Abstract
This study surveys the induction of RNA polymerase III (Pol III)–directed expression of short interspersed element (SINE) transcripts by various stresses in an animal model, silkworm larvae. Sublethal heat shock and exposure to several toxic compounds increase the level of Bm1 RNA, the silkworm SINE transcript, while also transiently increasing expression of a well-characterized stress-induced transcript, Hsp70 messenger RNA (mRNA). In certain cases, the Bm1 RNA response coincides with that of Hsp70 mRNA, but more often Bm1 RNA responds later in recovery. Baculovirus infection and exposure to certain toxic compounds increase Bm1 RNA but not Hsp70 mRNA, showing that SINE induction is not necessarily coupled to transcription of this particular heat shock gene. SINEs behave as an additional class of stress-inducible genes in living animals but are unusual as stress genes because of their high copy number, genomic dispersion, and Pol III–directed transcription.
INTRODUCTION
Short, interspersed, repetitive elements, called SINEs, have amplified in the genomes of most eukaryotes through retrotransposition of their RNA intermediates (Schmid 1996). Often considered junk DNA, approximately 1 million members of the Alu family of SINEs comprise 10% of the human genome (Schmid 1996). Likewise, approximately 20 000 members of the Bm1 SINE family are present in the silkworm genome (Adams et al 1986; Gao and Herrera 1996). Since they are ancestrally derived from genes for either signal recognition particle (SRP) RNA or transfer RNAs (Okada and Ohshima 1995), all SINEs contain internal promoter elements for RNA polymerase III (Pol III). Despite their abundance and corresponding transcriptional potential, some SINE RNAs are expressed at relatively low levels (Liu et al 1994; Li et al 1999).
In cell culture, mammalian SINE RNAs are induced by various stress treatments, including heat shock, virus infection, and translational inhibition, resulting in as much as a 50-fold increase in the accumulation of these RNAs (Singh et al 1985; Carey et al 1986; Fornace and Mitchell 1986; Jang and Latchman 1992; Jang et al 1992; Panning and Smiley 1993; Liu et al 1995; Li et al 1999). These same stresses increase the level of Bm1 RNA in silkworm cells (Kimura et al 1999). In most of these cases, a stress-induced increase in transcription causes much of the increase in SINE RNA. In silk worm cells, this accumulation is the result of an increase in transcription, the stability of transcript, or both, depending on the type of stress (Kimura et al 1999). In mice, the SINE stress response is subject to tissue-specific regulation and occurs during their recovery from either heat shock or acute ethanol toxicity, indicating this is a normal physiological response in living animals rather than a possible loss of transcriptional regulation in dying cells (Li et al 1999). The persistence of SINEs in eukaryotes and the conservation of this stress response suggest that these elements and their transcripts may have a role in cell stress recovery.
Because of their high copy number, genomic dispersion, and requirement for Pol III–directed transcription, SINEs would be an unusual class of stress genes. Interestingly, results in mice indicate that the induction of SINE transcription might be even more sensitive to stress than that of 2 classic heat shock genes, hsp70 and hsp27 (Li et al 1999). This sensitivity raises the possibility that the level of SINE RNA might be a useful indicator of organismal stress. To further examine these possibilities, we surveyed the effects of a range of stress treatments on SINE RNA expression in an intact organism. Silkworm larva was selected as an experimental model because it is an invertebrate that has a well-characterized SINE (Adams et al 1986; Gao and Herrera 1996; Okada et al 1997).
Several different Bm1 transcripts should be distinguished before considering this study (Adams et al 1986; Wilson et al 1988; Gao and Herrera 1996; Kimura et al 1999). Because of their ubiquitous distribution throughout the genome, SINEs are inevitably contained within Pol II–directed transcription units and abundantly represented in pre-mRNAs (Schmid 1996). This study concerns only SINE transcripts that result from Pol III–directed transcription from the internal promoter. Transcriptional initiation by Pol III precisely defines the 5′ end of the resulting transcripts, which can be assayed by primer extension using reverse transcriptase; control experiments confirm this assignment for Bm1 RNA transcripts (Kimura et al 1999). The 3′ ends of these transcripts are less well defined and variable. Pol III can terminate on encountering 4 or more consecutive T-residues, and a Pol III termination signal consisting of 5 T-residues is located at position 90 of the Bm1 consensus sequence immediately after the transfer RNA–related region of this SINE (Okada et al 1997). The corresponding 90-nt transcript (called scBm1 RNA) is observed, but this terminator is both leaky and not faithfully conserved in all members of the family. Therefore, longer Bm1 RNAs are transcribed (Wilson et al 1988; Kimura et al 1999). Another abundant transcript has a length of about 200 nt (Kimura et al 1999). This transcript might result from an unusual termination signal for Pol III or by processing of longer primary transcripts (Matsumoto et al 1989; Kimura et al 1999). Posttranscriptional processing is well established for primate and rodent SINE RNAs (Maraia and Sarrowa 1995). In the case of 1 Bm1 repeat, nonconsensus base substitutions create an internal termination signal, giving rise to a 354-nt transcript in vitro (Wilson et al 1988). Undoubtedly, other members of the Bm1 family contain nonconsensus terminators. In the event that Pol III does not terminate within an element, termination signals inevitably reside within the unique 3′ sequences that flank each member of the family, resulting in primary transcripts that are longer than the 440-nt Bm1 consensus sequence and heterogeneous in length. Several different length Bm1 transcripts having the same 5′ Pol III initiation site are anticipated.
