Abstract
The Hsp70-interacting protein Hip binds to the adenosine triphosphatase domain of Hsp70, stabilizing it in the adenosine 5′-diphosphate–ligated conformation and promoting binding of target polypeptides. In mammalian cells, Hip is a component of the cytoplasmic chaperone heterocomplex that regulates signal transduction via interaction with hormone receptors and protein kinases. Analysis of the complete genome sequence of the model flowering plant Arabidopsis thaliana revealed 2 genes encoding Hip orthologs. The deduced sequence of AtHip-1 consists of 441 amino acid residues and is 42% identical to human Hip. AtHip-1 contains the same functional domains characterized in mammalian Hip, including an N-terminal dimerization domain, an acidic domain, 3 tetratricopeptide repeats flanked by a highly charged region, a series of degenerate GGMP repeats, and a C-terminal region similar to the Sti1/Hop/p60 protein. The deduced amino acid sequence of AtHip-2 consists of 380 amino acid residues. AtHip-2 consists of a truncated Hip-like domain that is 46% identical to human Hip, followed by a C-terminal domain related to thioredoxin. AtHip-2 is 63% identical to another Hip-thioredoxin protein recently identified in Vitis labrusca (grape). The truncated Hip domain in AtHip-2 includes the amino terminus, the acidic domain, and tetratricopeptide repeats with flanking charged region. Analyses of expressed sequence tag databases indicate that both AtHip-1 and AtHip-2 are expressed in A thaliana and that orthologs of Hip are also expressed widely in other plants. The similarity between AtHip-1 and its mammalian orthologs is consistent with a similar role in plant cells. The sequence of AtHip-2 suggests the possibility of additional unique chaperone functions.
INTRODUCTION
Hip is one of several cochaperones that regulate activities of the Hsp70 chaperone family (Höhfeld et al 1995; Prapapanich et al 1996a, 1996b; Gebauer et al 1997; Bimston et al 1998). Hsp70s are virtually ubiquitous among tissues and organs of both animals and plants (Boorstein et al 1994; Boston et al 1996; Miernyk 1999), serving important roles in protein synthesis, assembly, and import. Hsp70s also function in signal transduction pathways in cooperation with other chaperones and cochaperones (Pratt 1998; Kimmins and McRae 2000; Smith 2000). In plants, members of the Hsp70 family have been identified in most subcellular compartments, including cytosol, nuclei, endoplasmic reticulum, mitochondria, chloroplasts, and peroxisomes (Boston et al 1996; Corpas and Trelease 1997; Miernyk 1999). Hsp70-interacting proteins function to mediate and differentiate the actions of Hsp70s among diverse pathways and cellular compartments (Bimston et al 1998; Takayama et al 1999), and recent studies indicate an intriguing complexity of cochaperone interactions regulating Hsp70s in eukaryotes (Gebauer et al 1997; Takayama et al 1999; Cheung and Smith 2000; Kimmins and MacRae 2000).
Interactions of Hip with Hsp70 and other cochaperones have been widely studied in animals (Höhfeld et al 1995; Prapapanich et al 1996a, 1996b, 1998; Irmer and Höhfeld 1997; Demand et al 1998; Kanelakis et al 2000), but not in plants. Sequence analysis of the Arabidopsis thaliana genome revealed 2 genes encoding distinct Hip-like proteins, including one similar to mammalian Hip and a second comprising a Hip-thioredoxin chimera, similar to a protein recently identified in grape. The 2 Hip orthologs are expressed in Arabidopsis and a variety of other plants.
The role of Hip in Hsp70 regulation
Previous studies have shown that Hip functions to stabilize Hsp70 in an adenosine 5′-diphosphate (ADP)–bound conformation that appears to prolong its interaction with substrate. Hsp70 cycles between 2 conformations in its role as a chaperone, an adenosine triphosphate (ATP)–bound form with low affinity for substrate and an ADP-bound form with high affinity for substrate (Palleros et al 1993). In bacteria the cochaperones DnaJ and GrpE mediate the reaction cycle of the Hsp70 ortholog DnaK (Frydman and Höhfeld 1997). DnaJ interacts with ATP-bound DnaK to promote hydrolysis of ATP to ADP, stimulating and stabilizing association of DnaK with its polypeptide substrate. Conversely, GrpE, a nucleotide exchange factor, promotes dissociation of ADP from DnaK, allowing ATP binding and subsequent release of the polypeptide substrate. Hsp70 and DnaJ-related proteins (Hsp40) are widespread and distributed throughout eukaryotic cells. However, orthologs of GrpE have been more difficult to identify in eukaryotes outside mitochondria (Ikeda et al 1994) and chloroplasts (Schlicher and Soll 1997). Recently, other proteins have been identified that could substitute for GrpE in promoting nucleotide exchange in other compartments of eukaryotic cells (Naylor et al 1998; Sondermann et al 2001).
Hip was originally identified by Höhfeld et al (1995) in a yeast 2-hybrid screen searching for GrpE-like proteins that interacted with the adenosine triphosphatase (ATPase) domain of rat heat shock cognate 70 (Hsc70). A protein identified in this screen was confirmed to bind Hsc70 and was designated Hip for Hsc70-interacting protein (Höhfeld et al 1995). Subsequently, a previously described p48 protein associated with mammalian progesterone receptor complexes (Smith 1993) was identified as Hip (Prapapanich et al 1996a), and a complementary DNA (cDNA) down-regulated in human colorectal cancer (Cao et al 1997) was found to encode Hip.
