Abstract
Comprehensive analysis of the Arabidopsis genome revealed a total of 13 sHsps belonging to 6 classes defined on the basis of their intracellular localization and sequence relatedness plus 6 ORFs encoding proteins distantly related to the cytosolic class CI or the plastidial class of sHsps. The complexity of the Arabidopsis sHsp family far exceeds that in any other organism investigated to date. Furthermore, we have identified a new family of ORFs encoding multidomain proteins that contain one or more regions with homology to the ACD (Acd proteins). The functions of the Acd proteins and the role of their ACDs remain to be investigated.
INTRODUCTION
The small Hsp (sHsp), or Hsp20, family of heat stress proteins is a nearly ubiquitous family of stress proteins that range in size from approximately 16–42 kDa. This diverse family of proteins, which includes the α-crystallin proteins (Acd proteins) of the vertebrate eye lens, is defined by a conserved C-terminal domain of approximately 90 amino acids referred to as the α-crystallin domain (ACD) (de Jong et al 1998). The ACD is flanked by an N-terminal domain and a short C-terminal extension (Nover 1991; Caspers et al 1995; Waters et al 1996; MacRae 2000). The sHsp N-terminal domains show no sequence conservation, except between evolutionarily closely related proteins. The sHsp family is, therefore, different from the Hsp70 and Hsp90 chaperone families, in which sequence identity, even between prokaryotic and eukaryotic members, is significant throughout the protein. Another defining feature of the sHsps is that, with few exceptions, all members of the family form homo-oligomeric complexes of 200–750 kDa (9 to >24 subunits), with the size dependent on the specific protein (Chen et al 1994; Lee et al 1995; Ehrnsperger et al 1997, 1999; Helm et al 1997; Kirschner et al 2000).
Plants are characterized by an unusual complexity of heat stress–inducible proteins belonging to the sHsp family. Plant sHsps appear to have evolved independently after the divergence of plants and animals (Waters 1995; de Jong et al 1998). Although in other organisms sHsps are found in the cytoplasm under most conditions, and at times in the nucleus, plants express both cytosolic sHsps and specific isoforms targeted to intracellular organelles. There are at least 2 forms of sHsps in the cytosol, referred to as class I and class II proteins (Vierling 1991), which share only approximately 50% identity in the ACD and are estimated to have diverged more than 400 million years ago (Waters and Vierling 1999). Three separate gene families have been described that encode mitochondrial, plastidial, and endoplasmic reticulum–localized sHsps, each with appropriate organelle-targeting signals. The organelle sHsp forms appear to be unique to plants, with the exception of Hsp22 in Drosophila, which was recently reported to be located in mitochondria (Morrow et al 2000).
Recent solution of the structure of Hsp16.5 from Methanococcus jannaschii at 2.9-Å resolution has provided general insight into the organization of sHsp monomers and oligomers (Kim et al 1998a). The ACD consists of a β-sandwich of 2 antiparallel β-sheets similar to the fold of the unrelated Fc fragment of immunoglobulin, although the exact folding topology is different (Deisenhofer 1981). The dominant β-structure of the sHsps was previously predicted from circular dichroism (C.D.) spectroscopy (Merck et al 1993; Dudich et al 1995; Sun et al 1997), secondary structure analysis of sHsp sequences (Caspers et al 1995; de Jong et al 1998), and site-directed spin-labeling studies (Berengian et al 1999; McHaourab et al 2000). This same β-sandwich fold is predicted to be present in all sHsps, and the putative location of β-strands in Arabidopsis and other plant sHsps are indicated in the alignment in Figure 1.
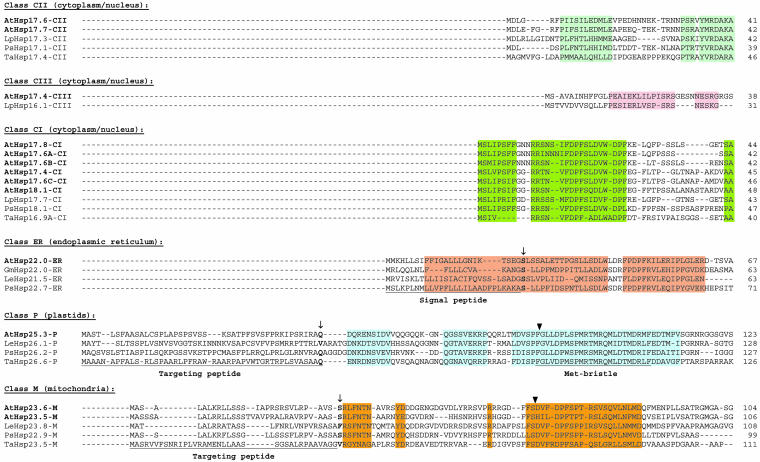
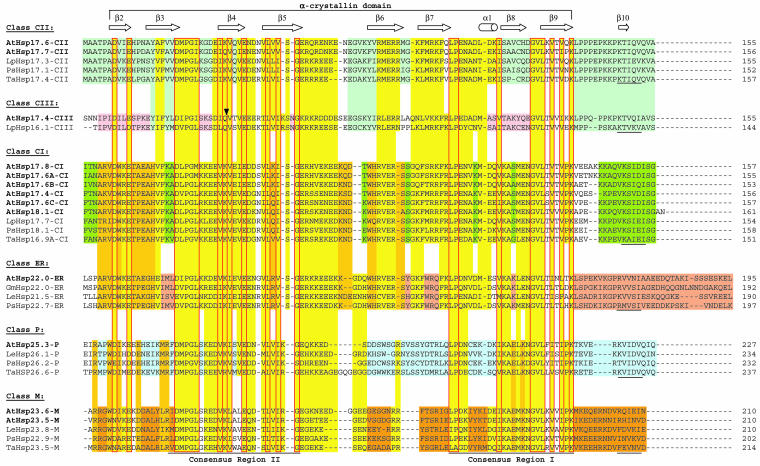
Fig 1.
