Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver; it ranks fifth among the most diagnosed cancers and represents the third most common cause of cancer-related deaths worldwide.[1]
The prevalence of HCC differs greatly by geographical locations reflecting variations in the main risk factors. Most cases of hepatocellular carcinoma (80%) arise in Eastern countries, such as those of the Asia-Pacific region, and sub-Saharan African regions where the dominant risk factor is chronic infection with hepatitis B virus (HBV), together with exposure to Aflatoxin B1.[2] By contrast, in Western countries the incidence has been rising rapidly in recent decades due to infection with hepatitis C virus (HCV) and alcohol use.[3] Other factors associated with HCC are diabetes and non-alcoholic fatty liver disease (NAFLD) and the more severe non-alcoholic steatohepatitis (NASH). NASH is characterized by fatty liver inflammation and is believed to cause fibrosis and cirrhosis, a known liver cancer risk factor.[4] Some population-based studies conducted in Western countries and Taiwan have shown up to two times increase in risk of HCC in obese individuals compared with non-obese individuals who lack history of diabetes.[5],[6] Furthermore, case-control studies and a few cohort studies suggest that patients with type II diabetes have an increased risk of developing HCC, as high as twice that of individuals with no history of diabetes.[7],[8] Hematochromatosis, alpha1-antitrypsin deficiency, autoimmune hepatitis, porphyrias and Wilson’s disease have also been associated with an increased incidence of HCC.
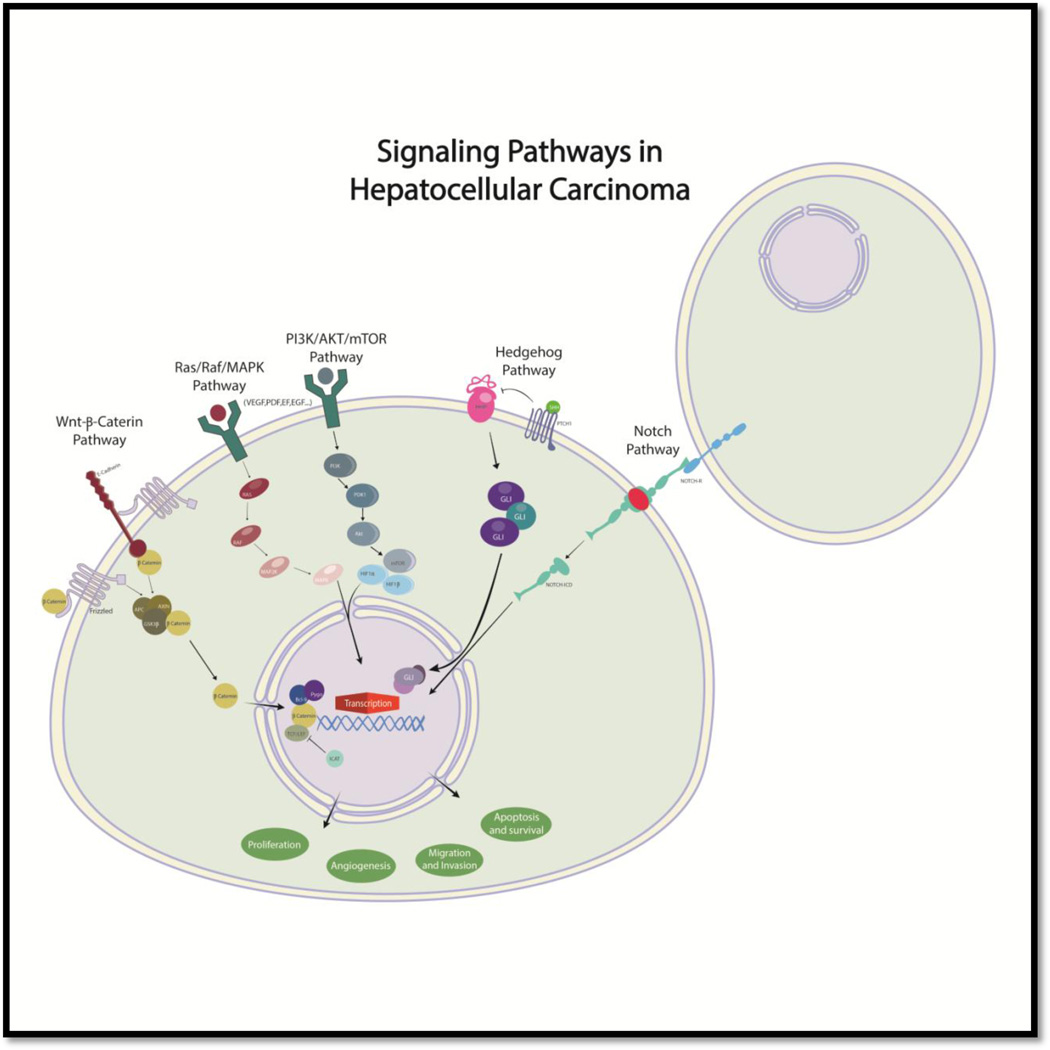
Numerous signaling pathways such as Ras/Raf/MAPK, WNT-β-catenin, EGFR, insulin-like growth factor receptor, AKT-mTOR, Notch, and Hedgehog have been implicated in hepatic carcinogenesis. Because of that, some of their components could represent important molecular targets for therapy in HCC. The Ras/Raf/MAPK pathway is typically activated in HCC as a result of increased signaling induced from upstream growth factors and due to inactivation of tumor suppressor genes.
In recent years, a clinical trial published in the New England Journal of Medicine suggests that sorafenib, a multikinase inhibitor which significantly inhibits the Ras/Raf/MAPK pathway, can slow down tumor progression and improve survival in patients with advanced HCC.[9]
The aim of this study is to review the literature on molecular directed therapies in HCC with special emphasis on the role of targeting the Ras/Raf/MAPK signaling pathway. Due to the number of ongoing studies and reagents being tested, it is important to stay abreast of the wide spectrum of possible treatment options.