Although Hip interacts with the ATPase domain of Hsc/Hsp70 (Höhfeld et al 1995; Prapapanich et al 1996b), as does GrpE, Hip differs from GrpE both structurally and functionally. In the eukaryotic Hsp70 reaction cycle a DnaJ-like protein, Hsp40, functions to stimulate ATP hydrolysis by Hsp70 (Frydman and Höhfeld 1997). Subsequent binding of Hip then appears to stabilize Hsp70 in its ADP conformation, prolonging its association with substrate (Höhfeld et al 1995). Hip does not bind to mutant Hsp70 that cannot hydrolyze ATP (Prapapanich et al 1996b, 1998). Hip also was active in luciferase refolding assays in vitro and thus appears to have chaperone activity independent of Hsp70 (Höhfeld et al 1995).
Hip-Hsp70 interaction within multichaperone complexes involved in progesterone receptor assembly in mammals has been extensively examined (Prapapanich et al 1996a, 1996b, 1998). Hip associates transiently with Hsp70 and the progesterone receptor at an intermediate stage in assembly of the functional receptor complex, and Hip-Hsp70 association stabilizes the receptor in a conformation that prevents premature interaction with hormone in the cytosol. Before dissociating from the complex, Hip and Hsp70 also promote association of the cochaperone Hop along with Hsp90 and other proteins (Krishna and Gloor 2001) with the progesterone receptor to form the mature, hormone-receptive complex (Prapapanich et al 1998; Smith 2000).
Recent studies have raised questions about whether Hip is required for steroid receptor assembly or plays a regulatory role in the pathway (Cheung and Smith 2000; Kanelakis et al 2000). The Bag-1 family of cochaperones, considered by some to substitute for GrpE function in eukaryotes (Höhfeld 1998; Sondermann et al 2001), competes with Hip for binding to the ATPase domain of Hsp70 (Gebauer et al 1997; Takayama et al 1997, 1999; Bimston et al 1998). In reconstituted heterocomplexes, Hip prevented inhibitory effects of Bag-1 on complex assembly and steroid binding, and Hip was proposed to play a regulatory role in opposing Bag-1 (Kanelakis et al 2000).
Structure of mammalian Hip
Studies of mammalian Hip have provided information about protein structure. Examination of quaternary structure indicated that Hip forms homodimers (Velten et al 2000). Subunits of Hip are composed of 369 amino acid residues with a calculated molecular mass of 41.3 kDa and are encoded in rat by messenger RNA (mRNA) of approximately 1.7 kb (Höhfeld et al 1995). The protein exhibits anomalous mobility in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), presenting a larger apparent molecular mass of 48–50 kDa (Höhfeld et al 1995; Prapapanich et al 1996a). Modeling of quaternary structure indicated that Hip forms an irregular elongated form in solution (Velten et al 2000).
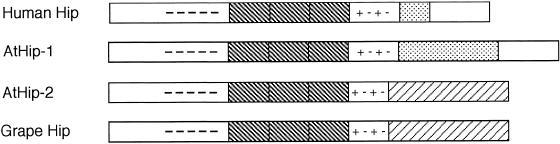
Structural and mutational analyses (Höhfeld et al 1995; Prapapanich et al 1996b, 1998; Irmer and Höhfeld 1997; Velten et al 2000) of mammalian Hip indicated the presence of multiple functional domains. From amino to carboxy termini these include a conserved amino terminal domain, a highly acidic region, 3 tetratricopeptide repeats (TPRs) with flanking charged regions, a domain of degenerate GGMP repeats, and a carboxy domain similar to yeast stress-induced protein Sti1 and its orthologs Hop and p60 (Fig 1). These domains are discussed later in relation to their conservation in Arabidopsis orthologs of Hip.
Fig 1.
Comparison of the overall structure of the human and plant Hip proteins. All representatives contain a conserved N-terminus (not labeled), a prominent acidic domain (dashes), 3 TPRs (dark hatched), and a highly charged region (plus, minus, plus, minus). Human Hip and AtHip-1 include a carboxy domain of degenerate GGMP repeats (stippled) and a Sti1-like C-terminus (not labeled). AtHip-2 and grape Hip contain a carboxy thioredoxin domain (light hatched)
Genes encoding Hip-like proteins in Arabidopsis
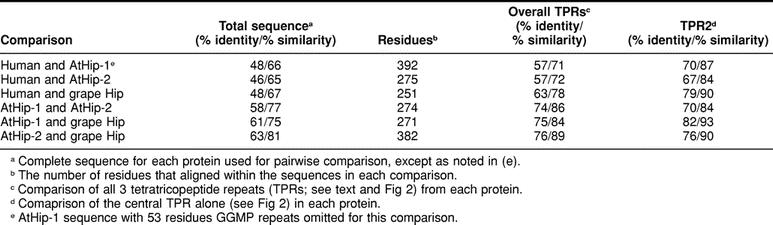
The Arabidopsis genome contains 2 genes with high degrees of similarity to mammalian Hip on chromosomes 4 and 3, designated AtHip-1 and AtHip-2, respectively (Table 1; Fig 1). The 2 genes do not share significant similarity in their nucleotide sequences. However, predicted protein sequences, based on revised annotation of gene sequences (see below), share substantial similarity with each other and with mammalian Hip and a Hip-like protein in grape (Fig 1; Table 2). AtHip-1 shares similarity to mammalian Hip over the entire length of the predicted protein. In contrast, AtHip-2 contains a truncated Hip-like domain followed by a carboxy thioredoxin–like domain, similar to the grape protein.