Sequence comparison of sHsps. Arabidopsis (At) and selected representatives of the sHsp family from tomato (Lp), pea (Ps), and wheat (Ta) are included. A color code marks sequence motifs conserved among the members of a given class or for all sHsps. Putative cleavage sites for removal of the targeting peptides of the organelle proteins (classes P, ER, and M) are indicated by arrows. The Met bristle was defined as a unique, methionine-rich sequence motif characteristic for all plastidial sHsp (Chen and Vierling 1991). Closed arrowheads point to the positions of introns in the ORF coding sequences. The ACD (yellow color code) comprises 2 homologous regions, consensus region II and I, separated by a linker, which is more variable in sequence and size. In the corresponding vertebrate genes, homologous regions II and I are encoded by 2 separate exons. Potential positions of secondary structural elements are indicated above the alignment based on positions of these elements in M jannaschii Hsp16.5. The conserved sequence motif, basic-x-I/V-x-I/V, which is partially involved in formation of the C-terminal β-strand (β10) is underlined for each class of sHsps
In M jannaschii the Hsp16.5 monomers form a 24-subunit, hollow spherical oligomer with an outer diameter of 120 Å and an inner diameter of 65 Å. Virtually the entire N-terminal domain (32 residues) of the proteins was disordered and not resolved. These 32 N-terminal residues (approximately 20% of the protein mass) could fill a significant portion of the interior space, and therefore the size of this space is debated. The basic building block of the M jannaschii oligomer is a dimer, another structural feature that is likely to be conserved in the plant sHsps. Within the resolved structure, the dimer interface has the strongest and most extensive contacts within the oligomer, being stabilized by both intermolecular hydrogen bonds and extensive hydrophobic contacts. The interface contacts comprise primarily an extended loop that forms the β6-strand, which binds antiparallel to β2 to extend one of the β-sheets in the β-sandwich fold. This loop region shows limited sequence conservation, but is primarily hydrophilic and capable of forming the necessary β-strand in all plant sHsps.
Interactions of the C-terminal extensions are critical in stabilizing the M jannaschii Hsp16.5 oligomer. A 3-residue β-strand (β10) in the C-terminus extends the β-sheet of another dimer by binding to β4. The β10-strand is part of a conserved sequence motif recognized by de Jong et al (1998), basic-x-I/V-x-I/V, which is present in all 6 defined plant sHsp classes (Fig 1), suggesting this same contact is involved in formation of the plant sHsp oligomers. In addition to intermolecular hydrogen bonds in this contact, the side chains of the hydrophobic residues in this motif make extensive hydrophobic contacts with a hydrophobic groove formed by strands β4 and β8 on one monomer of the interacting dimer. Recent solution of the structure of wheat Hsp16.9 (van Montfort et al, in preparation) will add considerably to our detailed understanding of plant sHsp structure and test current structural predictions.
Almost all sHsps function as chaperones in vitro to prevent irreversible protein aggregation and insolubilization and to interact otherwise with nonnative proteins in an adenosine triphosphate (ATP)–independent fashion (Horwitz 1992; Jakob et al 1993; Knauf et al 1993; Merck et al 1993; Lee et al 1995; Plater et al 1996; Kim et al 1998b). Early studies with crude cell protein fractions from plants suggested that sHsps could protect other proteins from heat-induced insolubilization (Jinn et al 1989). Very likely, sHsps function in the chaperone network to maintain denatured proteins in a folding competent state (Ehrnsperger et al 1997; Lee et al 1997). Substrate renaturation requires interaction with the Hsp70 chaperone system and ATP (Forreiter et al 1997; Leroux et al 1997; Veinger et al 1998; Lee and Vierling 2000). Interaction with substrate appears to involve heat-induced exposure of hydrophobic-binding sites on the sHsp subunits (Lee et al 1997), which occurs because of heat-induced oligomer dissociation or increased subunit exchange rates (Bova et al 1997, 2000; Ehrnsperger et al 1999; Haslbeck et al 1999). The sHsp complexes with denatured protein then reassembles into a larger complex, and reassembly may be critical for proper activity. This striking reorganization during stress may also initiate the assembly of cytosolic, multichaperone complexes, comprising 40-nm heat stress granules, which are found in heat-stressed plant cells (Nover et al 1983, 1989; Kirschner et al 2000). Full understanding of the importance of sHsp chaperone activity to plant stress tolerance will require identification of the critical substrates protected by these proteins.
MEMBERS OF THE ARABIDOPSIS sHsp FAMILY
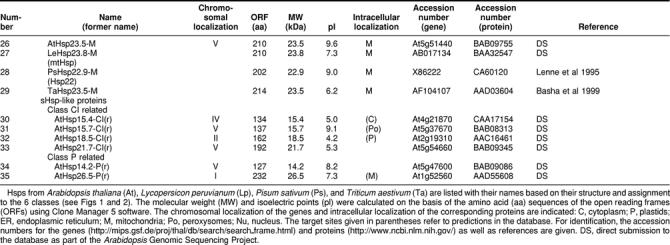
Searching the Arabidopsis genome revealed 19 open reading frames (ORFs) encoding proteins related to the previously characterized 5 classes of sHsps in plants (Vierling 1991; Waters et al 1996), of which 8 had already been cloned and characterized (see references given in Table 1). Details of the size, properties, and references to accession numbers and publications are compiled in Table 1 and Figure 1. The classification is based on the intracellular localization of the sHsps in the cytoplasmic-nuclear compartment (classes CI and CII), plastids (class P), endoplasmic reticulum (class ER), and mitochondria (class M). From the sequence comparison in Figure 1, which also includes selected representatives from other plants, it is evident that this classification is reflected also by characteristic sequence motifs found primarily in the N-terminal domain and C-terminal extensions. In addition, class-specific features of the ACD are also notable. There are 6 proteins of cytosolic class CI and 2 proteins of class CII. One representative each of the sHsp family is targeted to the chloroplasts and endoplasmic reticulum, and 2 are targeted to mitochondria. In addition, there is one protein (number 15 in Table 1) distantly related to class CII proteins, which we designated class CIII. A closely related member of this class was found in tomato by yeast 2-hybrid screening using a class CI sHsp as bait. It has unique properties, because it interacts with both class CI and class CII sHsps in the yeast 2-hybrid system, and it is preferentially localized in the nucleus (K. D. Scharf et al, in preparation). These 13 core members of the sHsp family are closely related to similar proteins from other plants (Figs 1 and 2). Six additional sHSPs included in Table 1 (numbers 30 to 35) and Figure 2 are more distantly related (Figs 2 and 4), but also appear to be bona fide members of this protein family.