Because of the heterogeneity of these cancers and the complex process involved in HCC carcinogenesis, some previously published data from preclinical studies suggest that combination therapy could be essential in HCC treatment. There are at least 35 combination therapy studies for advanced stage HCC ongoing in phases 1–3, and numerous reagents are being tested targeting novel signaling cascades (WNT-β-catenin and Notch).
Recently, there is an increasing interest in the cancer stem cell (CSC) theory. According to this hypothesis, cancer initiation, progression, recurrence, metastasis, and therapy resistance are unique properties implicit on CSC subsets. Focusing on targeting CSCs should bring important and revolutionary advances in cancer therapeutics. Our group and others have focused on ways of inducing inhibition of liver CSCs and differences in resistance patterns with non-liver CSC lines in vitro and in vivo.
Treatment strategies in HCC
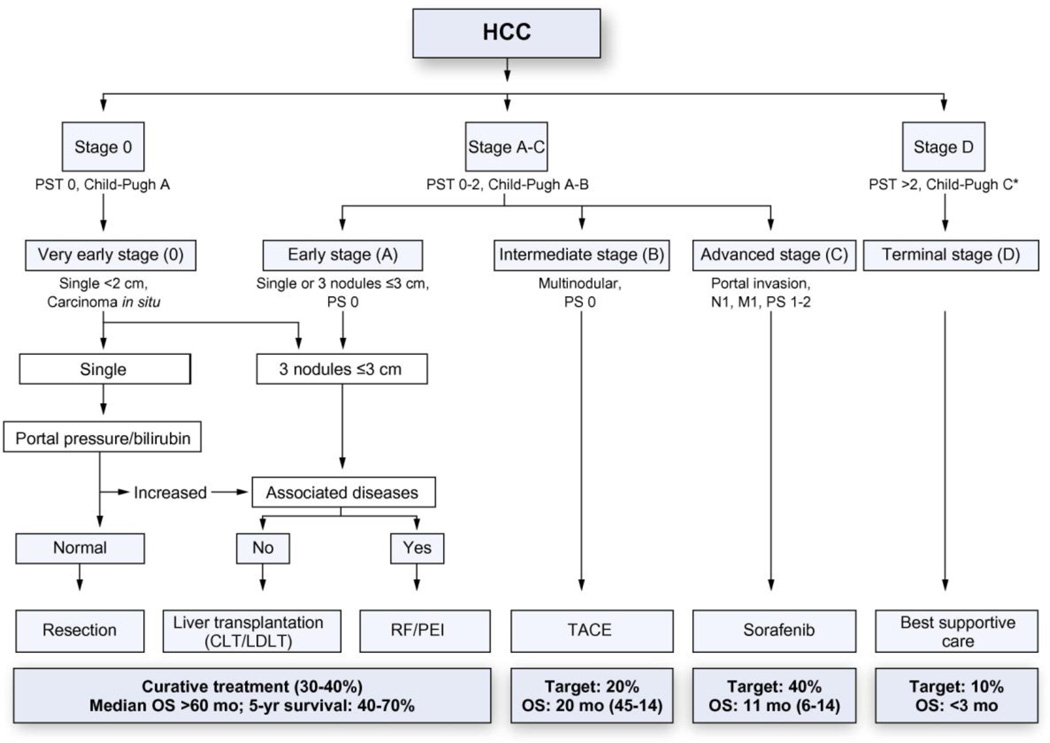
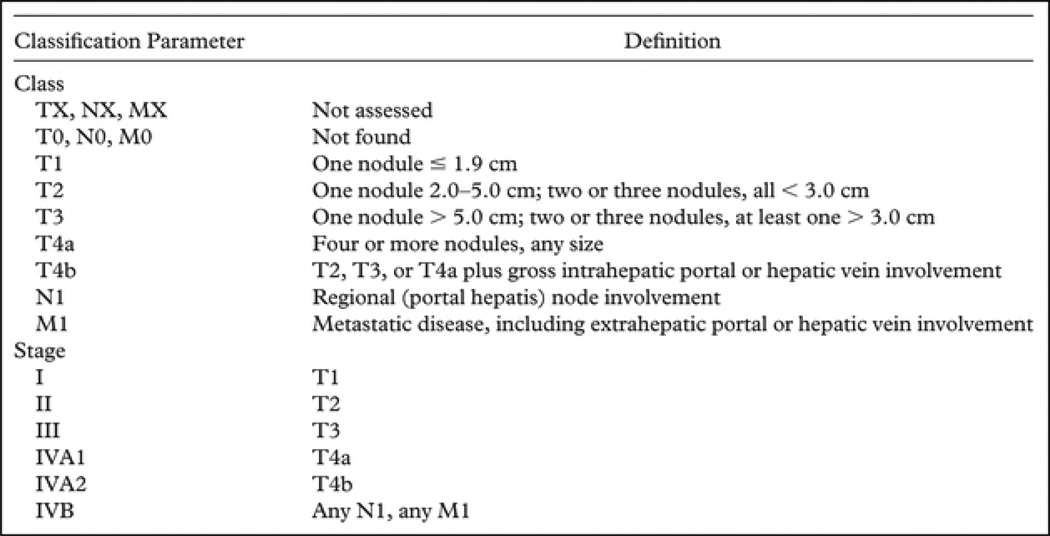
HCC treatment selection is guided by the stage of the disease and the expertise of the medical team.[10] The Barcelona clinic liver cancer (BCLC) staging system (Fig. 1) classifies HCC into 5 stages based on tumor burden (number and size of the nodules), health status, and liver function as defined by the Child-Pugh classification system.[11] This system not only addresses prognosis but also confers therapeutic strategies to each stage. The BCLC has been endorsed by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver[12] to guide decision making in the clinical setting. The American Liver Tumor Study Group modified the TNM classification and created a staging system that is currently used in the US and supported by the United Network of Organ Sharing (UNOS) (Fig. 2).[13]
Figure 1.
Updated BCLC staging system and treatment strategy, 2011. [20] (with permission from EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma Journal of Hepatology)
Figure 2.
American Liver Tumor Study Group Modified TNM Classification and Staging System[13].