Table 1.
Orthologs of Hip in Arabidopsis thaliana
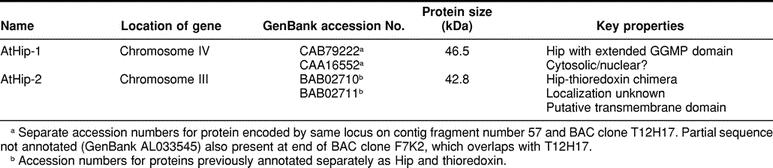
Table 2.
Sequence comparisons among human and plant orthologs of Hip
Revised annotation of Hip-like genes in Arabidopsis
Sequence analysis of clones identified from expressed sequence tags (ESTs) corresponding to Hip-related genes indicated a need for revising current annotations. Coding sequence for the gene on chromosome 4 in Arabidopsis was previously described as 1884 nucleotides in length, encoding a predicted protein of 628 amino acids, much longer at the amino end than mammalian Hip orthologs. Sequence alignment of cDNA clones from Arabidopsis EST collections with genomic sequence, as detailed below, indicated that the previously annotated coding sequence of this gene is actually 2 genes. However, we cannot rule out the possibility that a longer variant of Hip could be transcribed from the gene as it was previously annotated.
The cDNA R30271, which aligns with the 5′ end of this gene, was found to be only 700 nucleotides in length and is polyadenylated. The predicted amino acid sequence encoded by R30271 had no similarity to mammalian Hip. An ATG in the genomic sequence, located 371 nucleotides from the coding sequence of R30271, corresponded to the initiating Met codon of human Hip and coincided with an ATG in the predicted open reading frame of 2 independently identified Arabidopsis ESTs for this gene, H76638 and BE038428. Thus, it appears that the previously annotated gene encodes 2 genes and that protein coding sequence for AtHip-1 begins at the ATG aligning with human Hip. Revised coding sequence was determined by comparison with 5′ sequence from cDNAs H76638 and BE038428 and complete sequence obtained for H76919. The predicted amino acid sequence of 441 residues aligned with human Hip along its entire length, except that AtHip-1 contains an expanded region of GGMP repeats (Fig 1).
Coding sequence as annotated for the Hip-related gene in Arabidopsis on chromosome 3 is contiguous with sequence encoding a thioredoxin-like protein. Because we had identified a cDNA in grape encoding a chimeric Hip-thioredoxin protein, we investigated whether the coding sequences for Hip and thioredoxin actually comprised a continuous coding sequence. Structure of the AtHip-2 gene was clarified by comparison with the grape cDNA and analysis of EST cDNA clone BE527303. To determine the complete open reading frame for this gene, the cDNA BE527303 was sequenced in its entirety. Sequence obtained for BE527303 and a shorter polymerase chain reaction (PCR) product amplified from this clone included both Hip and thioredoxin coding sequence with no intervening stop codon. The predicted protein aligned with the Hip-thioredoxin protein from grape along its entire length. Thus, AtHip-2 appears to encode a similar chimera containing both Hip and thioredoxin-like domains (Fig 1). A Hip-thioredoxin chimera has not been reported in animals.
Hip-like protein in grape similar to AtHip-2
AtHip-2 shares 63% sequence identity with the product of a cDNA recently identified in grape (Vitis labrusca). The full-length grape cDNA encodes a protein of 385 amino acids consisting of an N-terminal Hip domain that is 48% identical to human Hip followed by a C-terminal thioredoxin domain. This unique combination of functional domains was confirmed by reverse transcriptase–PCR amplification of a single 850–base pair product obtained from total RNA from grape leaf using primers designed from the central TPR in the Hip domain and 3′ untranslated sequence. The sequence of this PCR-derived cDNA matched the original grape cDNA, including both Hip and thioredoxin sequences, confirming the presence and contiguous arrangement of the 2 domains.
A partial cDNA was first isolated (Cavaletto 1997) in a screen for genes encoding proteins associated with needle-shaped crystals of calcium oxalate, called raphides, that are produced in specialized cells of grape (Webb et al 1995, 1999). We subsequently obtained preliminary evidence that indicated an association of the Hip protein with grape raphides (Cavaletto and Webb, unpublished). If confirmed, these studies could point to a novel context for Hip function potentially relevant to AtHip-2. Although Arabidopsis does not normally accumulate calcium oxalate, small crystals have been observed at the base of developing ovules (Webb, unpublished).