Table 1.
Survey of the small Hsp family
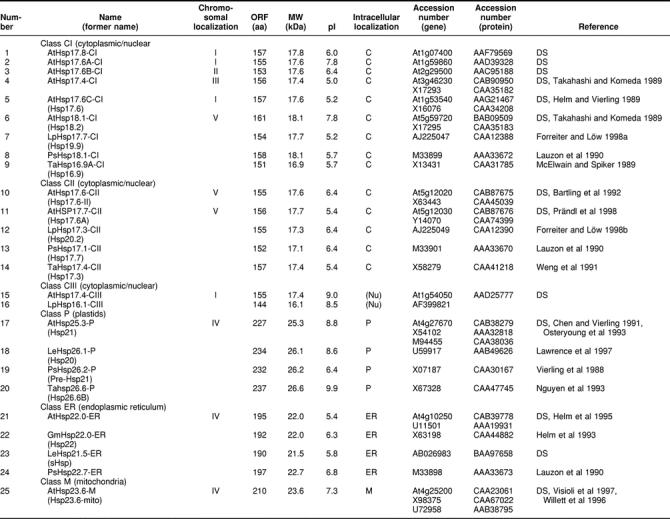
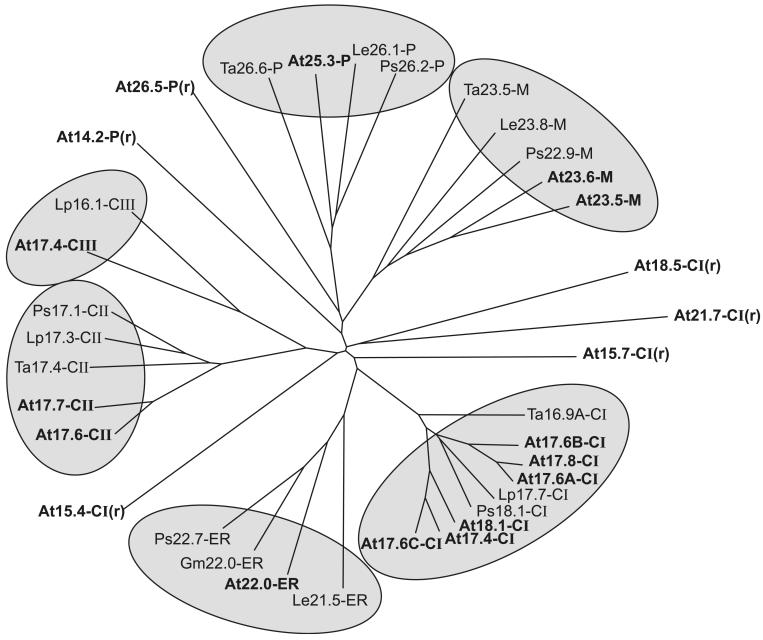
Fig 2.
Phylogenetic relationship of proteins of the sHsp family. The figure was created by Clustal alignment on the basis of the full-length amino acid sequences of all proteins compiled in Table 1. Proteins belonging to the 6 classes of sHsp are marked by shaded areas. The 6 proteins (numbers 30 to 35 in Table 1) with a more distant relationship to class CI and class P are clearly separated from the others
The number of sHsps found in different organisms varies significantly. Prokaryotes contain 1 or 2 sHsps, except species of Rhizobium, some of which have at least 10 shps (Munchbach et al 1999). Saccharomyces cerevisiae has 2 shsps encoding Hsp26 (Petko and Lindquist 1985) and Hsp42 (Wotton et al 1996), one of the largest family members. In humans, only 5 sHsps, including αA and αB-crystallin (de Jong et al 1998), were described, until recently when 4 additional transcribed sequences were identified (Boelens et al 1998; Suzuki et al 1998; Krief et al 1999; W. de Jong, personal communication). Caenorhabditis elegans has 16 proteins classified as sHsps (Ding and Candido 2000) and Drosophila melanogaster has 12 (Sébastien et al 2001).
A NEW FAMILY OF PROTEINS WITH ACDs (Acd PROTEINS)
An interesting new group of proteins is represented by 25 ORFs in the Arabidopsis genome encoding proteins with one or more ACDs. They are compiled in Table 2 as Acd proteins specified with numbers derived from their molecular weights. Although they are unrelated to the 6 classes of sHsps defined herein, it is an intriguing observation that a structural motif related to the ACD may be functional also in other proteins. Unfortunately, our knowledge about this new class of proteins is very limited. However, 3 examples indicate that this family deserves special attention.
Table 2.