MATERIALS AND METHODS
Silkworm rearing and stress treatments
Silkworm eggs (Sericulum, Sebastopol, CA, USA) were hatched at room temperature. Preliminary controls showed that the level of Bm1 SINE RNA is comparable in larvae (second through the fifth instar stages), pupa, and adult stages (data not shown). Fourth instar larvae weighing approximately 500 mg were selected for all experiments reported herein. For heat shock, a plastic tube containing larvae was submerged in a 42°C water bath for 1 hour and then returned to room temperature for various recovery times. Several heat shock mimetics and other toxins were administered by injection (20 μL) at the intersection of abdominal segments 5 and 6. The concentrations used were as follows: sodium arsenite, 0.1 mM (Nover 1984); mercuric chloride, 1 mM; cycloheximide, 75 μg/μL in 50% ethanol; aqueous cigarette tar (ACT), 8 mg/mL (UV absorbance = 61 at 258 nm; prepared as described by Stone et al 1994); aged catechol solution, 6 mM; and baculovirus (Bombyx mori nuclear polyhidrosis virus [NPV], 10 million active particles per injection). Sublethal concentrations for the different toxic compounds were empirically determined in preliminary experiments to select the doses described herein. Before injections, larvae were submerged in ice water for 15 minutes, making their bodies flaccid to accommodate the injection volume. Control experiments showed that this injection procedure and the injection of either water or ethanol carrier solutions did not affect the SINE RNA levels (data not shown).
RNA extraction and analysis
RNA was extracted from the larvae in 5 mL of Trizol (BRL) using a glass homogenizer according to the manufacturer's instructions. The RNA pellets that resulted from alcohol precipitation were dissolved in diethyl pyrocarbonate-treated water, and concentrations were measured using UV absorbance.
Northern blots were performed as described using probes Bm1 78 and Bm 5s 119 (Kimura et al 1999). Primer extension with reverse transcriptase was performed as described, using primers Bm1 146, Bm1 78, and Bm 5S 119 and Bm Hsp70 mRNA 116 (Kimura et al 1999). A primer (TCTTAATGGCCTTGATAGGTAT) to position 184 in the BmPV polyhedrin mRNA was also used (Maeda et al 1985). All primer extension products correspond to the expected and previously observed lengths. Gel images were recorded and processed with a Fuji Bas 1000 Phosphorimager screen, Molecular Dynamics hardware and software. The reported levels of Bm1 RNA primer extension products are all normalized to that of the 5S RNA primer extension product.
RESULTS
Lengths of Bm1 transcripts by Northern analysis
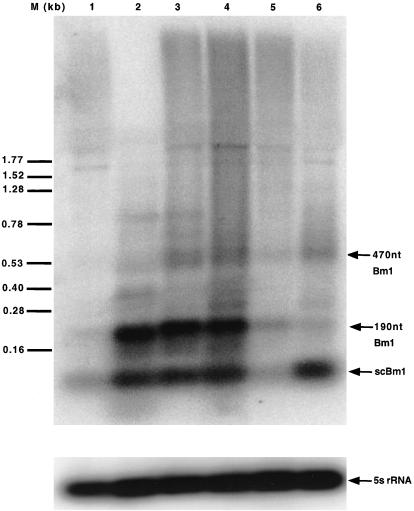
As expected, Northern analysis reveals several bands of Bm1 RNAs superimposed on a smear of heterogeneous length transcripts in silkworm larvae and cultured cells (Fig 1; Introduction). Three principal bands are 90-nt scBm1 RNA, a 190-nt transcript, and a 470-nt transcript, which approximates the consensus length of the Bm1 sequence (Introduction). The level of these transcripts is greater in cultured cells (lane 2) than in total larva RNA (lane 1). The signal for 5S RNA, an abundant, highly stable transcript, provides an RNA loading and processing control for this comparison and those in all subsequent experiments (Figs 1–7). We note herein that in all experiments, the 5S ribosomal RNA signal is essentially constant compared with that of stress-induced transcripts (Figs 1–7).
Fig 1.
Northern analysis of Bm1 RNAs induced by stress. RNA is from untreated BmN cells (lane 2) or from larvae that are untreated (lane 1), treated with cycloheximide (lane 3), infected with baculovirus for 5 days (lane 4), treated with sodium arsenite (lane 5), or recovering from heat shock for 1.5 hours (lane 6). This blot was hybridized with primer Bm1 78, washed, and rehybridized with primer 5S 119. Positions are indicated for scBm1, 190-nt Bm1, 470-nt Bm1 RNA, 5S ribosomal RNA, and length markers “M.”
Before performing primer extension assays, we were concerned that various stresses might cause different changes in the relative abundance of these Bm1 transcripts. This possibility is tested in a preliminary comparison using Northern analysis (Fig 1). Cycloheximide treatment (Fig 1; lane 3) and viral infection (lane 4) of larva increase the levels of scBm1 and 190-nt Bm1 RNAs to approximately the levels observed in cultured cells (lane 2). Heat shock causes a disproportionate increase in scBm1 RNA relative to the longer transcripts (lane 6). Arsenite has relatively little effect on any of these transcripts (lane 5). These results complement results from primer extension analysis (see below), which do not as readily distinguish between different length transcripts that share a common 5′ end.