Conservation of functional domains in Arabidopsis orthologs of Hip
AtHip-1 shares similarity with mammalian Hip over its entire length of 441 residues, including all previously identified functional domains, except that AtHip-1 has an extended region of degenerate GGMP repeats (Fig 1). AtHip-2, like grape Hip, contains a truncated Hip domain with substantial similarity to mammalian Hip followed by a carboxy thioredoxin domain (Fig 1). The Hip domains in AtHip-2 and grape Hip align with human Hip over 251 and 275 residues, respectively, including amino terminus, acidic domain, and TPRs and flanking highly charged regions. The region of GGMP repeats and the Sti1-like domain are replaced in AtHip-2 and grape Hip by thioredoxin. Pairwise sequence comparisons of AtHip-1, AtHip-2, grape Hip, and human Hip are summarized in Table 2. Alignments of complete sequence for Hip orthologs from Arabidopsis, grape, human, and selected other organisms can be viewed in online Figure 3 at http://www.cellstress.uconn.edu.
Fig 3.
Figure 3 is available online only
TPR structure critical to interaction with Hsp70 conserved in Arabidopsis
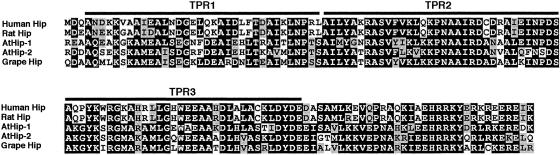
Physical interaction of Hip with Hsp70 is crucial to its function. Previous analyses of mammalian Hip have demonstrated that the TPRs, along with flanking charged regions, are required for binding to Hsp70 (Höhfeld et al 1995; Prapapanich et al 1996a, 1996b; Irmer and Höhfeld 1997; Velten et al 2000). AtHip-1 and AtHip-2 include 3 contiguous TPRs at the same location as in mammalian Hip (Figs 1 and 2). TPRs are degenerate 34-residue repeats that promote protein-protein interactions in a variety of cellular processes (Lamb et al 1995; Blatch and Lassle 1999). TPRs are defined by selected consensus residues within the motif, and intervening variable residues are typically more highly conserved among TPRs with similar function (Lamb et al 1995). Each TPR forms a pair of antiparallel α-helices that interact with target protein, which generally does not contain TPRs (Blatch and Lassle 1999). Modeling of Hip structure (Velten et al 2000) indicated that 6 antiparallel α-helices in Hip's 3 TPRs create a groove and that this groove and the flanking regions present an electrostatic surface complementary to a proposed binding site on the Hsp70 surface (Velten et al 2000).
Alignment of Hip sequences from Arabidopsis, grape, rat, and human showed that the TPRs and flanking charged regions are remarkably conserved (Fig 2). In particular, the central TPR exhibited the highest degree of sequence conservation (Fig 2; Table 2). Key charged residues within the TPRs and flanking sequence, predicted to form the electrostatic surface interacting with Hsp70, are not completely conserved in Arabidopsis and grape. However, the flanking charged region at the carboxy end includes 33 residues that are highly conserved in Arabidopsis and grape (Fig 2). Sequence alignments can help to delimit the functionally active part of the sequence, particularly if the truncated Hip domains in AtHip-2 and grape Hip are found to be competent to bind Hsp70.
Fig 2.
Comparison of the TPR motifs and the flanking charged region that are both necessary and sufficient for binding of Hip to the ATPase domain of Hsp70. Alignment illustrates the high degree of primary sequence conservation among animal and plant Hips. GenBank accession numbers are as follows: human Hip, U28918; rat Hip, X82021; AtHip-1, CAB79222; AtHip-2, BAB02710 and BAB02711; and grape Hip, AYO36906
The high degree of sequence conservation overall in the TPRs and flanking highly charged region indicates that mechanisms of Hip interaction with Hsp70 are likely to be conserved in Arabidopsis. Sequence variation within these motifs could reflect differences in interacting Hsp70s and in the functional contexts of Hip-Hsp70 interactions in plants.
Selected residues conserved within the amino terminus
In mammalian Hip, the amino terminus is necessary and sufficient for Hip dimerization. Mutants containing the first 124–150 residues alone formed oligomers, whereas those with N-terminal deletions did not (Höhfeld et al 1995; Prapapanich et al 1996b). Comparison of the amino terminus in Arabidopsis, grape, and mammalian Hip reveals that a high degree of conservation extends for the first 44 N-terminal residues (Fig 3). Such sequence comparisons will help to discern which amino acids could be critical for dimerization. Certain residues within the amino terminus are conserved between plant and animal Hips, whereas other residues are strictly conserved among plants or mammals but differ between the 2 groups.
The acidic domain enriched in Glu and Asp
A highly acidic domain between the amino terminus and the TPRs is particularly prominent in AtHip-1 (Fig 4). Functional significance of the acidic domain has not been determined. However, Hip mutants lacking this region did not have the altered mobility observed in wild-type Hip in SDS-PAGE (Prapapanich et al 1996b). Although amino acid sequence in this domain is not strictly conserved and precise delimitation of the domain is not clear, all representatives exhibit high percentages of Glu and/or Asp over approximately 44 amino acids. In addition, sequences include a remarkable string of 23 contiguous Glu residues in Plasmodium, 7 Glu and 1 Asp in AtHip-1, 6 Asp in AtHip-2, and 2 closely spaced Glu/Asp/Asp motifs in grape Hip.
Fig 4.