Survey of Arabidopsis Acd proteins, proteins with domain(s) homologous to the {α}-crystallin domain (ACD)
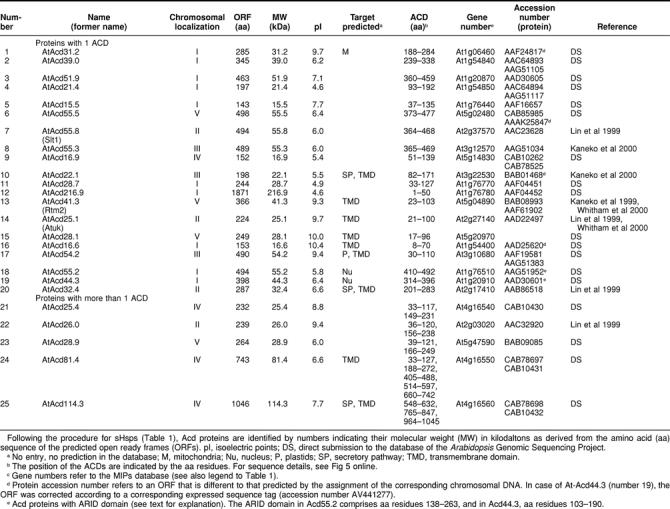
One Acd protein (RTM2 equals AtAcd41.3 in Table 2) was recently characterized as an essential component of a specific resistance mechanism against systemic spreading of tobacco etch virus in Arabidopsis (Whitham et al 2000). In addition to the N-terminal ACD, the RTM2 protein contains a central region with highly charged repeat motifs and a long C-terminal domain with a putative membrane-spanning domain. The RTM2 defect in the Arabidopsis mutant used for cloning of the gene is due to a point mutation introducing a stop codon in the ACD. The RTM2 protein is not heat shock inducible, and the rtm2 mutant plants are not impaired in their thermotolerance. The authors hypothesize that RTM2 has no typical chaperone function but rather functions as part of a localized high-molecular-weight complex restricting virus movement.
Another intriguing group in the Acd family is represented by Acd55.2 and Acd44.3 (numbers 18 and 19 in Table 2). They belong to the growing number of ARID proteins (ARID indicates AT-rich interaction domain, also called BRIGHT domain), which are DNA-binding proteins with a helix-turn-helix motif (Tucker et al 2000). Some of them were characterized as developmental regulators in D melanogaster and mammals (Tucker et al 1995; Treisman et al 1997). In addition, the p270 subunit of the SWI-SNF chromatin remodeling complex is a member of the ARID protein family (Kobayashi et al 2000). Two additional ARID proteins in the Arabidopsis genome (accession numbers AAF17649 with 338 amino acid residues and AAF40449 with 448 amino acid residues) have no ACD but an HMG box in their C-terminal parts.
The first examples of proteins with multiple ACDs were 2 major egg protein antigens of Schistosoma mansoni with 40 kDa and 2 ACDs (Nene et al 1986). Later, they were characterized as soluble Ca2+-binding proteins (Moser et al 1992). Similar to some members of the Arabidopsis Acd proteins, their duplicate ACDs are separated by a short proline-rich linker. Particularly interesting in this group of Arabidopsis Acd proteins is Acd114.3 (number 25 in Table 2) with 3 ACDs linked to a N-terminal domain exhibiting high-sequence homology to the tobacco protein CND41. This protein was described before as a chloroplast nucleoid DNA-binding protein (Nakano et al 1997) with aspartic protease activity (Murakami et al 2000). CND41 might be involved in down-regulation of transcription in chloroplasts.
A detailed sequence comparison and phylogenetic analysis of all proteins contained in Tables 1 and 2 are presented online in the attached material (Figs 4 and 5).
PROMOTER STRUCTURE AND EXPRESSION OF sHsps
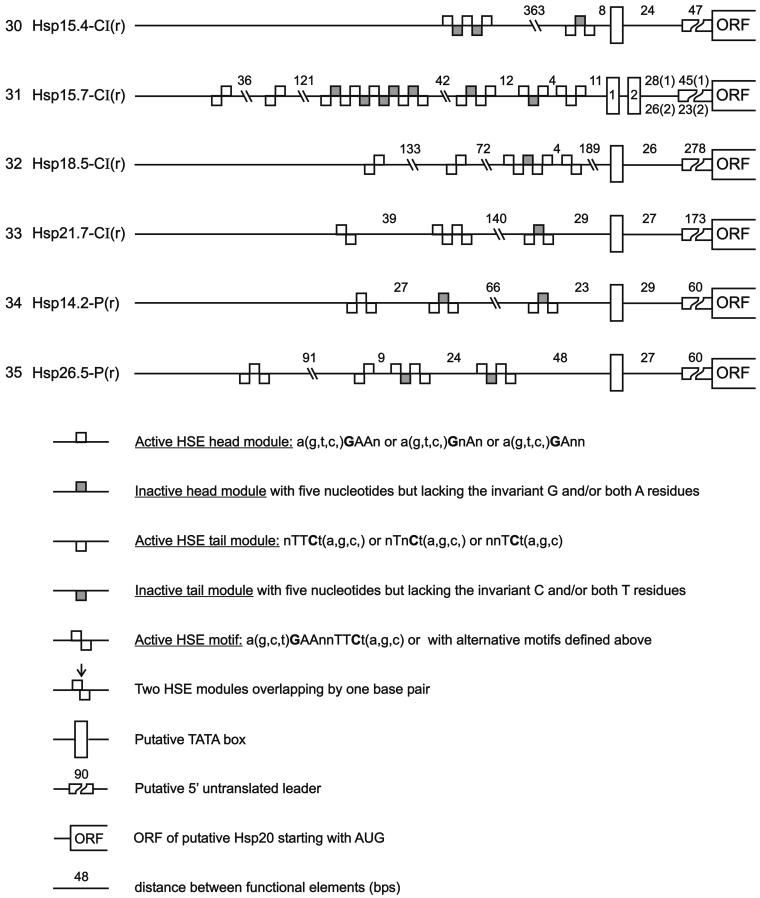
Except for some well-defined developmental stages, eg, during seed maturation (Hightower and Nover 1991; Waters et al 1996; Wehmeyer et al 1996), sHsps of plants are exclusively found after heat shock induction. We therefore analyzed the putative promoter structures of the genes listed in Table 1. Because in most cases experimental data about the transcription start site are lacking, we used the ATG start codon of translation as the only reliable point to search upstream for presumptive TATA box and heat shock element (HSE) motifs. The results are summarized in the block diagrams in Figure 3.