According to the BCLC algorithm, patients with early stage disease (BCLC0-A) are candidates for radical and curative therapies such as surgical resection, liver transplantation, and percutaneous ablation. Patients with single nodules (solitary lesion less than 2 cm in diameter and no vascular or distant metastases) without clinically significant portal hypertension undergo resection. Patients with a single nodule less than 5 cm or 3 nodules less than 3 cm (Milan Criteria) and portal hypertension or liver dysfunction are candidates for orthotopic liver transplantation. Patients with small tumor burden who are not candidates for resection or transplantation are usually treated with ablative procedures.
Patients with intermediate-stage disease (BCLC-B) are considered for transarterial chemoembolization (TACE),[14] which provides locoregional control of HCC and increases median survival times by 20–25 months.[15] TACE has been associated with systemic toxicity manifested with fever and abdominal pain due to hepatic ischemia, but recent progress in embolization devices, such as drug eluting beads have reduced the systemic side effects and have achieved an increase in local tumor response. Transarterial radioembolization with microspheres of yttrium-90 is under evaluation and based on cohort studies is a promising alternative to treat HCC.[16] A phase II study combining TACE and sorafenib therapy is currently ongoing.
Patients with advanced-stage HCC (BCLC-C) are considered for sorafenib, an oral multikinase inhibitor of BRAF, the VEGF receptor (VEGFR), and PDGF receptor (PDGFR). Two large randomized studies support sorafenib as the standard of care for patients with advanced HCC.[17],[18] Patients in terminal-stage HCC (BCLC-D) should receive palliative care only. These individuals have a median survival time of around 3 months.
Tumor recurrence is a major concern after resection, occurring in up to 70% of patients at 5 years after surgery.[19] Unfortunately, no agent has shown robust efficacy in preventing or delaying tumor recurrence. The tolerance of adjuvant therapy is sometimes a limiting factor in patients with cirrhosis and liver dysfunction. With no second-line treatment available for these patients with intolerance or failure to sorafenib, best supportive care or the inclusion in clinical trials is recommended.[20]
Signaling pathways in HCC proliferation and activation
Cytotoxic chemotherapy has not been found to be effective in the treatment of most patients with advanced HCC. In the last few years, the development of sorafenib has significantly stimulated the understanding of the different cell signaling pathways involved in the carcinogenesis.
Hepatocarcinogenesis is a complex multistep process in which several signaling cascades are altered, leading to a heterogeneous molecular profile.[21] Several studies have reported the different alterations that occur in the setting of a chronically injured liver after hepatitis B and hepatitis C virus infection and alcohol or aflatoxin exposure. Alterations in gene expression, chromosomal amplification, mutations, deletions, and copy number alterations (gain/loss), CpG hypermethylation, and DNA hypomethylation, as well as molecular abnormalities have been proposed. These changes lead to oncogene activation and tumor suppressor gene inactivation, progressing from a dysplastic nodule to HCC. [21]–[22]
Signal transduction pathways transmit extracellular signals to the cell nucleus where they regulate cell processes and gene expression patterns in order to induce context-specific cellular responses. Some pathways share a common structure (e.g., EGF receptor, insulin-like growth factor receptor, MET), in which a receptor with tyrosine kinase activity is phosphorylated upon binding to a specific extracellular ligand. On ligand binding, the activated receptor signals through second messengers (e.g,, RAS, AKT, etc) in a cascade fashion to regulate gene expression and cell responses. It comprises an extracellular, N-terminal region that binds ligands and a conserved C-terminal region that autophosphorylates to create binding sites for SH2 and other phosphotyrosine-binding proteins, such as Src. Tyrosine kinases inhibitors (TKIs) by either competitive binding with adenosine triphosphate or allosteric inhibition prevent the autophosphorylation of receptor tyrosine kinase.[23]
Numerous signaling pathways (Ras/Raf/MAPK, WNT-β-catenin, EGFR, insulin-like growth factor receptor, AKT-mTOR, Notch, Hedgehog) have been implicated in hepatic carcinogenesis, cell activation, and proliferation associated with tumor growth (Fig. 3). [24]
Figure 3.
Signaling pathways in HCC
Ras/Raf/MAPK signaling pathway
Recent publications indicate that HCC cell activation and proliferation is known to involve several different signaling pathways as previously mentioned.[22] Among them, the Ras/Raf/MAPK, and PI3K/AKT/mTOR are the most critical pathways in development and proliferation of HCC and have been extensively investigated.
The Ras/Raf/MAPK pathway is possibly the best-characterized signal transduction pathway in cell biology. Signals from the extracellular milieu are transduced to the cell nucleus through this pathway in order to regulate multiple cellular functions including proliferation, growth, and differentiation.[25] Dysregulation of this pathway leads to inappropriate cellular activities including enhanced cell growth, differentiation and survival, and ultimately to cancer.[26]
Oncogenic Ras genes in human cells include H-ras, N-ras and K-ras. The 21-kd transforming proteins H- and K- ras genes were first identified by Harvey and Kirsten as the counterparts of the oncogenes of rat sarcoma viruses, whereas the N-ras oncogene was isolated from a neuroblastoma.[27]
The Ras superfamily of genes encodes small GTP-binding proteins that function as binary molecular switches that control multiple downstream effectors in a cascade fashion. Ras binding to the cytoplasmic side of the plasma membrane and its subsequent activation requires farnesylation.[28],[29] Activation of the pathway begins when a signal binds to a protein tyrosine kinase receptor. EGF, PDGF, and the VEGF receptors are the best-known receptors in this pathway. However, multiple upstream receptors including other receptor tyrosine kinases, integrins, serpentine receptors, heterotrimeric G-proteins, and cytokine receptors are able to activate K-ras.[30] In normal quiescent cells, Ras is bound to GDP and is inactive (“off state”), while upon binding of a ligand, Ras binds to GTP (“on state”), which has an extra phosphate group than GDP. This extra phosphate induces dimerization of the receptor, a process that results in juxtaposition of the cytoplasmic, catalytic domains in a manner that allows activation of the kinase activity and transphosphorylation.[31] Following Ras activation, Raf is recruited to the cell membrane through binding to the switch I domain of Ras and also by lipid binding.[32]
Raf is a Ras effector member of a family of serine/threonine kinases that consists of three isoforms - A-Raf, B-Raf and C-Raf/Raf-1. In its GTP-bound conformation, Ras combines with Raf and mobilizes the inactive protein from the cytoplasm recruiting the Raf kinases (A-Raf, B-Raf and C-Raf/Raf-1) to the plasma membrane.[33],[34] The Ras-Raf complex is translocated to the cell membrane and Ras activates the serine/threonine kinase function of Raf isoforms.