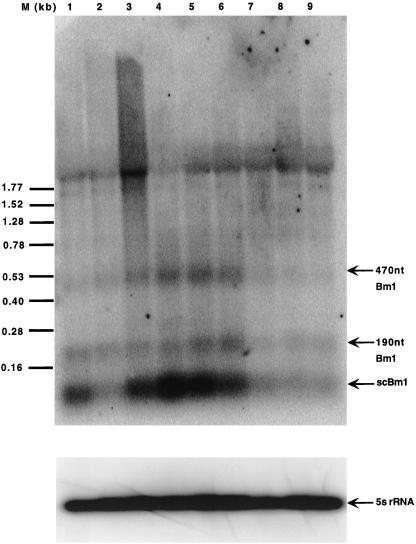
Shorter Bm1 transcripts may result from processing of longer precursors (Introduction), and depending on the type of stress, each RNA species might respond with different kinetics (Fig 2). Heat shock causes a very rapid transient increase in scBm1 RNA, with a maximum occurring at 1.5 hours of recovery (lane 4). The 2 longer transcripts reach their recovery maximums at either the same or a slightly later time. Interestingly, at 0.5 hour of recovery, there is an abrupt increase in hybridization to a heterogeneous smear of high-molecular-weight material, which then quickly fades as the shorter RNA bands start to appear (lane 3). This suggests a possible precursor-product relationship between primary Bm1 transcripts and the products of their processing (Introduction).
Fig 2.
Northern analysis of Bm1 RNAs nduced by heat shock. RNA was extracted from control larvae (lane 1) and larvae that had been heat shocked and allowed to recover at room temperature for 0 hour (lane 2), 0.5 hour (lane 3), 1.5 hours (lane 4), 4.5 hours (lane 5), 8 hours (lane 6), 12 hours (lane 7), 18 hours (lane 8), and 24 hours (lane 9). The blot was hybridized with oligonucleotide probes, and the band positions are labeled as described in Figure 1
Primer extension analysis of heat shock induction
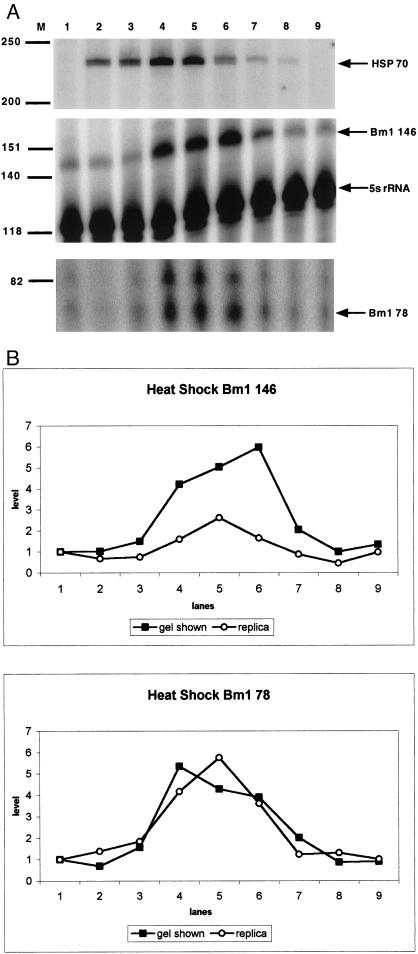
The levels of Bm1 and Hsp70 mRNAs were assayed by primer extension following heat shock of larvae (Fig 3 a,b). Larvae survived the heat shock without any apparent effects as did those in a replica experiment. Following heat shock, Hsp70 mRNA shows a transient increase, with a maximum occurring at 1.5 to 4.5 hours of recovery (Fig 3a, lanes 4 and 5). The level of Bm1 RNA was assayed using primers Bm1 78 and Bm1 146, which map to 2 positions within the consensus sequence (Fig 3 a,b). The maximum level (a 3- to 6-fold increase) of the 146-nt Bm1 RNA primer extension product occurs either at or slightly after the maximum for Hsp70 mRNA (Fig 3 a,b). The length of this product identifies the corresponding Bm1 transcripts as resulting from Pol III transcription (Kimura et al 1999). Primer Bm1 78 gives a doublet (Fig 3a). One of these 2 bands corresponds to consensus length of Pol III transcripts; the slightly longer product possibly results from a small (approximately 4-nt) nonconsensus sequence insertion that occurs in some members of the family (data not shown). The kinetics of the transient increase (6-fold) observed for the Bm1 78 doublet during heat shock recovery is indistinguishable from that of the 146-nt product, and both roughly coincide with that of Hsp70 mRNA (Fig 3 a,b). However, the kinetics of the Bm1 RNA heat shock response is qualitatively different in larvae and cultured cells. In cultured cells, the elevated level of Bm1 RNA (a 2.5-fold increase for a 43°C heat shock) is not transient as observed herein for larvae but persists after recovery (Discussion; Kimura et al 1999).
Fig 3.
