulated there. In accordance with this hypothesis, data from this study showed that subjects who have the same levels of UCd have higher levels of urinary NAG if they have lower induced MT expression levels in PBLs. However, 2 other renal effect indicators, B2M and RBP, did not show correlation with MT expression, even in the subpopulation with high UCd levels. This might be due to the difference in degree of renal damage, which each indicator reflects. NAG is located in the epithelial cells of renal tubules, and urinary NAG is considered to originate from these cells. Urinary NAG activity may reflect early histopathological changes of renal tubules (Nogawa et al 1986; Kawada et al 1989; Bernard et al 1995). On the other hand, B2M and RBP are low-molecular-weight proteins that are normally reabsorbed by the proximal tubule, so urinary B2M and urinary RBP mainly reflect the reabsorbance function of tubular cells (Jung et al 1993; Ikeda et al 2000; Oo et al 2000). In the subjects with UCd levels higher than 10 μg/g of creatinine, it is likely that cellular injuries, which cause leakage of NAG, have occurred in renal tubular cells, whereas reabsorbance functions of these cells have not been affected or have been affected only slightly. Such an interpretation is in accordance with previous studies concerning excretion of MT and other low-molecular-weight proteins in urine, in which it was found that urinary MT was related to urinary NAG but not to urinary B2M (Kawada et al 1990, 1995).
The relationship between MT expression and urinary NAG found in the present study suggests that MT synthesis in the kidney serves as a modifying factor in the susceptibility to the cadmium-induced renal dysfunction, thus it may represent the susceptibility of the body to the adverse effect caused by cadmium. Considering the difficulties in measuring renal MT expression in vivo, in vitro induced MT expression levels in PBLs provided a potential practical index.
In summary, the present study showed that both basal and induced MT expression levels in PBLs are closely related to cadmium exposure, confirming the validity of MT expression in PBLs as a biomarker of cadmium exposure. In addition, induced MT mRNA levels in PBLs seem to reflect the renal MT induction ability, thus providing a possible index of susceptibility to the adverse effect of cadmium. Further studies are warranted, including follow-up studies in a population with renal dysfunction and a normal population.
Acknowledgments
This work was partly performed as a Master's thesis at Shanghai Medical University (J.L.). Support from Swedish Agency for Research and Educational Cooperation with developing countries grants SWE-96-173 and SAREC-1997-0485, European Union grant ERB 3514 PL 971430, and the Karolinska Institutet is gratefully acknowledged.
REFERENCES
- Adamsson E. Long-term sampling of airborne cadmium dust in an alkaline battery factory. Scand J Work Environ Health. 1979;5:178–187. doi: 10.5271/sjweh.3091. [DOI] [PubMed] [Google Scholar]
- Aschner M, Cherian MG, and Klaassen CD. et al. 1997 Metallothionein in brain: the role in physiology and pathology. Toxicol Appl Pharmacol. 142:229–242. [DOI] [PubMed] [Google Scholar]
- Basun H, Lind B, Nordberg M, Nordström M, Sparring-Björkstén K, Winblad B. Cadmium in blood in Alzheimeŕs disease and non-demented subjects. Biometals. 1994;7:130–134. doi: 10.1007/BF00140482. [DOI] [PubMed] [Google Scholar]
- Bem EM, Orlowski C, Piotrowski JK, Januszewski K, Pajak J. Cadmium, zinc, copper, and metallothionein levels in the kidney and liver of inhabitants of upper Silesia (Poland) Int Arch Occup Environ Health. 1993;65:57–63. doi: 10.1007/BF00586060. [DOI] [PubMed] [Google Scholar]
- Bernard A, Thielemans N, Roels H, Lauwerys R. Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup Environ Med. 1995;52:177–180. doi: 10.1136/oem.52.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasch GA, Kretschmer E, Neidlinger P, Summer KH. Metallothionein in human liver and kidney: relationship to age, sex, diseases and tobacco and alcohol use. J Trace Elem Electrolytes Health Dis. 1988;2:233–237. [PubMed] [Google Scholar]