Raf activation stimulates a signaling cascade by phosphorylation of MAPK, also known as MEK, a serine/threonine kinase, to activate MEK1 and MEK2 which successively activate downstream proteins such as ERK1 and ERK2 kinases, which phosphorylate and activate a variety of nuclear transcription factors and kinases (including Elk-1, c-Ets1, c-Ets2, p90RSK1, MNK1, MNK2, as well as other proteins) involved in diverse cellular responses such as cell proliferation, survival, differentiation, motility, and angiogenesis. [35],[36]
Point mutations of the Ras family genes (H-RAS, K-RAS, and N-RAS) are not uncommon and comprise up to 30% of all human cancers.[37] Bos (et al) reported that 30% of HCC bear Ras mutations.[38] The most prevalent mutations in Ras genes are due to a substitution that renders the GTPase domain of Ras insensitive to inactivation by GDP and capable of autonomous activation (“on state”). [39],[40]
Proangiogenic pathways and other important signaling pathways in HCC activation and proliferation
Angiogenesis is a crucial event for a wide variety of physiological and pathological processes, from embryonic development and wound healing to cancer growth and metastasis. Angiogenesis is the consequence of a complex balancing process that is delicately regulated by promoting factors such as vascular endothelial growth factor (VEGF), angiopoietin, and fibroblast growth factor, and inhibitory factors like thrombospondin (TSP) and angiostatin.[41] The hypoxic condition in tumors induces increased expression and secretion of VEGFA by tumor cells.[42] Secreted VEGFA acts on endothelial cells (ECs) to induce EC proliferation, migration and survival, and finally angiogenesis that leads to tumor growth.[43]
The VEGF family consists of VEGF-A, B, C, D and E, and placental growth factor (PIGF). The VEGF-receptor family comprises VEGFR-1, VEGFR-2 and VEGFR-3. VEGF-A binds to VEGFR-1 and VEGFR-2 and is involved in angiogenesis and the maintenance of mature blood vessels, whereas VEGF-C and VEGF-D mainly bind to VEGFR-3 and are involved in lymphangiogenesis.[44]
HCC typically exhibits active angiogenesis. During the progression to differentiated HCC, angiogenesis and disruption of the vascular architecture occurs and contributes to increased vascular resistance, portal hypertension, and decreased hepatocyte perfusion.[45] In addition, during this process cancer cells develop the ability to invade vessels and metastasize.
Pro-angiogenic factors such as VEGF, angiopoietin, EGF, PDGF, FGF induce angiogenic signaling via RAS/RAF/MEK /ERK, mTOR, and Wnt signal transduction pathways. Moreover, recent studies demonstrated that VEGF expression is a prognostic factor in HCC. High VEGF expression has been associated with decreased survival.[46],[47]
The inhibition of proangiogenic pathways is a potential target for antitumor therapeutics, Bevacizumab, which directly targets VEGF, is currently being tested in multiple trials in combination with chemotherapy agents such as gemcitabine/oxaliplatin, capecitabine, with tyrosine kinase inhibitors such as sorafenib and erlotinib, and with transarterial chemoembolization. Ramucirumab, a monoclonal antibody against VEGFR2, is in an ongoing phase 3 trial in HCC patients.
Wnt-betacatenin pathway has been involved in tumor formation, in most cases through the dual function of β-catenin in cell adhesion and transcriptional activation of target genes.[48] This pathway regulates specific target genes such as c-myc, cyclin D, and surviving.[21] The Wnt pathway has been studied extensively in colon cancer, in which 90% of tumors contain a mutation of the APC gene.[49] In HCC, nuclear and/or cellular β-catenin accumulation, a hallmark of the activated canonical Wnt/FZ signaling has been observed in 33–67% of tumors. Mutations of APC are rare, only found in about 20–30%, suggesting that the predominant mechanism(s) activating Wnt/beta-catenin signaling pathway may be different from that found in colorectal cancers.[50] Mutations of β-catenin described in HCC are located in exon 3 of the CTNNB1 gene, the phosphorylation site for GSK3.[51]
The PI3K/AKT/mTOR signaling pathway plays an important role in HCC and is activated in 30–50% of HCC. The ribosomal protein S6 (RPS6), a target of p70S6K, is aberrantly activated in 50% of HCC.[52] The PI3K/Akt/mTOR pathway also plays a significant function in cell growth, survival regulation, metabolism, and anti-apoptosis. The PI3K/Akt/mTOR pathway is initiated by signaling inputs transduced to the inner cell after growth factor binding to a tyrosine kinase receptor (TKR) such as EGFR or IGFR.
The Hedgehog signaling pathway is a complex network of signaling molecules including both positive and negative regulatory proteins, fundamental to cell differentiation, regeneration, and stem cell biology, which has also been implicated in HCC proliferation. Similarly to β-catenin, after ligand stimuli, Gli accumulates inside the nucleus and induces transcription of genes related to cell cycle and growth, such as fibroblast and E, Bcl 2, N myc, β-catenin, and negative regulators ptch and Hh binding protein.[53]
The Notch pathway is an evolutionally conserved signaling pathway that has been implicated in a wide variety of processes, including cell-fate determination, tissue patterning and morphogenesis, cell differentiation, proliferation, and death.[54] Dysregulation of the Notch pathway has been involved in a growing number of highly aggressive hematopoietic and solid tumors.[54]–[55]
Another important regulator of organ size and tumorigenesis is the Hippo pathway. Dysregulation of the Hippo pathway contributes to the loss of contact inhibition observed in cancer cells, suggesting that YAP/TAZ plays a key role in the control of cell proliferation in response to cell contact. Interestingly, YAP protein is elevated and nuclear localized in some human liver and prostate cancers.[56]
The IGF/IGFR pathway is believed to play a key role in the pathogenesis of several tumors, including HCC. Most HCCs express insulin-like growth factors and their receptors (IGF-R). The ligands IGF-I and –II bind to their receptors IGF-1R and IGF-2R and are involved in DNA synthesis and cell proliferation. As genetic alterations in the IGF mediated signaling promotes survival, oncogenic transformation, and tumor growth and spread, it represents a potential target for innovative treatment strategies of HCC.[57],[58]
IGF targeting drugs are mainly anti-IGF-1R antibodies and are currently under development. Ongoing trials are testing cixutumumab (IMC-A12) in combination with sorafenib and MEDI-573 in combination with sorafenib, but the monoclonal antibody AVE1642 phase 1/2 trial was terminated by the sponsor.