Heat shock response of Bm1 RNA and Hsp70 mRNA. (a) Primer extension analysis was performed on RNA extracted from larvae using radiolabeled primers Bm Hsp70 116, Bm1 146, Bm 5S 119, and Bm1 78. RNAs are from an untreated control (lane 1) and heat shocked larvae following recovery for 0 hour (lane 2), 0.5 hour (lane 3), 1.5 hours (lane 4), 4.5 hours (lane 5), 8 hours (lane 6), 12 hours (lane 7), 18 hours (lane 8), and 24 hours (lane 9). The positions of the primer extension products and marker bands are indicated. (b) Phosphorimage analysis was performed on the 78-nt and 146-nt Bm1 RNA primer extension products from a replica experiment and those shown in panel a
Induction of SINE transcription by toxic compounds
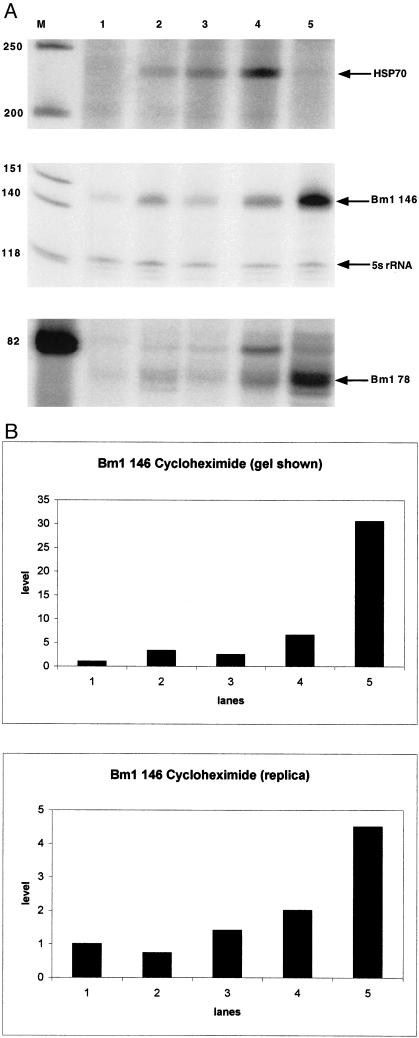
Cycloheximide induces a transient increase in Hsp70 mRNA, with a maximum occurring at 24 hours after injection (Fig 4a). There is also a marked increase in the extension products for Bm1 RNA when assayed using either primer Bm1 78 or Bm1 146 (Fig 4 a,b). However, the level of Bm1 RNA continues to accumulate after completion of the transient increase in Hsp70 mRNA (Fig 4a) is completed. In both this and the replica experiment, the animals refused food and became less mobile following cycloheximide treatment. The induction of these transcripts may be a later consequence of this drug's toxicity rather than an immediate response to an inhibition of protein synthesis. A similar persistent accumulation of Bm1 SINE RNA had been observed after exposing cultured cells to cycloheximide (Kimura et al 1999). The intensities of these responses are also similar: a 4- to 15-fold increase in Bm1 RNA after exposing the cultured cells to cycloheximide for 27 to 52 hours compared with 4.5- to 30-fold increases for larva following 48 hours of exposure (Fig 4b, Kimura et al 1999).
Fig 4.
Effect of cycloheximide on Bm1 RNA and Hsp70 mRNA. (a) Primer extension analysis was performed and the positions of the resulting products and marker bands are indicated as described in Figure 3. RNA was extracted from larvae that were untreated (lane 1) or exposed to cycloheximide for 4 hours (lane 2), 12 hours (lane 3), 24 hours (lane 4), and 48 hours (lane 5). (b) Phosphorimage analysis was performed on the 146-nt Bm1 RNA primer extension products from a replica experiment and those shown in panel a
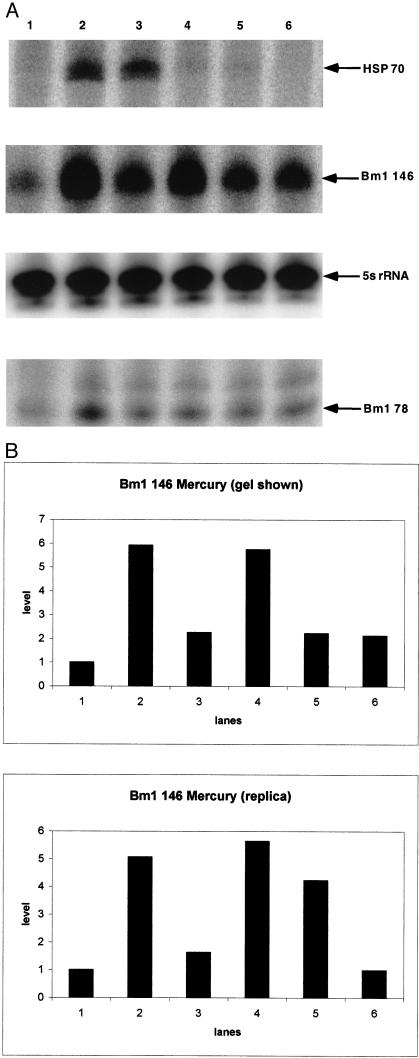
Mercuric chloride, a heat shock mimic, causes more rapid increases in both Hsp70 mRNA and Bm1 RNAs (Fig 5 a,b). There is a peculiar, apparently biphasic, response in the level of Bm1 RNA. Possibly, an initial response is similar to that caused by heat shock, and a subsequent more persistent response results from secondary effects of long-term mercury poisoning. In these experiments, the larvae appeared only slightly affected, eg, losing their appetite after a day of the trial. In virtually identical experiments, arsenite also caused marked increases in Hsp70 mRNA but no detectable increase in Bm1 RNA (data not shown, see Fig 1, lane 5). Although this suggests that the SINE RNA stress response may be separate from that of Hsp70 mRNA, we do not regard those experiments as being conclusive because we were unable to adjust the dose of arsenite to a subacute toxic level while maintaining an Hsp70 mRNA response.
Fig 5.