- Elinder CG, Kjellstrom T, Linnman L, Pershagen G. Urinary excretion of cadmium and zinc among persons from Sweden. Environ Res. 1978;15:473–484. doi: 10.1016/0013-9351(78)90126-3. [DOI] [PubMed] [Google Scholar]
- Enger MD, Hildebrand CE, Stewart CC. Cadmium++ responses of cultured human blood cells. Toxicol Appl Pharmacol. 1983;69:214–224. doi: 10.1016/0041-008x(83)90302-2. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Taioli E, Baranski B, Cohen B, Toniolo P, Garte SJ. Human metallothionein gene expression determined by quantitative reverse transcription-polymerase chain reaction as a biomarker of cadmium exposure. Cancer Epidemiol Biomarkers Prev. 1996;5:297–301. [PubMed] [Google Scholar]
- Hare RS. Endogenous creatinine serum and urine. Proc Soc Exp Biol Med. 1950;74:148–151. doi: 10.3181/00379727-74-17837. [DOI] [PubMed] [Google Scholar]
- Harley CB, Menon CR, Rachubinski RA, Nieboer E. Metallothionein mRNA and protein induction by cadmium in peripheral blood leucocytes. Biochem J. 1989;262:873–879. doi: 10.1042/bj2620873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand CE, Cram LS. Distribution of cadmium in human blood cultured in low levels of CdCl2: accumulation of cadmium in lymphocytes and preferential binding to metallothionein. Proc Soc Exp Biol Med. 1979;161:438–443. doi: 10.3181/00379727-161-40569. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Zhang ZW, Moon CS, Shimbo S, Watanabe T, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K. Possible effects of environmental cadmium exposure on kidney function in the Japanese general population. Int Arch Occup Environ Health. 2000;73:15–25. doi: 10.1007/pl00007933. [DOI] [PubMed] [Google Scholar]
- Jin T, Nordberg GF, Nordberg M. Influence of cadmium-metallothionein pretreatment on tolerance of rat kidney cortical cells to cadmium toxicity in vitro and in vivo. Pharmacol Toxicol. 1987a;60:345–349. doi: 10.1111/j.1600-0773.1987.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Jin T, Nordberg GF, Nordberg M. Resistance to acute nephrotoxicity induced by cadmium-metallothionein dependence on pretreatment with cadmium chloride. Pharmacol Toxicol. 1987b;61:89–93. doi: 10.1111/j.1600-0773.1987.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–536. [PubMed] [Google Scholar]
- Jin T, Nordberg G, Wu X, Ye T, Kong Q, Wang Z, Zhuang F, Cai S. Urinary N-acetyl-β-d-glucosaminidase isoenzymes as biomarker of renal dysfunction caused by cadmium in a general population. Environ Res. 1999;81:167–173. doi: 10.1006/enrs.1999.3959. [DOI] [PubMed] [Google Scholar]
- Jung K, Pergande M, Graubaum HJ, Fels LM, Endl U, Stolte H. Urinary proteins and enzymes as early indicators of renal dysfunction in chronic exposure to cadmium. Clin Chem. 1993;39:757–765. [PubMed] [Google Scholar]
- Kawada T, Koyama H, Suzuki S. Cadmium, NAG activity and β2-microglobulin in the urine of cadmium pigment workers. Br J Ind Med. 1989;46:52–55. doi: 10.1136/oem.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada T, Tohyama C, Suzuki S. Significance of the excretion of urinary indicator proteins for a low level of occupational exposure to cadmium. Int Arch Occup Environ Health. 1990;62:95–100. doi: 10.1007/BF00397855. [DOI] [PubMed] [Google Scholar]
- Kawada T, Tohyama C, Suzuki S. Effects of cadmium and lead exposure on urinary N-acetyl-beta-d-glucosaminidase, beta 2-microglobulin and metallothionein of workers. Asia Pacific J Public Health. 1995;8(2):91–94. doi: 10.1177/101053959500800206. [DOI] [PubMed] [Google Scholar]
- Koyama H, Satoh H, Suzuki S, Tohyama C. Increased urinary cadmium excretion and its relationship to urinary N-acetyl-beta-d-glucosaminidase activity in smokers. Arch Toxicol. 1992;66:598–601. doi: 10.1007/BF01973392. [DOI] [PubMed] [Google Scholar]
- Leibbrandt ME, Koropatnick J. Activation of human monocytes with lipopolysaccharide induces metallothionein expression and is diminished by zinc. Toxicol Appl Pharmacol. 1994;124:72–81. doi: 10.1006/taap.1994.1010. [DOI] [PubMed] [Google Scholar]
- Liu HC, He ZY, Tang YX, Mele CA, Veeck LL, Davis O, Rosenwaks Z. Simultaneous detection of multiple gene expression in mouse and human individual preimplantation embryos. Fertil Steril. 1997;67:733–741. doi: 10.1016/s0015-0282(97)81375-1. [DOI] [PubMed] [Google Scholar]