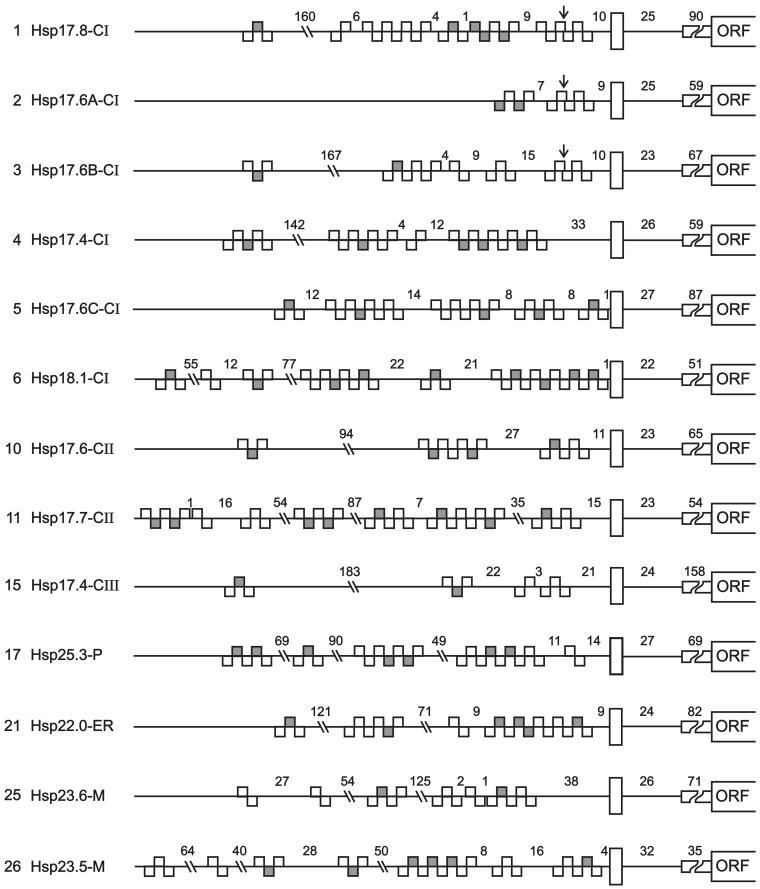
Fig 3.
Promoter architecture of Arabidopsis genes encoding sHsps with respect to the positioning of HSE clusters. For orientation and positioning of the putative TATA box, we used the well-defined start site of the ORFs and the data for genomic clones with experimentally defined transcription start sites. These are Hsp17.4-CI and Hsp18.1-CI (Takahashi and Komeda 1989) and Hsp25.3-P (Osteryoung et al 1993). Symbols are explained at the bottom of the figure. Numbers on the left refer to the corresponding numbers in Table 1
In practically all cases, putative promoter regions are characterized by clusters of HSEs frequently found in plant heat stress genes. These clusters are formed of palindromic repeats of the head (aGAAn) and tail (nTTCt) modules. Frequently, they are imperfect, because defective 5-nucleotide modules are interspersed (shaded blocks in Fig 3). In agreement with mutational analysis in our and other laboratories (Amin et al 1987; Bharti et al, unpublished data), modules are considered defective if the invariant G or C (letters underlined) and/or the 2 highly conserved A or T residues (uppercase letters) are lacking (see explanations given in Fig 3). The importance of such imperfect clusters for the regulatory fingerprint of the heat shock genes was recently demonstrated in our group by a strong synergistic activation of reporter gene expression with a mixture of tomato HsfA1 and HsfB1 (Bharti et al, unpublished data).
Searching in the Arabidopsis expressed sequence tag (EST) libraries, which were exclusively prepared from non–heat shock tissues, revealed ESTs for practically all cytoplasmic sHsps (Table 3). The only exception is Hsp17.6A-CI. Interestingly, no ESTs were found for the plastidial and mitochondrial representatives either. As expected, ESTs encoding class CI and class CII sHsps are detectable in libraries derived from RNAs from flower buds, green siliques, and dry seeds. However, surprisingly, some related ESTs were also found in rosette leaves. It is, of course, an open question, whether the presence of messenger RNAs also correlates with the accumulation of the corresponding proteins, which are usually not detectable in nonstressed leaves.
Table 3.
Expression of the genes encoding small Hsps (sHsps) in Arabidopsis: survey of expressed sequence tag (EST) clones
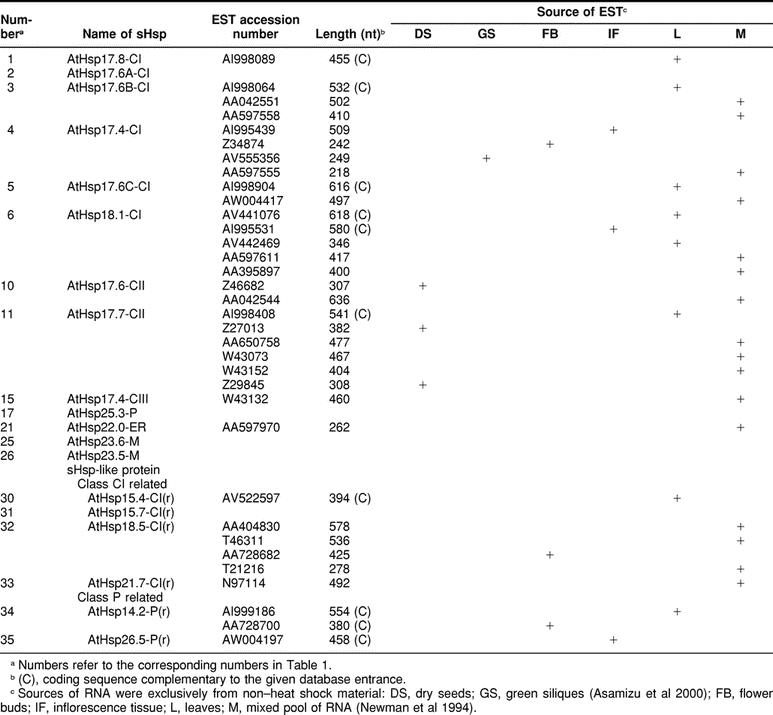
Fig 1.