Effect of mercury on Bm1 RNA and Hsp70 mRNA. Primer extension analysis was performed and the positions of the resulting products and marker bands are indicated as described in Figure 3. RNA was extracted from larvae that were untreated (lane 1) or exposed to mercury for 1 hour (lane 2), 3 hours (lane 3), 9 hours (lane 4), 24 hours (lane 5), and 48 hours (lane 6). (b) Phosphorimage analysis was performed on the 146-nt Bm1 RNA primer extension products from a replica experiment and those shown in panel a
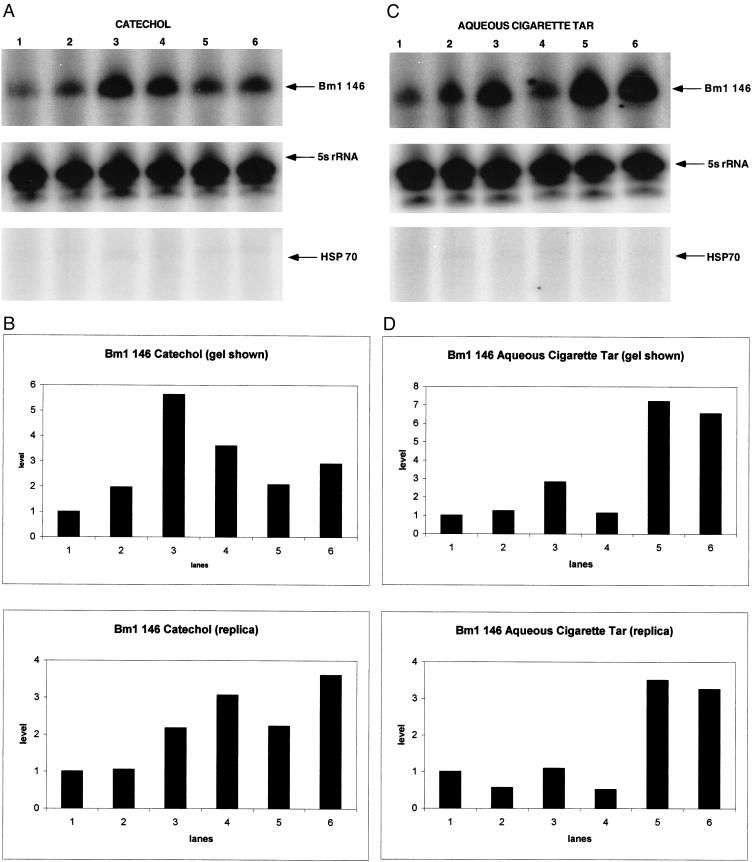
Tobacco smoke and its constituents induce Hsp70 in human cells (Bagchi et al 1995; Vayssier et al 1998; Pinot et al 1999). Oxidized catechol, a genotoxic stress agent and a major component of tobacco smoke, induces free radical–mediated DNA damage (Stone et al 1995; Pryor et al 1998). Catechol rapidly induces Bm1 RNA (compare lanes 1 and 3, Fig 6 a,b), but has no apparent effect on Hsp70 mRNA (Fig 6a). In these experiments, administration of this compound had no apparent effect on behavior. Conceivably, some other factor in tobacco smoke is responsible for inducing Hsp70 mRNA as assayed in human cells. Alternatively, silkworm may respond differently to this stress. To distinguish between these alternatives, the effect of ACT was tested on larvae (Fig 6 c,d). The larvae were visibly poisoned by ACT, which contains nicotine among other compounds (Pryor et al 1993, 1998; Stone et al 1995). Compared with catechol, cigarette tar causes a persistent increase in the level of Bm1 RNA (Fig 6 c,d). The persistence of this response might result from the acute poisoning of the animals. More importantly for the purpose of this study, cigarette tar, like catechol, has no detectable effect on Hsp70 mRNA (Fig 6 c,d), showing that Bm1 RNA can respond to a different stress-induced pathway.
Fig 6.
Effect of tobacco compounds on Bm1 RNA and Hsp70 mRNA. Primer extension analysis was performed and the positions of the resulting products and marker bands are indicated as described in Figure 3a. RNA was extracted from larvae that were untreated or exposed to either catechol (panel a) or ACT (panel c) for 1 hour (lane 2), 3 hours (lane 3), 9 hours (lane 4), 24 hours (lane 5), and 48 hours (lane 6). Phosphorimage analysis was performed on the 146-nt Bm1 RNA primer extension products from replica experiments and those shown in panels a and c to test the effects of catechol (panel b) and ACT (panel d)
Induction of SINE RNA by viral infection
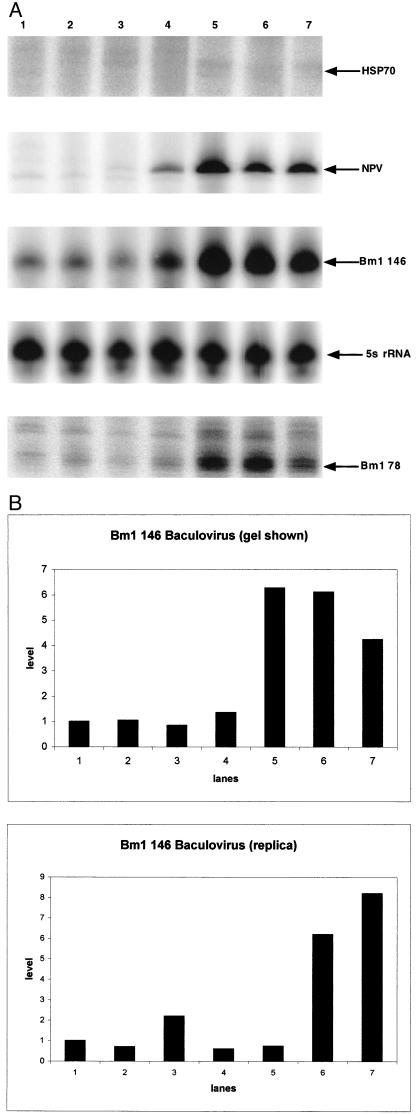
Infection of larvae with baculovirus increases the level of Bm1 RNA as assayed using the primers Bm1 146 or Bm1 78 (Fig 7 a,b). The highest level of Bm1 RNA (approximately 6- to 8-fold increase at days 4 and 5 after infection) temporally coincides with the highest level of expression of NPV polyhedrin mRNA (Fig 7 a,b). These animals are visibly ill on the fifth day of infection and died on the sixth day. Perhaps as a consequence of this morbidity, the Bm1 SINE RNA response is both more persistent and more robust in cultured cells, resulting in a 25-fold increase in its abundance following 5 days of infection (Kimura et al 1999). Viral infection has no effect on the level of Hsp70 mRNA (Fig 7a).