Targeting Ras/Raf/MAPK and the role of combination therapy
Cancer cells usually have more than one signaling pathway activated, and there is a distinct genetic susceptibility that somehow defines their response to therapy. As mentioned previously, sorafenib is the only drug that has been found to decrease tumor progression and improve outcomes in patients with advanced HCC. There is no doubt that among different pathways being targeted, Ras/Raf/MAPK and PI3K/AKT/mTOR are perhaps the most critical in development and proliferation of HCC and therefore have been extensively investigated. These pathways are both activated by upstream receptor ligands and frequently co-regulate many downstream targets in parallel.
In 2008, the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) study, a double-blind randomized controlled trial using overall survival as primary end point, showed that sorafenib significantly increased survival in patients with HCC, from 7.9 to 10.7 months, with a manageable profile of side effects.[17] Sorafenib is a multikinase inhibitor of more than a dozen kinases, which has been shown to inhibit tumor cell proliferation by inhibiting the Ras/Raf/MAPK pathway and to suppress angiogenesis by blocking VEGFR and PDGFR signaling. Despite the approval of sorafenib, based on a phase III clinical trial demonstrating overall survival benefits and its extensive application in clinical practice during the past few years, it is increasingly clear that the benefits of sorafenib remain modest.[59]
The SHARP study prompted interest in developing new therapies against the multiple pathways involved in HCC carcinogenesis. Numerous targeted therapies are currently under evaluation in different phases; these are summarized in Table 1. Effective inhibitors of specific key components of the Ras/Raf/MAPK and PI3K/AKT/mTOR pathways, have been developed and in many cases have been examined in clinical trials.[60] Nonetheless, improving the effectiveness of treating liver cancer patients with small molecule signal transduction inhibitors has proven to be difficult, and initial results have been negative with several different compounds. The Sunitinib trial, a global cooperative phase III study comparing sunitinib, a multikinase inhibitor that targets VEGFR-1, -2, -3, PDGFR and c-kit, was halted because of high toxicity and lack of superiority in terms of efficacy compared with sorafenib. Bivranib, an oral, selective, dual inhibitor of FGF-1,-2,-3, and VEGF-1,-2, and -3, failed to demonstrate survival benefit with placebo as a second-line therapy. The phase III trial testing the multikinase inhibitor targeting VEGFR and PDGFR families, linifanib, has therefore been terminated. The combination of sorafenib and erlotinib, an EGFR inhibitor, failed in comparison to sorafenib alone as a first line therapy.
Table 1.
Molecular Therapies (Including TKIs, mAbs, and Oligonucleotide Antisense) Currently Under Evaluation in HCC
Molecular Therapies Currently Under Evaluation in HCC (modified with permission from Villanueva and Llovet) [23]
| Drugs | Phase | Trials, n | Targets | |
|---|---|---|---|---|
| 1 | Sorafenib | 1, 1–2, 2, 3, 4 | 65 | BRAF, VEGFR, PDGFR |
| 2 | Erlotinib | 1, 1–2, 2, 3 | 13 | EGFR |
| 3 | Everolimus | 1, 1–2, 2, 3 | 7 | MTORC1 |
| 4 | Brivanib | 1, 2, 3 | 6 | FGFR, VEGFR, PDGFR |
| 5 | Sunitinib | 2, 3 | 6 | VEGFR, PDGFR, KIT |
| 6 | Linifanib | 2 | 1 | VEGF, PDGFR |
| 7 | PI-88 | 2, 3 | 2 | Endo-β-D-glucuronidase heparinase |
| 8 | Ramucirumab | 2, 3 | 2 | VEGFR2 |
| 9 | Bevacizumab | 1, 1–2, 2 | 20 | VEGF |
| 10 | AZD6244 | 1–2, 2 | 4 | MEK |
| 11 | Bortezomib | 1, 2 | 4 | Proteasome |
| 12 | Cediranib | 1, 2 | 3 | VEGFR |
| 13 | Cetuximab | 1, 2 | 3 | EGFR |
| 14 | Cixutumumab | 1, 2 | 4 | IGF-1R |
| 15 | Temsirolimus | 1, 2 | 12 | MTORC1 |
| 16 | ARQ197 | 1, 2, 3 | 6 | MET |
| 17 | BIBF1120 | 1,2 | 3 | VEGFR, PDGFR, FGFR |
| 18 | Dasatinib | 2 | 2 | BCR-ABL |
| 19 | GC33 | 1,2 | 3 | GPC3 |
| 20 | Gefitinib | 2 | 2 | EGFR |
| 21 | Lapatinib | 2 | 3 | EGFR, HER2/neu |
| 22 | Licartin | 2, 4 | 2 | HAb18G/CD147 |
| 23 | Pazopanib | 2 | 2 | VEGFR, PDGFR, KIT |
| 24 | Alvocidib | 1, 2 | 2 | Cyclin-dependent kinase |
| 25 | AEG35156 | 1–2 | 1 | XIAP |
| 26 | AMG386 | 2 | 1 | Angiopoietin |
| 27 | AZD8055 | 1–2 | 1 | MTORC1, MTORC2 |
| 28 | Regorafenib | 2,3 | 2 | VEGFR, TIE-2 |
| 29 | BIIB022 | 1–2 | 1 | IGF-1R |
| 30 | Belinostat | 1–2 | 1 | Histone deacetylase |
| 31 | CS-1008 | 2 | 1 | TRAIL |
| 32 | E7080 | 1,2,3 | 2 | VEGFR, FGFR, SCFR |
| 33 | Foretinib | 1 | 1 | MET |
| 34 | IDN-6556 | 2 | 1 | Caspase |
| 35 | IMC-1121B | 2 | 1 | VEGFR2 |
| 36 | Ispinesib | 2 | 1 | Kinesin spindel protein |
| 37 | LBH589 | 1 | 1 | Histone deacetylase |
| 38 | MLN8237 | 2 | 1 | Aurora kinase |
| 39 | Mapatumumab | 1–2 | 1 | TRAIL |
| 40 | Oblimersen | 2 | 1 | BCL2 |
| 41 | Panobinostat | 1 | 1 | Histone deacetylase |