- Lucier GW, Thompson CL. Issues in biochemical applications to risk assessment: when can lymphocytes be used as surrogate markers? Environ Health Perspect. 1987;76:187–191. doi: 10.1289/ehp.8776187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa K, Yamada Y, Kido T, Honda R, Ishizaki M, Tsuritani I, Kobayashi E. Signifcance of elevated urinary N-acetyl-b-d-glucosaminidase activity in chronic cadmium poisoning. Sci Total Environ. 1986;53:173–178. doi: 10.1016/0048-9697(86)90130-0. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Piscator M. Influence of long-term cadmium exposure on urinary excretion of protein and cadmium in mice. Environ Physiol Biochem. 1972;2:37–49. [Google Scholar]
- Nordberg GF, Garvey JS, Chang CC. Metallothionein in plasma and urine of cadmium workers. Environ Res. 1982;28:179–182. doi: 10.1016/0013-9351(82)90167-0. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Kjellstrom T, and Nordberg M 1985 Kinetics and metabolism. In: Cadmium and Health: A Toxicological and Epidemiological Appraisal, Vol I: Exposure, Dose, and Metabolism, ed Friberg L, Elinder CG, Kjellstrom T, Nordberg GF. CRC Press, Boca Raton, FL, 103–178. [Google Scholar]
- Nordberg GF 1992 Application of the “critical effect” and “critical concentration” concept to human risk assessment for cadmium. In: Cadmium in the Human Environment: Toxicity and Carcinogenicity, ed Nordberg GF, Herber RFM, Alessio L. IARC Scientific Publications, Lyon, France, 3–14. [PubMed] [Google Scholar]
- Nordberg M, Jin T, and Nordberg GF 1992 Cadmium, metallothionein and renal tubular toxicity. In: Cadmium in the Human Environment: Toxicity and Carcinogenicity, ed Nordberg GF, Herber RFM, Alessio L. IARC Scientific Publications, Lyon, France, 293–297. [PubMed] [Google Scholar]
- Nordberg M. Metallothioneins: historical review and state of knowledge. Talanta. 1998;46:243–254. doi: 10.1016/s0039-9140(97)00345-7. [DOI] [PubMed] [Google Scholar]
- Nordberg M, Nordberg GF. Toxicological aspects of metallothionein. Cell Mol Biol. 2000;46:451–463. [PubMed] [Google Scholar]
- Oo YK, Kobayashi E, Nogawa K, Okubo Y, Suwazono Y, Kido T, Nakagawa H. Renal effects of cadmium intake of a Japanese general population in two areas unpolluted by cadmium. Arch Environ Health. 2000;55:98–103. doi: 10.1080/00039890009603394. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Tohyama C. Urinary metallothionein as an indicator of cadmium body burden and of cadmium-induced nephrotoxicity. Environ Health Perspect. 1984;54:171–174. doi: 10.1289/ehp.8454171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh ZA, Tohyama C, Nolan CV. Occupational exposure to cadmium: effect on metallothionein and other biological indices of exposure and renal function. Arch Toxicol. 1987;59:360–364. doi: 10.1007/BF00295090. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Stewart TC, West AK. Effect of prior, low-level cadmium exposure in vivo on metallothionein expression in cultured lymphocytes. J Appl Toxicol. 1995;15:63–67. doi: 10.1002/jat.2550150114. [DOI] [PubMed] [Google Scholar]
- Stoeppler M, Brandt K 1980 Determination of lead and cadmium in whole blood by electro-thermal atomic absorption spectroscopy. In: Diagnosis and Therapy of Porphyrias and Lead Intoxication, ed Doss M. Springer Verlag, Heidelberg, Germany, 185–187. [Google Scholar]
- Tucker SM, Boyd PJR, Thompson AE. Automated assay of N-acetyl-β-glucosaminidase in normal and pathological human urine. Clin Chim Acta. 1975;62:333–339. doi: 10.1016/0009-8981(75)90245-4. [DOI] [PubMed] [Google Scholar]
- WHO/IPCS Environmental Health Criteria Document 134 Cadmium.. 1992 World Health Organization,. Geneva, Switzerland. [Google Scholar]
- Yoshida M, Ohta H, Yamauchi Y, Seki Y, Sagi M, Yamazaki K, Sumi Y. Age-dependent changes in metallothionein levels in liver and kidney of the Japanese. Biol Trace Elem Res. 1998;63:167–175. doi: 10.1007/BF02778875. [DOI] [PubMed] [Google Scholar]
- Yurkow EJ, DeCoste CJ. Effects of cadmium on metallothionein levels in human peripheral blood leukocytes: a comparison with zinc. J Toxicol Environ Health. 1999;58:313–327. doi: 10.1080/009841099157278. [DOI] [PubMed] [Google Scholar]