Fig 7.
Effect of baculovirus on Bm1 RNA and Hsp70 mRNA. (a) Except for the addition of an additional primer for NPV mRNA, primer extension analysis was performed and the positions of the resulting products and marker bands are indicated as described in Figure 3. RNA was extracted from larvae that were untreated (lane 1) or infected with baculovirus for 1, 2, 3, 4, 5, and 6 days (lanes 2–7, respectively). (b) Phosphorimage analysis was performed on the 146-nt Bm1 RNA primer extension products from a replica experiment and those shown in panel a
DISCUSSION
Heat shock of silkworm larvae and cultured cells
Here and in a previous study of silkworm cells (Kimura et al 1999), heat shock causes an increase in Bm1 transcripts while also inducing a transient increase in expression of Hsp70 mRNA. The steady-state abundance of a transcript is determined by both its transcription and stability. The accumulation of Bm1 RNA following heat shock (as well as other stresses, see below) results in part from its stabilization (Kimura et al 1999). Transcription and RNA stability could each be regulated very differently in isolated cells that represent a particular tissue compared with the mixed tissues from an intact animal. There is one significant difference between these sets of data. In contrast to the transient increase that is observed for larvae, the increased level of Bm1 RNA in cultured cells persists and does not decrease to its preheat shock basal level following recovery (Fig 3). This raises the possibility that BmN cells do not fully recover from heat shock and that the increase in Bm1 RNA observed in these cells might reflect a loss of regulated expression rather than an authentic heat shock response. Similarly, the basal level of expression of Bm1 RNA is higher in BmN cells than in larvae. Presumably, these differences are either an artifact of cell culture or a property of the particular tissue (ovary) from which these cells were derived. In either case, these differences in SINE RNA expression underscore the importance of using an animal model to describe the normal physiological response to stress.
In larvae, Bm1 RNA and Hsp70 mRNA respond to heat shock with similar but not identical kinetics. The initial induction and maximum expression of Bm1 transcripts occur slightly after those of Hsp70 mRNA, at least under the conditions of the particular heat shock protocol used herein. In addition, both RNAs exhibit transient responses returning to pre–heat shock basal levels after recovery is complete. By these criteria, Bm1 SINEs behave like classic heat shock genes, and Bm1 SINEs are possibly programmed for expression later in recovery than Hsp70 mRNA.
These findings complement similar results for the heat shock induction of mouse SINEs (Li et al 1999). In each case, the response occurs in living animals, a mammal and an insect, that recover from the initial heat shock. Mercury poisoning also induces the Bm1 SINE RNA stress response, showing that at least some other heat shock mimetics cause similar effects.
Bm1 SINE response to other stresses
In human, mouse, and rabbit cells, cycloheximide induces SINE RNA expression but does not induce Hsp70 mRNA (Liu et al 1995) so that the stress response for these 2 transcripts is not coupled in mammals. In silkworm larvae and cell culture, cycloheximide induces Bm1 SINE RNA but, unlike the case for mammals, also induces Hsp70 mRNA (Kimura et al 1999). This difference raises the question of whether SINE RNA can be induced independently of Hsp70 mRNA in silkworm.
Tobacco smoke extract and one of its principal constituents, catechol, rapidly induce Bm1 RNA expression without having any effect on Hsp70 mRNA. Bm1 RNA responds to stimuli and stress pathways that do not significantly induce Hsp70 transcription. Viral infection also induces Bm1 RNA transcription without causing a concomitant increase in Hsp70 mRNA, again showing that the 2 pathways are decoupled.
In addition to baculovirus, the SINE response to viral infection has been documented for several mammalian viruses: adenovirus, human immunodeficiency virus, herpes simplex virus, and simian vacuolating virus No. 40 (Singh et al 1985; Carey et al 1986; Jang and Latchman 1992; Jang et al 1992; Panning and Smiley 1993). Viral infection might activate many stress pathways. However, in one aspect, adenovirus infection resembles cycloheximide in causing stress by overloading the endoplasmic reticulum with incompletely synthesized proteins (Kaufman 1999). Unlike the heat shock response, endoplasmic reticulum stress does not necessarily cause an increase in Hsp70 mRNA (Brostrom and Brostrom 1998; Kaufman 1999). Bm1 SINEs evidently respond to a similar class of stress.