Continued
Fig 3.
Continued
Table 1.
Continued
Acknowledgments
We thank Lutz Nover (Frankfurt/Main, Germany) and Wilfried de Jong (Nijmegen, Netherlands) for many helpful discussions and comments during the preparation of the manuscript. The work was supported by grants from the Deutsche Forschungsgemeinschaft to K.-D.S. (SCHA 577/6-1) and by the Fonds der Chemischen Industrie. E.V. acknowledges research support from the National Institutes of Health (GM-42762) and the US Department of Agriculture (NRICGP) and support during preparation of this review from the National Science Foundation (POWRE Award), the Guggenheim Foundation, and the Dutch National Science Foundation.
REFERENCES
- Amin J, Mestril R, Schiller P, Dreano M, Voellmy R. Organization of the Drosophila melanogaster hsp70 heat shock regulation unit. Mol Cell Biol. 1987;7:1055–1062. doi: 10.1128/mcb.7.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamizu E, Nakamura Y, Sato S, Tabata S. A large scale analysis of cDNA in Arabidopsis: generation of 12,028 non-redundant expressed sequence tags from normalized and size-selected cDNA libraries. DNA Res. 2000;7:175–180. doi: 10.1093/dnares/7.3.175. [DOI] [PubMed] [Google Scholar]
- Bartling D, Bulter H, Liebeton K, Weiler EW. An Arabidopsis thaliana cDNA clone encoding a 17.6 kDa class II heat shock protein. Plant Mol Biol. 1992;18:1007–1008. doi: 10.1007/BF00019220. [DOI] [PubMed] [Google Scholar]
- Basha EM, Waters ER, Vierling E. Triticum aestivum cDNAs homologous to nuclear-encoded mitochondrion-localized small heat shock proteins. Plant Sci. 1999;141:93–103. [Google Scholar]
- Berengian AR, Parfenova M, McHaourab HS. Site-directed spin labeling study of subunit interactions in the α-crystallin domain of small heat-shock proteins—comparison of the oligomer symmetry in αA-crystallin, HSP 27, and HSP 16.3. J Biol Chem. 1999;274:6305–6314. doi: 10.1074/jbc.274.10.6305. [DOI] [PubMed] [Google Scholar]
- Boelens WC, Van Boekel MAM, de Jong WW. HspB3, the most deviating of the six known human small heat shock proteins. Biochim Biophys Acta Protein Struct Mol Enzymol. 1998;1388:513–516. doi: 10.1016/s0167-4838(98)00215-5. [DOI] [PubMed] [Google Scholar]
- Bova MP, Ding LL, Horwitz J, Fung BKK. Subunit exchange of αA-crystallin. J Biol Chem. 1997;272:29511–29517. doi: 10.1074/jbc.272.47.29511. [DOI] [PubMed] [Google Scholar]
- Bova MP, McHaourab HS, Han Y, Fung BKK. Subunit exchange of small heat shock proteins: analysis of oligomer formation of αA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- Caspers GJ, Leunissen JAM, de Jong WW. The expanding small heat-shock protein family, and structure predictions of the conserved α-crystallin domain. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- Chen Q, Osteryoung K, Vierling E. A 21-kDa chloroplast heat shock protein assembles into high molecular weight complexes in vivo and in organelle. J Biol Chem. 1994;269:13216–13223. [PubMed] [Google Scholar]
- Chen Q, Vierling E. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol Gen Genet. 1991;226:425–431. doi: 10.1007/BF00260655. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragent B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JAM. Genealogy of the α-crystallin-small heat-stress superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Ding L, Candido EPM. HSP25, a small heat shock protein associated with dense bodies and M-lines of body wall muscle in Caenorhabditis elegans. J Biol Chem. 2000;275:9510–9517. doi: 10.1074/jbc.275.13.9510. [DOI] [PubMed] [Google Scholar]
- Dudich IV, Zav'yalov VP, Pfeil W, Gaestel M, Zav'yalova GA, Denesyuk AI, Korpela T. Dimer structure as a minimum cooperative subunit of small heat-shock proteins. Biochim Biophys Acta Protein Struct Mol Enzymol. 1995;1253:163–168. doi: 10.1016/0167-4838(95)00135-x. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Lilie H, Gaestel M, Buchner J. The dynamics of Hsp25 quarternary structure. J Biol Chem. 1999;274:14867–14874. doi: 10.1074/jbc.274.21.14867. [DOI] [PubMed] [Google Scholar]
- Forreiter C, Kirschner M, Nover L. Stable transformation of an Arabidopsis cell suspension culture with firefly luciferase providing a cellular system for analysis of chaperone activity in vivo. Plant Cell. 1997;9:2171–2181. doi: 10.1105/tpc.9.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forreiter C, Löw D. Cloning and characterization of two different cDNAs coding for cytoplasmic small heat stress proteins in Lycopersicon peruvianum. Plant Physiol. 1998a;117:718. [Google Scholar]
- Forreiter C, Löw D. Characterization of a cDNA coding for cytoplasmic class II small heat stress protein in Lycopersicon peruvianum. Plant Physiol. 1998b;117:1125. [Google Scholar]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KW, LaFayette PR, Nagao RT, Key JL, Vierling E. Localization of small HSPs to the higher plant endomembrane system. Mol Cell Biol. 1993;13:238–247. doi: 10.1128/mcb.13.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KW, Lee GJ, Vierling E. Expression and native structure of cytosolic class II small heat-shock proteins. Plant Physiol. 1997;114:1477–1485. doi: 10.1104/pp.114.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KW, Schmeits J, Vierling E. An endomembrane-localized small heat-shock protein from Arabidopsis thaliana. Plant Physiol. 1995;107:287–288. doi: 10.1104/pp.107.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KW, Vierling E. An Arabidopsis thaliana cDNA clone encoding a low molecular weight heat shock protein. Nucleic Acids Res. 1989;17:7995. doi: 10.1093/nar/17.19.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L, Nover L 1991 Heat Shock and Development. Springer, Berlin. [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jinn TL, Yeh YC, Chen YM, Lin C-Y. Stabilization of soluble proteins in vitro by heat shock proteins-enriched ammonium sulfate fraction from soybean seedlings. Plant Cell Physiol. 1989;30:463–469. [Google Scholar]