Comparison of the N-terminal dimerization motif of the Hip proteins. Key residues are conserved among plant and animal orthologs of Hip. GenBank accession numbers are the same as in Figure 2
These sequences exhibit features common to high-capacity calcium-binding motifs (Michalak et al 1998). Previous studies have shown that Hip-Hsp70 interaction is disrupted by high salt (Prapapanich et al 1996b), but effects of calcium on the interaction have not been examined. Sequence characteristics indicate a need to examine whether Hip binds calcium and to ascertain any potential role(s) of the acidic domain in mediating regulation of Hip by calcium. Alternatively, the acidic domain could be involved in another kind of electrostatic interaction.
Extended domain of GGMP repeats in AtHip-1
Near the carboxy terminus, AtHip-1 contains a region of degenerate GGMP repeats that extend for 96 residues, approximately 3 times longer than the GGMP domain in mammalian Hip (Fig 1). This lengthy GGMP domain is similar to prominent GGMP repeats in Hip-like orthologs in Plasmodium berghei (Uparanukraw et al 1993) and Plasmodium chabaudi (Langsley et al 1993), which contain 68 and 90 residues of these repeats, respectively. The function of GGMP repeats has not been established (Prapapanich et al 1996a, 1996b). GGMP repeats of unknown function are also found at the carboxy end of cytosolic Hsp70s in eukaryotes (Boorstein et al 1994). The number and degeneracy of similar PGQM (GQMP) repeats in synexins appeared related to tissue-specific expression or localization (Srivastava et al 1996). The facility of Arabidopsis for examining mutational effects in vivo will aid in clarifying the functional significance of this domain.
Sti1-like carboxy domain in AtHip-1
A short Sti1-like domain at the carboxy terminus of Hip (Höhfeld et al 1995) is present in AtHip-1 but not AtHip-2 (Fig 1). Sti1 is the yeast ortholog of Hop/p60, a mammalian cochaperone that mediates interaction between Hsp70 and Hsp90 in multichaperone heterocomplexes. The Sti1 domain from AtHip1 is aligned with yeast Sti1, human Hip, and 2 Arabidopsis orthologs of Sti1 in Figure 5. The Sti1-like domain of human Hip contains 2 DPEV motifs shown to function in assembly of the mature, hormone-responsive progesterone receptor in mammals (Prapapanich et al 1998). Mutation of 2 DPEV motifs within the Sti1 domain of Hip to APAV inhibited maturation of progesterone receptor complexes by blocking entry of Hsp90 into the complex (Prapapanich et al 1998). One of the DPEV motifs is conserved in AtHip-1, whereas the other is altered to DPEL, a substitution that might not be functionally relevant. The remainder of the Sti1-like domain is reasonably conserved between AtHip-1 and mammalian Hips.
Fig 5.
The acidic domains of selected Hip sequences. The domains are variable but all contain a high percentage of charged residues, including contiguous strings of glutamate (E) and aspartate (D) residues. See the legend of Figure 2 for GenBank accession numbers, except for Plasmodium protein, which has the GenBank accession number AAK14819
Thioredoxin domain in AtHip-2 and grape Hip
A novel feature distinguishing both AtHip-2 and grape Hip from other known Hip orthologs is the thioredoxin-like domain at the carboxy terminus in place of GGMP repeats and the Sti1 domain. Although BLAST analysis indicates that the thioredoxin domain in each is similar to cytosolic h-type thioredoxins, both AtHip-2 and grape Hip include a putative membrane-spanning region within this domain. Substitution of GGMP and Sti1 domains with thioredoxin and potential for membrane association suggest that AtHip-2 and grape Hip differ from other Hip orthologs in cellular location and function in vivo. Both AtHip-2 and grape Hip include the thioredoxin active site (Fig 5) mediating redox regulation through oxidation and reduction of sulfhydryl groups (Holmgren 1989; Meyer et al 1999). Other chaperones and cochaperones that contain thioredoxin-like domains (Nigam et al 1994; de Crouy-Chanel et al 1995; Ferrari and Soeling 1999) function in protein folding and cellular redox regulation. Further research will clarify effects conferred on Hip function by the carboxy thioredoxin domain along with its putative membrane-spanning region.
Thioredoxin domains of AtHip-2 and grape Hip share a higher degree of sequence conservation with each other than with other thioredoxins in plants (Fig 6). The AtHip-2 domain shares 68% sequence identity and 85% sequence similarity with grape Hip. In contrast, the AtHip-2 domain has 43% sequence identity and 69% sequence similarity with the most closely related thioredoxin in Arabidopsis. Other thioredoxins closely related to AtHip-2 included h-type thioredoxins in rice phloem (45% identity, 70% similarity) and tobacco (42% identity, 69% similarity).
Fig 6.
Comparison of the C-terminal Sti1/Hop/p60 domain of selected proteins. The GenBank accession numbers for AtHip1 and human Hip are in the legend for Figure 2; the number for Saccharomyces cerevisiae Sti1 is P15705; for AtHop-2, AC007190; and for AtTic40, BAB10189
Expression of Hip orthologs in Arabidopsis and other plants
ESTs in Arabidopsis corresponding to both AtHip-1 and AtHip-2 have been identified, indicating that both genes are expressed. AtHip-1 aligns with numerous ESTs from a variety of mRNA sources, including untreated rosettes (AI99689), etiolated seedlings (Z25650), sodium chloride–treated plants (BE038428), and pooled mRNA from different sources (H76638, H76919, AA605571; Newman et al 1994). This abundance of ESTs suggests that AtHip-1 is widely expressed in Arabidopsis. In contrast, only one EST has been identified for AtHip-2 from a collection of ESTs enriched in specificity for developing seeds (BE527303; White et al 2000). This could indicate that AtHip-2 is only expressed in seeds or perhaps is expressed at low levels elsewhere.