Unusual features of SINEs as stress genes
The expression of SINE RNA is increased by a variety of stresses in both living mice and silkworm, and the resulting induction of SINE RNA resembles that of classic Hsp mRNAs, identifying SINEs as a class of stress genes. Although some stresses may increase the stability of Bm1 RNA (Kimura et al 1999), the primary mechanism by which stress increases the expression of mammalian SINEs is by increasing the rate of their transcription (Singh et al 1985; Carey et al 1986; Fornace and Mitchell 1986; Panning and Smiley 1993; Russanova et al 1995; Li et al 1999). Sequence analysis shows that the SINE stress response does not result from just a few extremely active loci; instead, many members of these repetitive sequence families are transcribed (Liu et al 1995; Kimura et al 1999).
As stress genes, SINEs are unusual because of their high copy number, genomic dispersion, and transcription by Pol III rather than Pol II. These 3 features might be essential for the basal regulation and stress-induced transcription of SINEs. SINE transcriptional regulation has been most thoroughly studied for human Alu repeats, which provide a simple model. Alu repeats are subject to multiple levels of transcriptional control, including especially repression by chromatin (Russanova et al 1995; Schmid 1998; Li et al 2000). Other Pol III–directed genes contain dedicated cis-acting elements, which stimulate their transcription, but Alus generally lack such sequences because of their random genomic dispersion (Schmid 1996, 1998). Consequently, Alu transcription relies almost exclusively on the strength of the weak internal Pol III promoter; therefore, Alus are effectively silenced by their chromatin condensation (Russanova et al 1995; Schmid 1998, Li et al 2000). Stresses that activate Alu transcription also open their chromatin as assayed by either their in vitro transcription template activity or accessibility to restriction enzyme digestion (Russanova et al 1995; Li et al 2000). Because of their very high copy number and genomic dispersion, the internal promoter elements of some Alu members will invariably become accessible and transcriptionally derepressed as a result of these stress-induced changes in chromatin (Russanova et al 1995; Li et al 2000).
Since there are several different length Bm1 transcripts, the stress response of silkworm SINE RNA is likely to be more complicated than that of human Alus (Introduction; Kimura et al 1999). Although at least one of these transcripts probably results from alternative termination, others might be posttranscriptionally processed. Furthermore, the relative abundance and temporal appearance of each species apparently depend on the type of stress. Since the significance of these different transcripts is entirely unknown, an investigation of these possibilities currently would be premature.
Even more significantly, the function of SINE RNA in the stress response is not known, although in certain instances Alu RNA can stimulate expression of a reporter protein (Chu et al 1998). Since regulated changes in protein synthesis are involved in many aspects of the stress response, one very attractive possibility is that SINE RNA serves a role in translational regulation. Although its function remains to be determined, the SINE stress response extends from mammals to insects.
Acknowledgments
We are grateful to Thomas Angel, Alan Wheelright, and Ferris Lang for preparing the ACT solution, Jennifer Roberts for rearing the silkworm and preparing RNA, and Dr Shizuo G. Kamita for his guidance on silkworm injection methods. We would also like to thank Roger Bernhardt (Sericulum) for providing us with high-quality silkworm eggs and his expertise on rearing methods. This research was supported by US Public Health Service grant GM21346 (C.S.), a research assistantship from the University of California Tobacco Related Disease Research Program grant DT-0174 (R.K.), and the Agricultural Experiment Station of the University of California (C.S.).
REFERENCES
- Adams DS, Eickbush TH, Herrera RJ, Lizardi PM. A highly reiterated family of transcribed oligo(A)-terminated, interspersed DNA elements in the genome of. Bombyx mori. J Mol Biol. 1986;187:465–478. doi: 10.1016/0022-2836(86)90327-x. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Bagchi D, Adickes E, Stohs SJ. Chronic effects of smokeless tobacco extract on rat liver histopathology and production of Hsp 90. J Environ Pathol. 1995;14:61–68. [PubMed] [Google Scholar]
- Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- Carey MF, Singh K, Botchan M, Cozzarelli NR. Induction of specific transcription by RNA polymerase III in transformed cells. Mol Cell Biol. 1986;6:3068–3076. doi: 10.1128/mcb.6.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WM, Ballard R, Carpick BW, Williams BRG, Schmid CW. Potential Alu function: regulation of the activity of double-stranded RNA-activated kinase PKR. Mol Cell Biol. 1998;18:58–68. doi: 10.1128/mcb.18.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace AJ, Mitchell JB. Induction of B2 RNA polymerase III transcription by heat shock: enrichment for heat shock induced sequences in rodent cells by hybridization subtraction. Nucleic Acid Res. 1986;14:5793–5811. doi: 10.1093/nar/14.14.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Herrera RJ. Enrichment of middle repetitive element Bm-1 transcripts in translationally active RNA fractions of the silkmoth, Bombyx mori. Genetica. 1996;97:173–182. doi: 10.1007/BF00054624. [DOI] [PubMed] [Google Scholar]