| 42 | Resminostat | 2 | 1 | Histone deacetylase |
| 43 | TSU-68 | 1–2 | 1 | VEGFR, FGFR, PDGFR |
| 44 | Talabostat | 1 | 1 | Dipeptidyl peptidases |
| 45 | Tremelimumab | 2 | 1 | B7-CD28 |
| 46 | Vandetanib | 2 | 1 | EGFR, VEGFR, RET |
| 47 | Vorinostat | 1 | 1 | Histone deacetylase |
| 48 | Z-208 | 1–2 | 1 | RAR |
| 49 | E7050 | 1–2 | 1 | c-MET, VEGFR |
| 50 | TRC105 | 1–2 | 2 | Anti CD-105 antibody |
| 51 | Bavituximab | 1–2 | 1 | Phosphatidylserine monoclonal antibody |
| 52 | RO5323441 | 1 | 1 | Anti-PIGF |
| 53 | BAY86-9766 | 2 | 1 | MEK |
| 54 | Perifosine | 1 | 1 | Akt, PI3K |
| 55 | MEDI-573 | 1 | 1 | IGF-1,-2 |
Recently, our group demonstrated that although monotherapy is effective in vitro, the patterns of response to drugs are different between different stem and non-stem cell HCC lines using different molecules to block Ras/Raf/MAPK and PI3K/akt/mTOR pathways. PKI-587, a dual inhibitor of PI3K and mTOR in combination with sorafenib is superior to monotherapy in stem (CD133+, CD44+ and CD24+) cells and non- liver cancer stem HCC lines in vitro. Interestingly, we demonstrated that blockage/inhibition of only one of the main pathways PI3K/mTOR or Ras/Raf/MAPK, separately, could result in activation of the other pathway which could result in cell survival, thus highlighting the importance of drug combination in HCC therapy.[61] We also evaluated PI-103, another dual inhibitor of the PI3K/mTOR pathway, in combination with sorafenib and demonstrated excellent results in vitro and in vivo.[62] Other inhibitors of the PI3K/akt/mTOR pathway have been used alone and in combination on different HCC cell lines in vitro and in vivo, such as AZD-8055 (mTOR inhibitor), BKM-120 (PI3K inhibitor), BEZ-235, and GDC-0980 (dual PI3K/mTOR inhibitor).
There are at least 35 combination therapy studies for advanced stage HCC treatment ongoing in phases 1–3 (Table 2). Numerous reagents are being tested targeting novel signaling cascades (WNT-β-catenin, Notch, etc).[63]
Table 2.
Combination therapy as first line treatment of advanced HCC.
| ID | Study | Phase |
|---|---|---|
| NCT00956436 | BIIB022 + Sorafenib | Phase I |
| NCT01029418 | AZD6244 + Sorafenib | Phase 1 / Phase 2 |
| NCT01008917 | Temsirolimus + Sorafenib | Phase I |
| NCT00943449 | 4SC-201 (Resminostat) + Sorafenib (Shelter) | Phase 2 |
| NCT01271504 | E7050 + Sorafenib | Phase 1 / Phase 2 |
| NCT01306058 | TRC105 + Sorafenib | Phase 1 / Phase 2 |
| NCT00882869 | AEG35156 + Sorafenib | Phase 1 / Phase 2 |
| NCT01687673 | Temsirolimus + Sorafenib | Phase 2 |
| NCT00712855 | Mapatumumab + Sorafenib | Phase 1 |
| NCT01258608 | Mapatumumab + Sorafenib | Phase 2 |
| NCT00872014 | AMG 386 + Sorafenib | Phase 2 |
| NCT01335074 | Temsirolimus + Sorafenib | Phase 1 / Phase 2 |
| NCT00976170 | GC33 + Sorafenib | Phase 1 |
| NCT01033240 | CS1008 + Sorafenib | Phase 2 |
| NCT01264705 | Bavituximab + Sorafenib | Phase 1 / Phase 2 |
| NCT01308723 | RO5323441 + Sorafenib | Phase 1 |
| NCT00906373 | IMC-A12 + Sorafenib | Phase 2 |
| NCT01004003 | BIBF 1120 + Sorafenib | Phase 2 |
| NCT01498952 | MEDI-573 + Sorafenib | Phase 1 |
| NCT00823290 | LBH589 + Sorafenib | Phase 1 |
| NCT00901901 | Erlotinib + sorafenib (SEARCH) | Phase 3 |
| NCT01204177 | BAY86-9766 + Sorafenib (BASIL) | Phase 2 |
| NCT01013519 | Temsirolimus + Sorafenib Tosylate | Phase 1 |
| NCT00867321 | Bevacizumab + Sorafenib | Phase 1 / Phase 2 |
| NCT01008566 | Cixutumumab + Sorafenib Tosylate | Phase 1 |
| NCT01005199 | Sorafenib + Everolimus | Phase 2 |
| NCT00881751 | Bevacizumab + Erlotinib or Sorafenib | Phase 2 |
| NCT01035229 | Everolimus + Sorafenib | Phase 3 |
| NCT00827177 | ARQ 197 (Tivantinib) + Sorafenib | Phase 1 |
| NCT00398814 | Perifosine + Sorafenib | Phase 1 |
| NCT00775073 | Bevacizumab + Everolimus | Phase 2 |
| NCT00287222 | Bevacizumab + Erlotinib | Phase 2 |
| NCT01010126 | Temsirolimus + Bevacizumab | Phase 2 |
| NCT00467194 | Sirolimus + Bevacizumab | Phase 1 |
BIIB022 (anti-IGF-1R antibody); AZD6244 (Selumetinib MEK inhibitor); 4SC-201 (Resminostat HDAC inhibitor); E7050 (c-Met and VEGFR inhibitor); TRC105 (IgG1 anti-CD105 monoclonal antibody); AEG35156 (inhibitor of apoptosis protein - antisense oligonucleotide); AMG 386 (Selective Angiopoietin ½ Neutralizing Peptibody); GC33 (monoclonal antibody against GPC3); CS1008 (IgG1 monoclonal antibody against human death receptor 5 (DR5)); RO5323441 (monoclonal antibody against PlGF-induced VEGFR-1 phosphorylation); IMC-A12 (anti-IGF-1R antibody); BIBF 1120 (Nintedanib TKI of VEGF, PDGF and FGF); MEDI-573 (anti-IGF-1R antibody); LBH589 (Panobinostat HADC inhibitor); BAY86-9766 (Refametinib MEK inhibitor); ARQ 197 (Tivantinib c-Met inhibitor)
Refametinib (BAY86-9766) and selumetinib (AZD6244) are MEK inhibitors that induce apoptosis and cease cellular proliferation of HCC cellular lines. They are currently being tested in combination with sorafenib in a phase 2 trial.