Plant EST databases also contain numerous other Hip-like sequences revealed by tblastn analysis (Hip protein queried against translated database), including cDNAs in tomato, soybean, cotton, rice, corn, barley, and pine. These sequences provide evidence that Hip is widely expressed in plants, including a gymnosperm and divergent taxa among angiosperms. For many of these orthologs, limited 5′ sequence does not support distinction among human Hip/AtHip-1 and Hip/thioredoxin proteins, AtHip-2, and grape Hip. In other orthologs 3′ sequence tags indicate similarity to AtHip-1 and human Hip. Selected ESTs in wheat, cotton, and Medicago span portions of Hip and thioredoxin domains, suggesting that Hip-thioredoxin proteins are expressed in these plants.
What is the role of Hip in plants?
Numerous questions arise from identification of Hip orthologs in Arabidopsis, grape, and other plants. If Hip-Hsp70 interactions are conserved in plants, how might Hip function to regulate Hsp70 activity in plant cells? Multichaperone Hsp70-Hsp90 complexes are present in plants (Stancato et al 1996; Reddy et al 1998; Krishna and Gloor 2001), but their function is not known. It will be critical to determine whether Hip associates with these complexes in plants, as in animals. Alternatively, Hip could regulate Hsp70 in a different context in plants.
The striking similarity of AtHip-1 to mammalian Hip supports the idea that it could play a similar role in plant cells, and this needs to be investigated. Steroids, including sex hormones and their derivatives, are common in plants (Heftmann 1974; Agarwal 1993; Groeneveld 1999), and steroid biosynthetic enzymes are conserved between animals and plants (Li et al 1997). A putative ortholog of the mammalian mineralocorticoid receptor has been identified in Chlamydomonas (Mirshahi et al 1992). Although the biological activities of many plant steroids are not well understood, they affect a variety of physiological and developmental processes (Heftmann 1974; Geuns 1982; Mirshahi et al 1992; Fujioka et al 1997). Recent focus on brassinosteroids in Arabidopsis has demonstrated their importance in signaling pathways in plant growth and development (Schumacher and Chory 2000). A brassinolide receptor in the plasma membrane has recently been identified and characterized (Friedrichsen et al 2000; He et al 2000).
Our studies of the Hip-thioredoxin chimera in grape indicate that it is associated with calcium oxalate crystals (Cavaletto 1997; Cavaletto and Webb, unpublished), which accumulate in intravacuolar membrane compartments (Webb 1999). During fertilization in animals, a calcium channel in the sperm plasma membrane is activated by progesterone via a membrane-associated progesterone receptor (Falkenstein et al 1996; Buddhikot et al 1999; Christ et al 1999). Similar membrane-bound receptors are common in a variety of cell types involved in bone synthesis and resorption, processes regulated by progesterone and other steroid hormones (MacNamara et al 1995; Bland 2000). Membrane receptors of this sort might interact with chaperones for stabilization or regulation, but this has not yet been examined in animals or plants.
The facility of genetic and mutational analysis in Arabidopsis will aid in defining roles for the Hip orthologs in Arabidopsis. Comparative analyses of AtHip-1 and AtHip-2 expression and localization will help to clarify functional differences between the 2 orthologs. Identification of interacting proteins for Hip orthologs and examination of the cellular contexts of Hip interactions are critical to advance understanding of Hip functions in plants.
Fig 7.
Alignment of the thioredoxin domains of AtHip-2 and grape Hip with closely related thioredoxins (Trxs) from plants. The thioredoxin active site is indicated by asterisks. GenBank accession numbers are as follows: AtHip-2, BAB02711; grape Hip, AY036906; rice Trx, Q42443; tobacco Trx, P29449; Arabidopsis Trx-1, P29448; and Arabidopsis Trx-2, BAB09200
Acknowledgments
We acknowledge Dr Victoria Goodwin for constructing the grape cDNA library and Ms Valerie Kinney for assistance in analysis of Arabidopsis EST clones. We thank Dr Brian Larkins and Dr Karen Schumaker for use of laboratory facilities and useful discussions. We gratefully acknowledge support from the National Science Foundation (MCB-9418608) and from Purdue University Agricultural Research Programs. This is paper number 16502 of Purdue University Agricultural Research Programs.