- Kaneko T, Katoh T, Sato S, Nakamura Y, Asamizu E, Kotani H, Miyajima N, Tabata S. Structural analysis of Arabidopsis thaliana chromosome 5, IX: sequence features of the region 1,011,550 bp covered by seventeen P1 and TAC clones. DNA Res. 1999;6:183–195. doi: 10.1093/dnares/6.3.183. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Katoh T, Sato S, Nakamura Y, Asamizu E, Tabata S. Structural analysis of Arabidopsis thaliana chromosome 3, II: sequence features of the 4,251,695 bp regions covered by 90 P1, TAC and BAC clones. DNA Res. 2000;7:217–221. doi: 10.1093/dnares/7.3.217. [DOI] [PubMed] [Google Scholar]
- Kelley LA, MacCallum RM, Sternberg MJE. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J Mol Biol. 2000;299:501–522. doi: 10.1006/jmbi.2000.3741. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998a;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kim R, Kim KK, Yokota H, Kim SH. Small heat shock protein of Methanococcus jannaschii, a hyperthermophile. Proc Natl Acad Sci U S A. 1998b;95:9129–9133. doi: 10.1073/pnas.95.16.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Winkelhaus S, Thierfelder J, Nover L. Transient expression and heat stress induced aggregation of endogenous and heterologous small heat stress proteins in tobacco protoplasts. Plant J. 2000;24:397–412. doi: 10.1046/j.1365-313x.2000.00887.x. [DOI] [PubMed] [Google Scholar]
- Knauf U, Jakob U, Engel K, Buchner J, Gaestel M. Stress- and nitrogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J. 1993;13:54–60. doi: 10.1002/j.1460-2075.1994.tb06234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Dallas PB, Pacchione S, Wilsker D, Bowrin V, Moran E. The human SWI-SNF complex protein p270 is an ARID family member with non-sequence-specific DNA binding activity. Mol Cell Biol. 2000;20:3137–3146. doi: 10.1128/mcb.20.9.3137-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Faivre JF, and Robert P. et al. 1999 Identification and characterization of cvHsp: a novel human small stress proteins selectively expressed in cardiovascular and insulin-sensitive tissues. J Biol Chem. 274:36592–36600. [DOI] [PubMed] [Google Scholar]
- Lauzon LM, Helm K, Vierling E. A cDNA clone from Pisum sativum encoding a low molecular weight heat shock protein. Nucleic Acids Res. 1990;18:4274. doi: 10.1093/nar/18.14.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SD, Cline K, Moore GA. Chromoplast development in ripening tomato fruit—identification of cDNAs for chromoplast-targeted proteins and characterization of a cDNA encoding a plastid-localized low-molecular-weight heat shock protein. Plant Mol Biol. 1997;33:483–492. doi: 10.1023/a:1005785321165. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Pokala N, Vierling E. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem. 1995;270:10432–10438. doi: 10.1074/jbc.270.18.10432. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000;122:189–198. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne C, Block MA, Garin J, Douce R. Sequence and expression of the mRNA encoding Hsp22, the mitochondrial small heat-shock protein in pea leaves. Biochem J. 1995;311:805–813. doi: 10.1042/bj3110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux MR, Ma BJ, Batelier G, Melki R, Peter E, Candido M. Unique structural features of a novel class of small heat shock proteins. J Biol Chem. 1997;272:12847–12853. doi: 10.1074/jbc.272.19.12847. [DOI] [PubMed] [Google Scholar]
- Lin X, Kaul S, and Rounsley SD. et al. 1999 Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature. 402:761–768. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/alpha-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain EF, Spiker S. A wheat cDNA clone which is homologous to the 17kd heat-shock protein gene family of soybean. Nucleic Acids Res. 1989;17:1764. doi: 10.1093/nar/17.4.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaourab HS, Berengian AR, Koteiche HA. Site-directed spin-labeling study of the structure and subunit interactions along a conserved sequence in the α-crystallin domain of heat-shock protein 27: evidence of a conserved subunit interface. Biochemistry. 2000;36:14627–14634. doi: 10.1021/bi971700s. [DOI] [PubMed] [Google Scholar]
- Merck KB, Groenen PJTA, Voorter CEM, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine α-crystallin and mouse small heat-shock protein: a family of chaperones. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- Morrow G, Inaguma Y, Kato K, Tanguay RM. The small heat shock protein Hsp22 of Drosophila melanogaster is a mitochondrial protein displaying oligomeric organization. J Biol Chem. 2000;275:31204–31210. doi: 10.1074/jbc.M002960200. [DOI] [PubMed] [Google Scholar]
- Moser D, Doenhoff MJ, Klinkert MQ. A stage-specific calcium-binding protein expressed in eggs of Schistosoma mansoni. Mol Biochem Parasitol. 1992;51:229–238. doi: 10.1016/0166-6851(92)90073-s. [DOI] [PubMed] [Google Scholar]
- Munchbach M, Nocker A, Narberhaus F. Multiple small heat shock proteins in Rhizobia. J Bacteriol. 1999;181:83–90. doi: 10.1128/jb.181.1.83-90.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Kondo Y, Nakano T, Sato F. Protease activity of CND41, a chloroplast nucleoid DNA-binding protein, isolated from cultured tobacco cells. FEBS Lett. 2000;468:15–18. doi: 10.1016/s0014-5793(00)01186-8. [DOI] [PubMed] [Google Scholar]