The PI3K/Akt/mTOR inhibitors currently used in combination with sorafenib include agents in early stages of development, like the Akt inhibitor perifosine in a phase 1 trial, but also everolimus (RAD001), sirolimus (Rapamune), and temsirolimus are being tested in phase 1 and 2 clinical trials.
Bevacizumab, a recombinant, humanized monoclonal antibody directed against VEGF, has emerged as an important therapeutic agent in several malignancies and has been approved in the treatment of colorectal cancer, non-small-cell lung cancer and breast carcinoma. Bevacizumab has been evaluated as a single agent in HCC with no encouraging results, but its combination with sorafenib is being tested in a phase 1/2 clinical trial.
Bavituximab, an anti-phosphatidylserine monoclonal antibody, is also being tested in combination with sorafenib in a phase 1/2 clinical trial. Llovet (et al) reported the use of panobinostat, a pan-HDAC-inhibitor, alone and in combination with sorafenib in an in vitro and in vivo model with promising results.[64] Now panobinostat and resminostat are being tested in combination with sorafenib in a phase 1 and phase 2 clinical trials, respectively.
The IGF/IGFR system plays an important role in cell proliferation and resistance to chemotherapy. IGF-targeting drugs are at this time under development and mainly include anti-IGF antibodies. BIIB022, MEDI-573, and IMC-A12 (cixutumumab) are in phase 1 and 2 clinical trials in combination with sorafenib.
Dysregulation of the c-MET receptor and its ligand HGF, critical for hepatocyte regeneration after liver injury, is a common event in HCC. Tivantinib, a c-Met inhibitor, and E7050, a dual c-MET and VEGFR-2 tyrosine kinase inhibitor, are presently being tested in combination with sorafenib in a phase 1/2 trial.
A lot of effort has been made to identify small molecules capable of disrupting aberrant Wnt/β-Catenin pathway to treat HCC. Recently, our group has studied inhibition of the WNT-β-catenin pathway with FH535 on HCC cell lines Huh7, Hep3B and PLC, and on liver CSCs with very promising results in vitro (manuscript in press, Anticancer Research). The small molecular agent FH535 is a dual inhibitor of peroxisome proliferator-activated receptor (PPAR) and β-catenin/TCF/LEF.
Additional targeted therapies are being studied at the present time, such as the extrinsic/intrinsic apoptotic pathway, Hedgehog signaling, JAK/STAT signaling, TGF-β signaling, Notch pathway, ubiquitin-proteasome pathway, nuclear factor-κB signaling, and cell cycle control.
Conclusion
HCC has proven to be a very heterogeneous tumor. Regardless of the recent advances in the understanding of the pathophysiology, HCC carcinogenesis remains uncertain. Numerous signaling pathways (Ras/Raf/MAPK, WNT-β-catenin, EGFR, insulin-like growth factor receptor, AKT-mTOR, Notch, Hedgehog) have been studied in hepatic carcinogenesis and their components represent molecular targets for therapy in HCC. Most patients with HCC have underlying cirrhosis, which has an adverse impact on the overall survival and makes them vulnerable to toxicity. The management of HCC has changed substantially in recent times, and the development of sorafenib represented a significant breakthrough that has prompted further expansion on molecular targeted therapies that potentially inhibit different pathways in hepatocarcinogenesis. Numerous agents are under various phases of clinical development, and combination therapy is gaining importance with multiple trials currently ongoing. Recently, significant interest in the CSC theory is increasing; and according to the CSC hypothesis, cancer initiation, progression, recurrence, metastasis, and therapy resistance are unique properties implicit on CSC subsets. Focusing on targeting CSCs could probably bring important and revolutionary advances in cancer therapeutics.