- Jang KL, Collins MK, Latchman DS. The human immunodeficiency virus tat protein increases the transcription of human Alu repeated sequences by increasing the activity of the cellular transcription factor TFIIIC. J Acquired Immune Defic Syndr. 1992;5:1142–1147. [PubMed] [Google Scholar]
- Jang KL, Latchman DS. The herpes simplex virus immediate-early protein ICP27 stimulates the transcription of cellular Alu repeated sequences by increasing the activity of transcription factor TFIIIC. Biochem J. 1992;284:667–673. doi: 10.1042/bj2840667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kimura RH, Choudary PV, Schmid CW. Silkworm Bm1 SINE RNA increases following cellular insults. Nucleic Acid Res. 1999;27:3381–3387. doi: 10.1093/nar/27.16.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TH, Spearow J, Rubin CM, Schmid CW. Physiological stresses increase mouse short interspersed element (SINE) RNA expression in vivo. Gene. 1999;239:367–372. doi: 10.1016/s0378-1119(99)00384-4. [DOI] [PubMed] [Google Scholar]
- Li TH, Kim C, Rubin CM, Schmid CW. K562 cells implicate increased chromatin accessibility in Alu transcriptional activiation. Nucleic Acid Res. 2000;28:3031–3039. doi: 10.1093/nar/28.16.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WM, Chu WM, Choudary PV, Schmid CW. Cell stress and translational inhibitors transiently increase the abundance of mammalian SINE transcripts. Nucleic Acid Res. 1995;23:1758–1765. doi: 10.1093/nar/23.10.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WM, Maraia RJ, Rubin CM, Schmid CW. Alu transcripts: cytoplasmic localization and regulation by DNA methylation. Nucleic Acid Res. 1994;22:1087–1095. doi: 10.1093/nar/22.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kawai T, Obinata M, Fujiwara H, Horiuchi T, Saeki Y, Sato Y, Furusawa M. Production of human alpha-interferon in silkworm using a baculovirus vector. Nature. 1985;315:592–594. doi: 10.1038/315592a0. [DOI] [PubMed] [Google Scholar]
- Maraia RJ, Sarrowa J 1995 Alu-family SINE RNA: interacting proteins and pathways of expression. In: The Impact of Short Interspersed Elements (SINEs) on the Host Genome, ed Maraia R. Springer, New York, 163–196. [Google Scholar]
- Matsumoto K, Takii T, Okada N. Characterization of a new termination signal for RNA polymerase III responsible for generation of a discrete-sized RNA transcribed from salmon total genomic DNA in a HeLa cell extract. J Biol Chem. 1989;264:1124–1131. [PubMed] [Google Scholar]
- Nover L 1984 Heat Shock Response of Eukaryotic Cells. Springer-Verlag, Berlin. [Google Scholar]
- Okada N, Hamada M, Ogiwara I, Ohshima K. SINEs and LINEs share common 3′ sequences: a review. Gene. 1997;205:229–243. doi: 10.1016/s0378-1119(97)00409-5. [DOI] [PubMed] [Google Scholar]
- Okada N, Ohshima K 1995 Evolution of tRNA-derived SINEs. In: The Impact of Short Interspersed Elements (SINEs) on the Host Genome, ed Maraia R. Springer, New York, 61–80. [Google Scholar]
- Panning B, Smiley JR. Activation of RNA polymerase III transcription of human Alu repetitive elements by adenovirus type 5: requirement for the E1b 58-kilodalton protein and the products of E4 open reading frames 3 and 6. Mol Cell Biol. 1993;13:3231–3244. doi: 10.1128/mcb.13.6.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinot F, Bachelet M, Francois D, Polla BS, Walti H. Modified natural porcine surfactant modulates tobacco smoke induced stress response in human monocytes. Life Sci. 1999;64:125–134. doi: 10.1016/s0024-3205(98)00542-6. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stome K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11:441–448. doi: 10.1021/tx970159y. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stone K. Oxidants in cigarette smoke. Tobacco Smoking Nutr. 1993;686:12–28. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- Russanova VR, Driscoll CT, Howard BH. Adenovirus type 2 preferentially stimulates polymerase III transcription of Alu elements by relieving repression: a potential role for chromatin. Mol Cell Biol. 1995;270:4282–4290. doi: 10.1128/mcb.15.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CW. Alu: structure, origin, evolution, significance and function of one-tenth of human DNA. Prog Nucleic Acid Res Mol Biol. 1996;53:283–319. doi: 10.1016/s0079-6603(08)60148-8. [DOI] [PubMed] [Google Scholar]
- Schmid CW. Does SINE evolution preclude Alu function? Nucleic Acid Res. 1998;20:4541–4550. doi: 10.1093/nar/26.20.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Carey M, Saragosti S, Botchan M. Expression of enhanced levels of small RNA polymerase III transcripts encoded by the B2 repeats in simian virus 40-transformed mouse cells. Nature. 1985;314:553–556. doi: 10.1038/314553a0. [DOI] [PubMed] [Google Scholar]
- Stone KK, Bermudez E, Pryor WA. Aqueous extracts of cigarette tar containing the tar free radical cause DNA nicks in mammalian cells. Environ Health Perspect. 1994;102:173–178. doi: 10.1289/ehp.94102s10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KK, Bermudez E, Zang LY, Carter KM, Queenan KE, Pryor WA. The ESR properties, DNA nicking, and DNA association of aged solutions of catechol versus aqueous extracts of tar from cigarette smoke. Arch Biochem Biophys. 1995;319:196–203. doi: 10.1006/abbi.1995.1282. [DOI] [PubMed] [Google Scholar]
- Vayssier M, Banzet N, Francois D, Bellman K, Polla BS. Tobacco smoke induces both apoptosis and necrosis mammalian cells: differential aspects of Hsp. Am J Physiol. 1998;275:L771–L779. doi: 10.1152/ajplung.1998.275.4.L771. [DOI] [PubMed] [Google Scholar]
- Wilson ET, Condliffe DP, Sprague KU. Transcriptional properties of BmX, a moderately repetitive silkworm gene that is an RNA polymerase III template. Mol Cell Biol. 1988;8:624–631. doi: 10.1128/mcb.8.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]