REFERENCES
- Agarwal MK. Receptors for mammalian steroid hormones in microbes and plants. FEBS Lett. 1993;322:206–210. doi: 10.1016/0014-5793(93)81570-p. [DOI] [PubMed] [Google Scholar]
- Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 1998;17:6871–6878. doi: 10.1093/emboj/17.23.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland R. Steroid hormone receptor expression and action in bone. Clin Sci. 2000;98:217–240. [PubMed] [Google Scholar]
- Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Boorstein WR, Ziegelhoffer T, Craig EA. Molecular evolution of the HSP70 multigene family. J Mol Evol. 1994;38:1–17. doi: 10.1007/BF00175490. [DOI] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- Buddhikot M, Falkenstein E, Wehlin M, Meizel S. Recognition of a human sperm surface protein involved in the progesterone-initiated acrosome reaction by antisera against an endomembrane progesterone binding protein from porcine liver. Mol Cell Endocrinol. 1999;158:187–193. doi: 10.1016/s0303-7207(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Cao J, Cai X, Zheng L, Geng L, Shi Z, Pao CC, Zheng S. Characterization of colorectal-cancer-related cDNA clones obtained by subtractive hybridization screening. J Cancer Res Clin Oncol. 1997;123:447–51. doi: 10.1007/BF01372549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletto J 1997 Raphide-associated proteins in grape (Vitis labrusca). MS Thesis, Purdue University, West Lafayette, IN 47907. [Google Scholar]
- Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489. [DOI] [PubMed] [Google Scholar]
- Christ M, Haseroth K, Falkenstein E, Wehling M. Nongenomic steroid actions: fact or fantasy? Vitam Horm. 1999;57:325–373. doi: 10.1016/s0083-6729(08)60647-0. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Trelease RN. The plant 73kDa peroxisomal membrane protein, PMP73, is immunorelated to molecular chaperones. Eur J Cell Biol. 1997;73:49–57. [PubMed] [Google Scholar]
- de Crouy-Chanel A, Kohiyama M, Richarme G. A novel function of Escherichia coli chaperone DnaJ. J Biol Chem. 1995;270:22669–22672. doi: 10.1074/jbc.270.39.22669. [DOI] [PubMed] [Google Scholar]
- Demand J, Lüders J, Höhfeld J. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;229:86–89. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- Ferrari DM, Soeling H-D. The protein disulphide isomerase family: unravelling a string of folds. Biochem J. 1999;339:1–10. [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen DM, Joazeiro CAP, Li J, Hunter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 2000;123:1247–1255. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman J, Höhfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Li J, and Choi YH. et al. . 1997 The Arabidopsis deetiolated 2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 9:1951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer M, Matthias Z, Gehring U. Proteins interacting with the molecular chaperone hsp70/hsc70: physical associations and effects on refolding activity. FEBS Lett. 1997;417:109–113. doi: 10.1016/s0014-5793(97)01267-2. [DOI] [PubMed] [Google Scholar]
- Geuns JMC. Plant steroid hormones—what are they and what do they do? Trends Biochem Sci. 1982;7:7–9. [Google Scholar]
- Groeneveld HW. Tracing steroid synthesis in plants. Crit Rev Biochem Mol Biol. 1999;34:59–69. doi: 10.1080/10409239991209192. [DOI] [PubMed] [Google Scholar]
- He Z, Wang Z-Y, Li J, Ahu Q, Lamb C, Ronald P, Chory J. Perception of brassinosteroids by the extracellular domain of the receptor kinas BRI1. Science. 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- Heftmann E. Recent progress in the biochemistry of plant steroids other than sterols (saponins, glycoalkaloids, pregnane derivatives, cardiac glycosides, and sex hormones) Lipids. 1974;9:626–639. doi: 10.1007/BF02532513. [DOI] [PubMed] [Google Scholar]
- Höhfeld J. Regulation of the heat shock cognate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights. Biol Chem. 1998;379:269–274. [PubMed] [Google Scholar]
- Höhfeld J, Minami Y, Hartl F-U. Hip, a new cochaperone involved in the eukaryotic hsc70/hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- Ikeda E, Yoshida S, Mitsuzawa H, Uno I, Toh-e A. YGE1 is a yeast homologue of Escherichia coli grpE and is required for maintenance of mitochondrial functions. FEBS Lett. 1994;339:265–268. doi: 10.1016/0014-5793(94)80428-1. [DOI] [PubMed] [Google Scholar]
- Irmer H, Höhfeld J. Characterization of functional domains of the eukaryotic co-chaperone Hip. J Biol Chem. 1997;272:2230–2235. doi: 10.1074/jbc.272.4.2230. [DOI] [PubMed] [Google Scholar]
- Kanelakis KC, Murphy PMJ, Galigniana MD, Morishima Y, Takayama S, Reed JC, Toft DO, Pratt WB. Hsp70 interacting protein Hip does not affect glucocorticoid receptor folding by the hsp90-based chaperone machinery except to oppose the effect of BAG-1. Biochemistry. 2000;39:14317–14321. doi: 10.1021/bi001671c. [DOI] [PubMed] [Google Scholar]
- Kimmins S, MacRae TH. Maturation of steroid receptors: an example of functional cooperation among molecular chaperones and their associated proteins. Cell Stress Chaperones. 2000;5:76–86. doi: 10.1379/1466-1268(2000)005<0076:mosrae>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna P, Gloor G. The Arabidopsis thaliana family of Hsp90 proteins. Cell Stress Chaperones. 2001;6:238–246. doi: 10.1379/1466-1268(2001)006<0238:thfopi>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- Langsley GW, Barale J-C, Mattei D. Isolation from a Plasmodium chabaudi chromosome 7 specific library of a novel gene encoding a protein with multiple GGMP repeats homologous to hsp70. Mol Biochem Parasitol. 1993;59:331–334. doi: 10.1016/0166-6851(93)90232-m. [DOI] [PubMed] [Google Scholar]