Acknowledgment
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003 Dec;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology. 2012 May;142(6):1264.e1–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N. Engl. J. Med. 2011 Sep;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Karin M. Obesity, inflammationm, and liver cancer. J. Hepatol. 2012 Mar;56(3):704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Yang H, Yang W, Liu C, Chen P, You S, Wang L, Sun C, Lu S, Chen D, Chen C. Metabolic Factors and Risk of Hepatocellular Carcinoma by Chronic Hepatitis B/C Infection: A Follow-up Study in Taiwan. Gastroenterology. 2008 Jul;135(1):111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003 Apr;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004 Feb;126(2):460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. Off. Clin. Pr. J. Am. Gastroenterol. Assoc. 2006 Mar;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz J-F, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008 May;134(6):1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin. Liver Dis. 2010 Feb;30(1):61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatol. Baltim. Md. 2005 Nov;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 13.Clark HP, Carson WF, Kavanagh PV, Ho CPH, Shen P, Zagoria RJ. Staging and Current Treatment of Hepatocellular Carcinoma1. Radiographics. 2005 Oct;25(suppl 1):S3–S23. doi: 10.1148/rg.25si055507. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52(2):762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatol. Baltim. Md. 2003 Feb;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 16.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J. Hepatol. 2012 Jun;56(6):1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz J-F, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008 Jul;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang T-S, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009 Jan;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 2005;25(2):181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 20.“EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012 Apr;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin. Liver Dis. 2007 Feb;27(1):55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 22.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006 Jun;25(27):3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 23.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011 May;140(5):1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo M. Current status of molecularly targeted therapy for hepatocellular carcinoma: clinical practice. Int. J. Clin. Oncol. 2010 Jun;15(3):242–255. doi: 10.1007/s10147-010-0089-y. [DOI] [PubMed] [Google Scholar]
- 25.Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2006 Jan;1(1):7–9. [PubMed] [Google Scholar]
- 26.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EWT, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Bäsecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007 Aug;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbacid M. ras genes. Annu. Rev. Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BE, Heymach JV. Farnesyl transferase inhibitors for patients with lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004 Jun;10(12 Pt 2):4254s–4257s. doi: 10.1158/1078-0432.CCR-040016. [DOI] [PubMed] [Google Scholar]
- 29.Caponigro F. Farnesyl transferase inhibitors: a major breakthrough in anticancer therapy? Naples, 12 April 2002. Anticancer. Drugs. 2002 Sep;13(8):891–897. doi: 10.1097/00001813-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Cantrell DA. GTPases and T cell activation. Immunol. Rev. 2003 Apr;192:122–130. doi: 10.1034/j.1600-065x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 31.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010 Jun;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marais R, Light Y, Paterson HF, Marshall CJ. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. Embo J. 1995 Jul;14(13):3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat. Rev. Cancer. 2010 Dec;10(12):842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chong H, Vikis HG, Guan K-L. Mechanisms of regulating the Raf kinase family. Cell. Signal. 2003 May;15(5):463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 35.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007 May;26(22):3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 36.Crews CM, Alessandrini A, Erikson RL. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 37.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012 Jan;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep;49(17):4682–4689. [PubMed] [Google Scholar]
- 39.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001 Jan;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 40.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta Bba - Rev. Cancer. 2003 Mar;1603(2):47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000 Sep;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 42.Harris AL. Hypoxia — a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002 Jan;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 43.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Publ. Online 29 April 1993 Doi101038362841a0. 1993 Apr;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N, Davis-Smyth T. The Biology of Vascular Endothelial Growth Factor. Endocr. Rev. 1997 Feb;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 45.Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J. Hepatol. 2009 Mar;50(3):604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br. J. Cancer. 2009 May;100(9):1385–1392. doi: 10.1038/sj.bjc.6605017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tseng P-L, Tai M-H, Huang C-C, Wang C-C, Lin J-W, Hung C-H, Chen C-H, Wang J-H, Lu S-N, Lee C-M, Changchien C-S, Hu T-H. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J. Surg. Oncol. 2008 Oct;98(5):349–357. doi: 10.1002/jso.21109. [DOI] [PubMed] [Google Scholar]
- 48.Gavert N, Ben-Ze’ev A. beta-Catenin signaling in biological control and cancer. J. Cell. Biochem. 2007 Nov;102(4):820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 49.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta. 2003 Jun;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 50.Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front. Biosci. J. Virtual Libr. 2006;11:1901–1915. doi: 10.2741/1933. [DOI] [PubMed] [Google Scholar]
- 51.Zucman-Rossi J, Jeannot E, Nhieu JTV, Scoazec J-Y, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, Wendum D, Chiche L, Fabre M, Mellottee L, Laurent C, Partensky C, Castaing D, Zafrani ES, Laurent-Puig P, Balabaud C, Bioulac-Sage P. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatol. Baltim. Md. 2006 Mar;43(3):515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 52.Mínguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr. Opin. Gastroenterol. 2009 May;25(3):186–194. doi: 10.1097/MOG.0b013e32832962a1. [DOI] [PubMed] [Google Scholar]
- 53.Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol. Biosyst. 2010 Jan;6(1):44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- 54.Yin L, Velazquez OC, Liu Z-J. Notch signaling: emerging molecular targets for cancer therapy. Biochem. Pharmacol. 2010 Sep;80(5):690–701. doi: 10.1016/j.bcp.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol. Cancer Ther. 2006 Mar;5(3):483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 56.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai Z-C, Guan K-L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007 Nov;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J-M, Chen W-S, Liu Z-P, Luo Y-H, Liu W-W. Effects of insulin-like growth factors-IR and -IIR antisense gene transfection on the biological behaviors of SMMC-7721 human hepatoma cells. J. Gastroenterol. Hepatol. 2003 Mar;18(3):296–301. doi: 10.1046/j.1440-1746.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- 58.Höpfner M, Huether A, Sutter AP, Baradari V, Schuppan D, Scherübl H. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem. Pharmacol. 2006 May;71(10):1435–1448. doi: 10.1016/j.bcp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Zhu AX. New agents on the horizon in hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2013 Jan;5(1):41–50. doi: 10.1177/1758834012458480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Milella M, Tafuri A, Bonati A, Evangelisti C, Cocco L, Martelli AM, McCubrey JA. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011 Mar;2(3):135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J. Surg. Res. 2012 Aug;176(2):542–548. doi: 10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 62.Gedaly R, Angulo P, Chen C, Creasy KT, Spear BT, Hundley J, Daily MF, Shah M, Evers BM. The role of PI3K/mTOR inhibition in combination with sorafenib in hepatocellular carcinoma treatment. Anticancer Res. 2012 Jul;32(7):2531–2536. [PubMed] [Google Scholar]
- 63. [Accessed: 13-Feb-2013];Home - ClinicalTrials.gov. [Online]. Available: http://clinicaltrials.gov/.
- 64.Lachenmayer A, Toffanin S, Cabellos L, Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai H-W, Ward SC, Thung S, Friedman SL, Llovet JM. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J. Hepatol. 2012 Jun;56(6):1343–1350. doi: 10.1016/j.jhep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]