- Nakano T, Murakami S, Shoji T, Yoshida S, Yamada Y, Sato F. A novel protein with DNA binding activity from tobacco chloroplast nucleoids. Plant Cell. 1997;9:1673–1682. doi: 10.1105/tpc.9.9.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Dunne DW, Johnson KS, Taylor DW, Cordingley JS. Sequence and expression of a major egg antigen from Schistosoma mansoni: homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- Newman T, deBruijn FJ, and Green P. et al. 1994 Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 106:1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Weng J, Joshi CP. A wheat (Triticum aestivum) cDNA clone encoding a plastid-localized heat-shock protein. Plant Physiol. 1993;103:675–676. doi: 10.1104/pp.103.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L 1991 Heat Shock Response. CRC Press, Boca Raton, FL. [Google Scholar]
- Nover L, Miernyk J 2001 A genomic approach to the chaperone system of Arabidospis thaliana. Cell Stress Chaperones 6: 173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol. 1983;3:1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989;9:1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Sundberg H, Vierling E. Pola(A) tail length of heat shock protein RNA is increased by severe heat stress, but intron splicing is unaffected. Mol Gen Genet. 1993;239:323–333. doi: 10.1007/BF00276930. [DOI] [PubMed] [Google Scholar]
- Petko L, Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1985;45:885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Plater ML, Goode D, Crabbe MJC. Effects of site-directed mutations on the chaperone-like activity of αB-crystallin. J Biol Chem. 1996;271:28558–28566. doi: 10.1074/jbc.271.45.28558. [DOI] [PubMed] [Google Scholar]
- Prändl R, Hinderhofer K, Eggers-Schumacher G, Schöffl F. Hsf3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol Gen Genet. 1998;258:269–278. doi: 10.1007/s004380050731. [DOI] [PubMed] [Google Scholar]
- Sébastien M, Geneviève M, Julie M, and Tanguay RM 2001 Drosophila small heat shock proteins: cell and organelle-specific chaperones? In: Small Stress Proteins, ed Arrigo A-P and Müller WEG. Heidelberg, Springer-Verlag. [DOI] [PubMed] [Google Scholar]
- Sun T-S, Das BK, Liang JJN. Conformational and functional differences between recombinant human lens αA- and αB-crystallin. J Biol Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sugiyama Y, and Hayashi Y. et al. 1998 MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J Cell Biol. 140:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Komeda Y. Characterization of two genes encoding small heat-shock proteins in Arabidopsis thaliana. Mol Gen Genet. 1989;219:365–372. doi: 10.1007/BF00259608. [DOI] [PubMed] [Google Scholar]
- Treisman JE, Luc A, Rubin GM, Heberlein U. Eyelid antagonizes wingless signaling during Drosophila development and has homology to the Bright family of DNA-binding proteins. Genes Dev. 1997;11:1949–1962. doi: 10.1101/gad.11.15.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker PW, Herrscher RF, Kaplan MH, Lelsz DL, Das C, Scheuermann R. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev. 1995;9:3067–3082. doi: 10.1101/gad.9.24.3067. [DOI] [PubMed] [Google Scholar]
- Tucker PW, Kortschak RD, Saint R. ARID proteins come in from the desert. Trends Biochem Sci. 2000;25:294–299. doi: 10.1016/s0968-0004(00)01597-8. [DOI] [PubMed] [Google Scholar]
- Veinger L, Diamant S, Buchner J, Goloubinoff P. The small heat-shock protein IbpB from Escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaperone network. J Biol Chem. 1998;273:11032–11037. doi: 10.1074/jbc.273.18.11032. [DOI] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Vierling E, Nagao RT, DeRocher AE, Harris LM. A heat shock protein localized to chloroplasts is a member of a eucaryotic superfamily of heat shock proteins. EMBO J. 1988;7:575–581. doi: 10.1002/j.1460-2075.1988.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli G, Maestri E, Marmiroli N. Differential display-mediated isolation of a genomic sequence for a putative mitochondrial LMW HSP specifically expressed in condition of induced thermotolerance in Arabidopsis thaliana(L.) Heynh. Plant Mol Biol. 1997;34:517–527. doi: 10.1023/a:1005824314022. [DOI] [PubMed] [Google Scholar]
- Waters ER. The molecular evolution of small heat-shock proteins in plants. Genetics. 1995;141:785–795. doi: 10.1093/genetics/141.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–338. [Google Scholar]
- Waters ER, Vierling E. The diversification of plant cytosolic small heat shock proteins preceded the divergence of mosses. Mol Biol Evol. 1999;16:127–139. doi: 10.1093/oxfordjournals.molbev.a026033. [DOI] [PubMed] [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Synthesis of small heat shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Wang ZF, Nguyen HT. A Triticum aestivum cDNA clone encoding a low-molecular weight heat shock protein. Plant Mol Biol. 1991;17:273–275. doi: 10.1007/BF00039504. [DOI] [PubMed] [Google Scholar]
- Whitham SA, Anderberg RJ, Chrisholm ST, Carrington JC. Arabidopsis RTM2 gene is necessary for specific restriction of Tobacco Etch Virus and encodes an unusual small heat shock-like protein. Plant Cell. 2000;12:569–582. doi: 10.1105/tpc.12.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett DA, Basha EM, Vierling E. Nucleotide sequence of a cDNA encoding a mitochondrion-localized small Hsp from Arabidopsis thaliana: AtHsp23.6. Plant Physiol. 1996;112:1399. [Google Scholar]
- Wotton D, Freeman K, Shore D. Multimerization of Hsp42p, a novel heat shock protein of Saccharomyces cerevisiae, is dependent on a conserved carboxyl-terminal sequence. J Biol Chem. 1996;271:2717–2723. doi: 10.1074/jbc.271.5.2717. [DOI] [PubMed] [Google Scholar]