- Li J, Biswas MG, Chao A, Russell DW, Chory J. Conservation of function between mammalian and plant steroid 5-reductases. Proc Natl Acad Sci U S A. 1997;94:3554–3559. doi: 10.1073/pnas.94.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara P, O'Shaughnessy C, Manduca P, Loughrey HC. Progesterone receptors are expressed in human osteoblast-like cell lines and in primary human osteoblast cultures. Calcif Tissue Int. 1995;57:436–441. doi: 10.1007/BF00301947. [DOI] [PubMed] [Google Scholar]
- Meyer Y, Verdoucq L, Vignols F. Plant thioredoxins and glutaredoxins: identity and putative roles. Trends Plant Sci. 1999;4:388–394. doi: 10.1016/s1360-1385(99)01475-2. [DOI] [PubMed] [Google Scholar]
- Michalak M, Mariani P, Opas M. Calreticulin, a multifunctional Ca++ binding chaperone of the endoplasmic reticulum. Biochem Cell Biol. 1998;76:779–785. doi: 10.1139/bcb-76-5-779. [DOI] [PubMed] [Google Scholar]
- Miernyk JA. Protein folding in the plant cell. Plant Physiol. 1999;121:695–703. doi: 10.1104/pp.121.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshahi M, Mirshahi A, Nato A, Agarwal MK. Receptor mediated mineralocorticoid action in alga cell mutants. FEBS Lett. 1992;314:237–240. doi: 10.1016/0014-5793(92)81479-6. [DOI] [PubMed] [Google Scholar]
- Naylor DJ, Stines AP, Hoogenraad NJ, Hoj PB. Evidence for the existence of distinct mammalian cytosolic, microsomal, and two mitochondrial GrpE-like proteins, the co-chaperones of specific Hsp70 members. J Biol Chem. 1998;273:21169–21177. doi: 10.1074/jbc.273.33.21169. [DOI] [PubMed] [Google Scholar]
- Newman T, Bruijn FJ, and Green P. et al. 1994 Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 106:1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman MY. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones include Ca2+-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- Palleros DR, Reid KL, Shi L, Welch WJ, Fink AL. ATP-induced protein-hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365:664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Nair SC, Rimerman RA, Smith DF. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an hsp70-binding protein. Mol Endocrinol. 1996a;10:420–431. doi: 10.1210/mend.10.4.8721986. [DOI] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Smith DF. Mutation of Hip's carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly. Mol Cell Biol. 1998;18:944–952. doi: 10.1128/mcb.18.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Toran EJ, Rimerman RA, Smith DF. Mutational analysis of the hsp70-interacting protein Hip. Mol Cell Biol. 1996b;16:6200–6207. doi: 10.1128/mcb.16.11.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- Reddy RK, Kurek I, Silverstein AM, Chinkers M, Breiman A, Krishna P. High-molecular-weight FK506-bindiroteins are components of heat-shock protein 90 heterocomplexes in wheat germ lysate. Plant Physiol. 1998;118:1395–1401. doi: 10.1104/pp.118.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicher T, Soll J. Chloroplastic isoforms of DnaJ and GrpE in pea. Plant Mol Biol. 1997;33:181–185. doi: 10.1023/a:1005784115363. [DOI] [PubMed] [Google Scholar]
- Schumacher K, Chory J. Brassinosteroid signal transduction: still casting the actors. Curr Opin Plant Biol. 2000;3:79–84. doi: 10.1016/s1369-5266(99)00038-2. [DOI] [PubMed] [Google Scholar]
- Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- Smith DF. Chaperones in progesterone receptor complexes. Cell Dev Biol. 2000;11:45–52. doi: 10.1006/scdb.1999.0350. [DOI] [PubMed] [Google Scholar]
- Sondermann H, Scheufler C, Schneider C, Höhfeld J, Hartl F-U, Moarefi I. Structure of a Bag/Hsc70 complex: convergent evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–1557. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Zhang-Keck Z-Y, Caohuy H, McPhie P, Pollard HB. Novel isoforms of synexin in Xenopus laevis: multiple tandem PGQM repeats distinguish mRNAs in specific adult tissues and embryonic stages. Biochem J. 1996;316:729–735. doi: 10.1042/bj3160729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancato LF, Hutchison KA, Krishna P, Pratt WB. Animal and plant cell lysates share a conserved chaperone system that assembles the glucocorticoid receptor into a functional heterocomplex with hsp90. Biochemistry. 1996;35:554–561. doi: 10.1021/bi9511649. [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Zhihua X, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- Uparanukraw P, Toyoshima T, Aikawa M, Kumar N. Molecular cloning and localization of an abundant novel protein of Plasmodium berghei. Mol Biochem Parasitol. 1993;59:223–234. doi: 10.1016/0166-6851(93)90220-r. [DOI] [PubMed] [Google Scholar]
- Velten M, Villoutreix BO, Ladjimi MM. Quaternary structure of the hsc70 cochaperone Hip. Biochemistry. 2000;39:307–315. doi: 10.1021/bi9917535. [DOI] [PubMed] [Google Scholar]
- Webb MA. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell. 1999;11:751–761. doi: 10.1105/tpc.11.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MA, Cavaletto JM, Carpita NC, Lopez L, and Arnott HJ 1995 The intravacuolar organic matrix associated with calcium oxalate crystals in leaves of Vitis. Plant J. 7:633–648. and cover. [Google Scholar]
- White JA, Todd J, and Newman T. et al. 2000 A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol. 124:1582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]